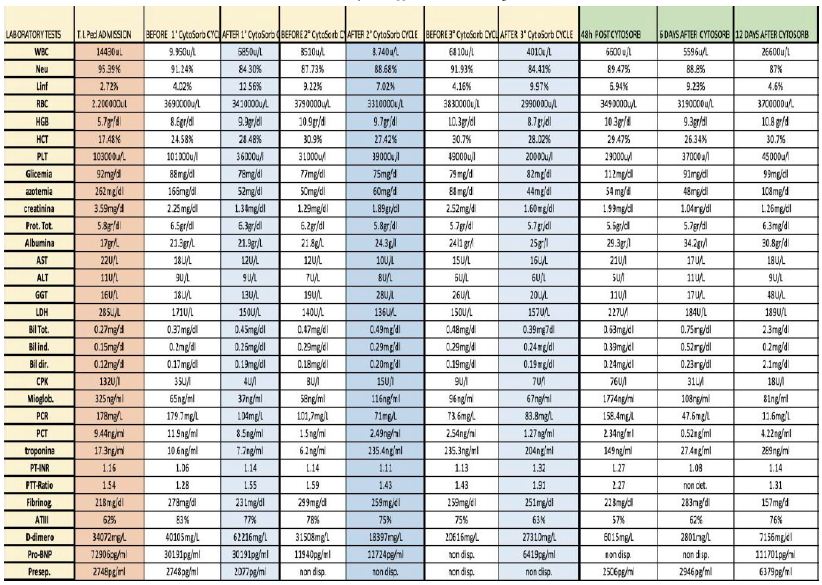
Abstract
Background: Despite the availability of efficacious drugs, tuberculosis remains a major public health problem in low- and middle-income countries. This study was aimed to determine the prevalence of tuberculosis in Massawa Hospital, Eritrea.
Methods: Laboratory and medical records of tuberculosis patients in Massawa Hospital were reviewed. All patients who did sputum exam by Xpert Gene from January 01, 2018 to May 1, 2021 in Massawa Hospital were enrolled in this study. Categorical variables were presented in percent, frequencies, Chi-square test, and odds ratio with 95% confidence interval. P value <0.05 was considered significant.
Results: Sputum examination was done on 2178 patients and the prevalence of bacteriologically positive tuberculosis was 7%. Moreover, the prevalence of rifampicin resistant tuberculosis among the total tested and bacteriologically positive patients was 0.4% and 5.9% respectively. The main reason for sputum examination was presumptive diagnosis of tuberculosis (85.5%). Tuberculosis spondylitis (15.6%) and adenitis (13.6%) were found to be the most common types of extra pulmonary tuberculosis. The prevalence of tuberculosis in HIV patients was 5.2% and all started highly active antiretroviral therapy. Patients aged 15 to 24 years were having higher prevalence of tuberculosis (8.8%, 95%CI 0.68-4.72, OR-1.79). And, those from Ghelaelo subzone were having about two times higher prevalence of tuberculosis (9.9%, 95%CI 1.39-3.06, OR-2.06). Patients who had previous history of tuberculosis were having about five times higher prevalence of tuberculosis (27.5%, 95%CI 2.65-11.17. OR-5.4, p<0.001) and Rifampicin resistant tuberculosis (9.1%, p<0.002).
Conclusion: The prevalence of tuberculosis and the multidrug resistant tuberculosis among the confirmed cases was comparatively increased than the average WHO estimates for Eritrea and similar to a study conducted in Nakfa subzone, Eritrea. The prevalence of tuberculosis in HIV patients was higher to the WHO estimates and previous studies in the country. Previous history of tuberculosis was significantly associated with the prevalence tuberculosis and multidrug resistant tuberculosis. Further prospective studies to evaluate the national prevalence of tuberculosis and rifampicin resistant tuberculosis are highly recommended.
Keywords
Tuberculosis, Prevalence, Multidrug resistance tuberculosis, Eritrea
Introduction
Tuberculosis (TB) is a life-threatening disease caused by Mycobacterium tuberculosis having an increased prevalence in developing countries [1]. It is a major global health problem and ranks alongside HIV as a leading cause of mortality worldwide [2]. Mycobacterium tuberculosis is an intracellular bacterium, causing respiratory illness, tuberculosis. The bacilli infect about one-third of the world’s population, leaving the majority with an asymptomatic state of a disease called latency [3]. While only a few proportions (10%) of those latently infected develop active TB, the majority (90%) will remain asymptomatic [4].
According to World Health Organization (WHO) 2019 tuberculosis report, there are an estimated 10 million people infected with TB and 1.2 million tuberculosis deaths among peoples living with HIV/AIDS globally [5]. Worldwide, millions of people continue to fall sick and die from TB, a preventable and curable infectious disease [6].
Treatment outcome is an important indicator to evaluate the effect of TB prevention and control program. Globally, in 2012, the treatment success rate (TSR) was 86% among all new TB cases and in the African region, it was 81% [7]. WHO recommends that at least 90% TSR for all persons diagnosed with TB and initiated on TB treatment services [8]. The latest global TB treatment outcome data for new bacteriologically confirmed pulmonary TB cases indicates a global fall in TSR from 86% in 2014 to 83% in 2017 [9].
According to the recent WHO estimate report, a total of 3100 new TB cases were present in 2018 in Eritrea which correspond to 89 cases per 100,000 populations [10]. Of these cases 140 were patients with TB and HIV co-infection and 66 of the cases had multidrug resistant TB. Nationally, 550 patients died from tuberculosis and related complications and 47 of them had HIV [10]. The 2018 conducted survey also revealed that the incidence rate of estimated proportion of TB cases with MDR-TB was 2% and 4.1% of them were from previously treated cases [10].
Study conducted in Nakfa subzone, one of the 58 subzones of Eritrea; showed prevalence of smear positive pulmonary TB cases was 7.8%, relatively increased prevalence of smear positive pulmonary tuberculosis than the average WHO estimate for the country [11]. This study also showed that females (8.2%), the adult age group of 41- 60 years (11%) and during the year 2014 (16.8%) had the highest rate of pulmonary TB infection [11].
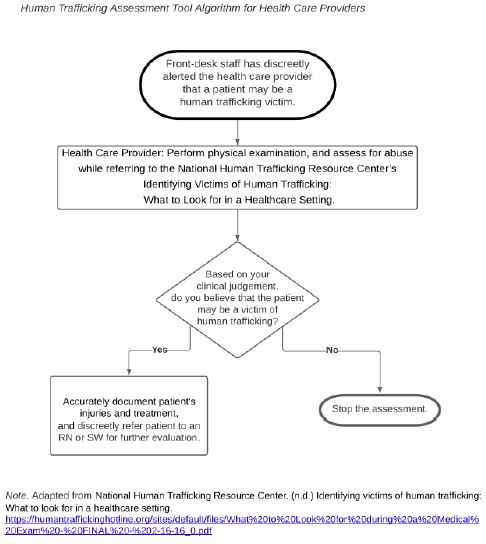
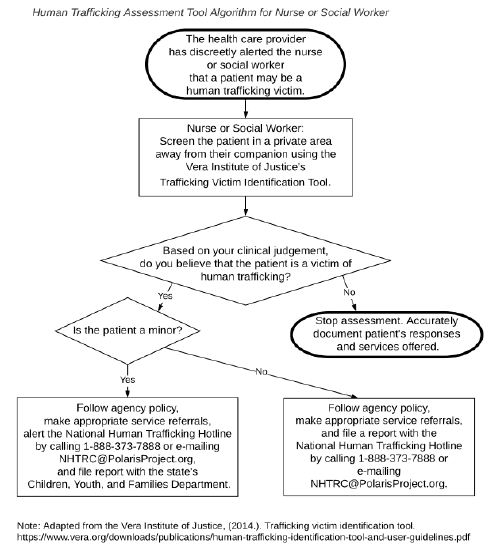
From observational point of view and work experience, the prevalence of pulmonary, extra pulmonary and multi-drug resistant tuberculosis seems higher in Massawa Hospital starting in 2020. This study was aimed to determine the prevalence of tuberculosis in Massawa Hospital, Eritrea.
Materials and Methods
Study Design and Population
This was a retrospective cross-sectional type of study that medical profile and laboratory results of all TB suspected patients, who did sputum exam by Xpert Gene from January 01, 2018 to May 1, 2021 in Massawa Hospital was retrieved, reviewed and analyzed in the study.
Data Collection, Analysis and Interpretation
Sputum results of patients were retrieved from the Xpert Gene machine register by experienced laboratory technicians from May 2-20, 2021. The socio-demographic characteristics and treatment outcomes were collected from the medical profile of patient’s treatment cards using a pre designed checklist.
Data was entered in CSPro 7.3 and analyzed by SPSS software. Categorical variables were presented in proportions, frequencies and Chi-squared test were implemented to assess association between the dependent and independent variables. Besides, odds ratio with 95% confidence interval was also presented and P value <0.05 was considered significant.
Ethical Clearance
Ethical approval was obtained from the Ministry of Health Ethical Review and Clearance Committee on 03/05/2021. Patient’s data confidentiality was kept secured and personal identifiers were not collected. A unique number was assigned to each patient in the data set and it was coded and interpreted in aggregates. Further permission was requested from the Zonal and Hospital Medical Directors.
Results
Socio Demographic Characteristics of the Patients
A sputum exam of 2178 patients was retrieved and about forty percent of them were requested by medical doctors. Majority of the patients who did sputum exam were in the age group of 35-54 years (36.1%) and the main reason was presumptive diagnosis of tuberculosis (85.5%). The prevalence of bacteriologically positive tuberculosis was 7%. The prevalence of Rifampicin resistant tuberculosis from all those who did sputum exam and the confirmed cases was 0.4% and 5.9% respectively (Table 1).
Table 1: Socio demographic characteristics of patients
|
Variables |
Categories |
Frequency (N) |
Percent (%) |
|
Diagnosis year |
2018 |
404 |
18.5 |
| 2019 |
562 |
25.8 |
|
| 2020 |
878 |
40.3 |
|
| May 2021 |
334 |
15.3 |
|
|
Age of respondent (years) |
< 15 |
98 |
4.5 |
| 15-34 |
701 |
32.2 |
|
| 35-54 |
785 |
36.1 |
|
| 55 and above |
594 |
27.2 |
|
|
Sex of respondent |
Female |
1086 |
49.9 |
| Male |
1092 |
50.1 |
|
|
Subzone of respondent |
Massawa |
1087 |
49.9 |
| Ghelaelo |
535 |
24.6 |
|
| Foro |
336 |
15.4 |
|
| Others |
220 |
10.1 |
|
|
Reason for sputum exam |
Presumptive |
1796 |
82.5 |
| Previous TB |
40 |
1.8 |
|
| contact trace |
121 |
5.6 |
|
| Others |
221 |
10.2 |
|
|
Requested by |
Doctor |
961 |
44.1 |
| Nurse degree |
572 |
26.3 |
|
| Nurse | 645 | 29.6 | |
|
Sputum result by Xpert Gene |
MTB detected | 152 | 7.0 |
| MTB not detected | 2026 | 93.0 | |
|
Rifampicin Resistance |
Resistant | 9 | 0.4 |
| Indeterminate | 12 | 0.6 | |
| Sensitive | 131 | 6.0 | |
| Total | 2178 | 100.0 | |
Association of Background of Patients with Prevalence of Tuberculosis
The prevalence of bacteriologically confirmed tuberculosis cases was higher on patients aged 15 to 24 years (8.8%, 95%CI 0.68-4.72, OR-1.79). Patients from Ghelaelo subzone were having about two times higher prevalence of tuberculosis compared to patients from Massawa subzone (9.9%, 95%CI 1.39-3.06, OR-2.06). Patients who had previous history of tuberculosis were having about five times higher prevalence of tuberculosis compared to the other groups (27.5%, 95%CI 2.65-11.17, OR-5.4, p<0.001). Other background of patients did not show significant association with the prevalence of tuberculosis (Table 2).
Table 2: Association of background characteristics with prevalence of Tuberculosis
| Variables | Sputum result N (%) | P value | Odds Ratio | 95%CI | |
| Negative | Positive | ||||
| Diagnosis year | |||||
| 2018 | 373 (92.3) | 31 (7.7) |
0.802 |
1 | |
| 2019 | 527 (93.8) | 35 (6.2) | 0.80 | (0.48-1.32) | |
| 2020 | 814 (92.7) | 64 (7.3) | 0.95 | (0.61-1.48) | |
| May, 2021 | 312 (93.4) | 22 (6.6) | 0.08 | (0.48-1.50) | |
| Age of respondents (years) | |||||
| < 15 | 93 (94.9) | 5 (5.1) |
0.828 |
1 | |
| 15-24 | 333 (91.2) | 32 (8.8) | 1.79 | (0.68-4.72) | |
| 25-34 | 314 (93.5) | 22 (6.5) | 1.30 | (0.48-3.54) | |
| 35-44 | 345 (92.7) | 27 (7.3) | 1.46 | (0.55-3.88) | |
| 45-54 | 385 (93.2) | 28 (6.8) | 1.35 | (0.51-3.60) | |
| 55-64 | 288 (93.8) | 19 (6.2) | 1.23 | (0.45-3.38) | |
| 65 and above | 268 (93.4) | 19 (6.6) | 1.31 | (0.48-3.63) | |
| Sex | |||||
| Female | 1008 (92.8) | 78 (7.2) |
0.710 |
1 | |
| Male | 1018 (93.2) | 74 (6.8) | 0.94 | (0.68-1.31) | |
| Subzone | |||||
| Massawa | 1032 (94.9) | 55 (5.1) |
0.018 |
1 | |
| Ghelaelo | 482 (90.1) | 53 (9.9) | 2.06 | (1.39-3.06) | |
| Foro | 310 (92.3) | 26 (7.7) | 1.57 | (0.97-2.55) | |
| Others* | 202 (91.2) | 18 (8.2) | 1.67 | (0.96-2.91) | |
| Reason for sputum exam | |||||
| Presumptive | 1679 (93.5) | 117 (6.5) |
0.001 |
1 | |
| Previous TB | 29 (72.5) | 11 (27.5) | 5.4 | 2.65-11.17 | |
| contact trace | 119 (98.35) | 2 (1.65) | 0.24 | 0.06-0.98 | |
| Others* | 199 (90.0) | 22 (10.0) | 1.59 | 0.98-2.56 | |
| Requested by | |||||
| Nurse degree | 531 (92.8) | 41 (7.2) |
0.238 |
1 | |
| Doctor | 903 (94.0) | 58 (6.0) | 0.83 | (0.55-1.26) | |
| Nurse | 592 (91.8) | 53 (8.2) | 1.16 | (0.76-1.77) | |
| Total | 2026 (93.0) | 152 (7.0) | 2178 (100.0) | ||
| *Dahlak, Gindae, Afabet, Nakfa, Shieb
**Follow up, HIV patient, unresponsive Smear negative |
|||||
Association of Background of Patients with MDR Tuberculosis
The highest prevalence of Rifampicin resistant (MDR) tuberculosis was detected in 2020 (9.4%) and was higher on patients aged 55 years an above (10.5%). The prevalence of MDR tuberculosis was higher on males (9.5%) and, residents of Foro and Ghelaelo subzones showed higher MDR prevalence compared to the other subzones. Patients who had history of tuberculosis were having higher prevalence of MDR tuberculosis (p<0.002). Background of patients did not show significant association to the MDR-TB prevalence. Patients’ with indeterminate Rifampicin resistance results were put on follow up and sputum exam was repeated after an interval of several days in which all of them were negative for rifampicin resistant tuberculosis (Table 3).
Table 3: Association of background characteristics with MDR Tuberculosis
| Variables | Rifampicin resistance N (%) | P value | ||
| Indeterminate | Sensitive | Resistant | ||
| Diagnosis year | ||||
| 2018 | 0 (0.0) | 30 (96.8) | 1 (3.2) |
0.085 |
| 2019 | 1 (2.9) | 32 (91.4) | 2 (5.7) | |
| 2020 | 7 (10.9) | 51 (79.7) | 6 (9.4) | |
| 2021 | 4 (18.2) | 18 (81.8) | 0 (0.0) | |
| Age of respondents (years) | ||||
| Under 15 | 1 (20.0) | 4 (80.0) | 0 (0.0) |
0.335 |
| 15-34 | 3 (6.8) | 39 (88.6) | 2 (4.6) | |
| 35-54 | 5 (9.1) | 47 (85.5) | 3 (5.5) | |
| 55 and above | 3 (7.9) | 31 (81.6) | 4 (10.5) | |
| Sex | ||||
| Female | 4 (5.1) | 72 (92.3) | 2 (2.6) |
0.071 |
| Male | 8 (10.8) | 59 (79.7) | 7 (9.5) | |
| Subzone | ||||
| Ghelaelo | 5 (9.4) | 44 (83.0) | 4 (7.5) |
0.927 |
| Massawa | 3 (5.5) | 49 (89.1) | 3 (5.5) | |
| Foro | 1 (3.8) | 23 (88.5) | 2 (7.7) | |
| Others | 3 (16.7) | 15 (83.3) | 0 (0.0) | |
| Reason for sputum exam | ||||
| Presumptive | 10 (7.8) | 110 (86.0) | 8 (6.2) |
0.002 |
| Previous TB | 0 (0.0) | 10 (90.9) | 1 (9.1) | |
| Others | 2 (15.4) | 11 (84.6) | 0 (0.0) | |
| Requested by | ||||
| Nurse degree | 1 (2.4) | 37 (90.2) | 3 (7.3) |
0.205 |
| Doctor | 4 (6.9) | 49 (84.5) | 5 (8.6) | |
| Nurse | 7 (13.2) | 45 (84.9) | 1 (1.9) | |
| Total | 12 (7.9) | 131 (86.2) | 9 (5.9) | 152 (100.0) |
Treatment Success of Tuberculosis
A total of 154 patients had started treatment for tuberculosis in the hospital in the study time and about nineteen percent were below 15. Two third (66.2%) of patients had pulmonary tuberculosis, and tuberculosis spondylitis (15.6%) and adenitis (13.6%) were the most common causes of extra pulmonary tuberculosis. The prevalence of tuberculosis in HIV patients was 5.2% and all patients these tested positive for HIV had started anti-retroviral treatment and co-trimoxazole preventive therapy. The prevalence of Rifampicin resistant tuberculosis from these started treatments was 5.8% and all were referred to Merhano National MDR-TB treatment hospital for management. The treatment success of tuberculosis was 81.2% with a death rate of 7.1% (Table 4).
Table 4: Treatment successes of tuberculosis patients
| Variables | Categories | Frequency (N) | Percent (%) |
|
Address |
Massawa | 131 | 85.1 |
| Foro | 19 | 12.3 | |
| Others | 4 | 2.5 | |
|
Age of respondent (years) |
< 15 | 29 | 18.8 |
| 15-34 | 39 | 25.3 | |
| 35-54 | 51 | 33.1 | |
| 55 and above | 16 | 22.7 | |
|
Sex of respondent |
Female | 81 | 52.6 |
| Male | 73 | 47.4 | |
|
Treatment started year |
2018 | 52 | 33.8 |
| 2019 | 40 | 26.0 | |
| 2020 | 62 | 40.3 | |
|
Site of infection |
Extra pulmonary | 52 | 33.8 |
| Pulmonary | 102 | 66.2 | |
|
If Extra pulmonary; Specify |
Bone TB | 24 | 15.6 |
| Skin TB | 6 | 3.9 | |
| TB adenitis | 21 | 13.6 | |
|
Type of patient |
New | 147 | 95.5 |
| Relapse | 7 | 4.5 | |
|
HIV status |
Negative | 146 | 94.8 |
| Positive | 8 | 5.2 | |
| Ante-retroviral therapy started | No | 146 | 94.8 |
| Yes | 8 | 5.2 | |
| Co-trimoxazole preventive therapy | No | 146 | 94.8 |
| Yes | 8 | 5.2 | |
|
Rifampicin resistant |
No | 118 | 94.2 |
| Yes | 9 | 5.8 | |
|
Treatment outcome |
Cured + Completed | 125 | 81.2 |
| Died | 11 | 7.1 | |
| Failure | 1 | 0.6 | |
| Not evaluated | 8 | 5.2 | |
| Referred | 9 | 5.8 | |
| Total | 154 | 100.0 | |
Discussion
Determining the prevalence of tuberculosis is very crucial for the management of the disease. This study was aimed to evaluate this challenge in Massawa Hospital. The prevalence of bacteriologically confirmed tuberculosis was 7%, (152 cases per 2178). This was almost similar to a study conducted in Nakfa subzone, Eritrea; which showed that the overall prevalence of 7.8% [11]. According to the recent WHO estimate report, the prevalence of tuberculosis in 2018 in Eritrea corresponds to 89 cases per 100,000 populations [10]. Estimated tuberculosis incidence in the Horn of Africa ranges from 65 cases per 100,000 people per year in Eritrea [12]. This higher prevalence could be attributed to the higher detection rate of tuberculosis after the introduction of diagnostic modalities like Xpert Gene and increasing community awareness about the disease and early health seeking behavior. The contributions of the community tuberculosis agents are also remarkable [13].
The prevalence of Rifampicin resistant tuberculosis in the bacteriologically confirmed new cases and previously treated was 5.9% and 9.1% respectively. This was higher to the national average based on the WHO estimates for Eritrea; the prevalence of MDR-TB among new cases and previously treated cases was 2.6% and 18% respectively [14]. It was also higher to a preliminary survey done in Eritrea that Rifampicin resistance among new cases and previously treated cases was 2% and 7.5% [14]. The introduction of the new diagnostic modalities could have a value on the higher incidence of MDR cases.
This study revealed that the prevalence of extra-pulmonary cases was 33.8% and tuberculosis spondylitis and adenitis were the most common cause. This was similar to the national average that the proportion of extra pulmonary notified in 2016 was 34% but higher to 16% for Africa [14]. This result showed that the disease is not contained in the lung which seeds it to different extra pulmonary sites that leads to different complications.
The prevalence of tuberculosis in HIV patients was 5.2%, which was slightly lower to the national average (6%) in 2017 [14] and higher to other study (3.7%) [13]. This shows that the impact of the previously introduced strategy by the Ministry of Health to screen all TB patients for HIV had increased to detect the co-infection and burden of these diseases.
This study showed that 85.7% of patients that were on treatment were new TB cases and 4.5% were with relapse. This was lower to other study that 92.6% of the patients were new TB cases, but there were 1.9% relapses cases [13]. Furthermore, this research reported that, 76.6% of the patients were bacteriologically positive before starting treatment. This was higher to the national average that of these notified 58% were bacteriologically positive compared to 64% in Africa in 2015 [14]. This was also higher to other study that 73% [15] and 45.1% [13] of the patients were bacteriologically positive pulmonary TB. This result explained that, some cases of the extra pulmonary cases, mostly the TB adenitis and skin TB were bacteriologically positive for mycobacterium tuberculosis.
This study revealed that the treatment success rate was 81.2%. Even though this was lower to the national average in 2016, 90% [14], it was similar to the 2012 treatment success rate in the African region, which was 81% [7]. When compared to other countries, this was similar to studies in South Africa 80% [16], Ethiopia 79.4% [17] and 81.8% [18]. But, it was lower to studies in South Africa 82.2% [19] and Ethiopia 90.1% [20], 86.8% [21]. It was higher to studies in Uganda 39% [22], Zimbabwe 70% [23], Nigeria 57.7% [24], and Russia 77% [25]. Since some patients (5.8%) were referred for MDR treatment and some were not evaluated (5.2%) as they didn’t yet complete their treatment, this could significantly decreased the success rate from the national average.
This study reported that majority of tuberculosis cases were in the age group of 15-54 years (58.4%) and children less than 15 years contribute 18.8%. This was similar to the national average in Eritrea in 2017 that the age of 15-54 years and children < 15 years was 61.5% and 17.1% respectively [14]. A study in Nakfa, Eritrea; showed that the adult age group of 41-60 years had the highest rate of pulmonary TB infection [11]. The higher prevalence in the pediatric and geriatric population could be due to their poor containment of the latent infection that leads to pulmonary or extra-pulmonary tuberculosis.
The mortality rate of tuberculosis in the hospital was 7.1%. This was higher to other studies, 5% [25] and 3.7% [13]. This higher rate of mortality could be due to other comorbid diseases, delayed health seeking behaviors and treatment complications.
Patient with previous history of tuberculosis showed significant association with the prevalence of tuberculosis and MDR tuberculosis. This could be mainly due to the presence of other smear positive cases in the family which didn’t get treated and spread the infection. Besides, treatment defaulters could be another cause for the higher prevalence of MDR cases in the previously treated patients.
Conclusion
The prevalence of tuberculosis was higher to the national average and the WHO estimates for the African region. Majority of the bacteriologically positive tuberculosis patients were new cases. The prevalence of extra pulmonary and tuberculosis in HIV patients was slightly lower to the national average but higher to other studies and WHO estimates for Eritrea. The treatment success rate was lower to the national average and patients with previous history of tuberculosis had showed significant association to the prevalence of tuberculosis and MDR tuberculosis.
Recommendations
To estimate the current prevalence of tuberculosis and MDR– tuberculosis, a national survey is highly recommended. Awareness of the community about adherence, disease and treatment complications are essential. Further prospective studies to evaluate the difference of tuberculosis prevalence by subzone and the impact of nutritional status are indispensable. Routine contact tracing for MDR-tuberculosis and the directly observed treatment strategy should be advocated to decrease the relapse and MDR-tuberculosis.
Declarations
Ethics Approval and Consent to Participate
Ethical approval was obtained from the Ministry of Health Ethical Review and Clearance Committee on 03/05/2021 and that informed consent was obtained from all subjects and/or their legal guardian (s). All methods were carried out in accordance with relevant guidelines and regulations.
Acknowledgment
Authors acknowledges for the patients for using their data.
Author’s Contribution
The proposal was drafted by BT, FK and further edition was done by all the authors. Data was analyzed by FK and all authors have participated on data interpretation. The draft of the manuscript was written by BT and the final form was shaped by BT, HG and FK. All authors have contributed by interpretation, analysis, critical discussion and approved for publication.
Abbreviations
TB: Tuberculosis, MDR: Multi Drug Resistant, TSR: Treatment Success Rate, HIV: Human Immunodeficiency Virus, ART: Antiretroviral Therapy, WHO: World Health Organization, MTB: Mycobacterium Tuberculosis, CSPro: Census and Survey Processing System, SPSS: Statistical Package for the Social Sciences.
References
- Kassa J, Dedefo M, Korsa A, Dibessa T (2018) Factors affecting treatment outcome of tuberculosis among tuberculosis patients in West J Bioanal Biomed. 10(1): 24-29. [crossref]
- Taye G, Defar A, Taddele T, et al. (2018) Treatment outcome and associated factors among TB patients in Ethiopia: hospital-based retrospective Am J Epidemiol Infect Dis 6 (1): 14-19.
- Dorhoi A, Reece ST, Kaufmann SH (2011) For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol Rev 240 (1): 235-251. [crossref]
- Lee S-W, Wu LS-H, Huang G-M, Huang K-Y, Lee T-Y (2016) Gene expression profiling identifies candidate biomarkers for active and latent tuberculosis. BMC Bioinform 17: S3. [crossref]
- World Health Organization. Global tuberculosis report; WHO/CDS/TB/2019:
- World Health Organization Global tuberculosis report 2018. Geneva, Switzerland, 2018: 27-67.
- World health organization, Global tuberculosis report. Geneva, Switzerland: WHO; 2015
- Stop TB Partnership First Global Plan to End TB Progress Report shows need for huge efforts and scale-up to reach 90-(90)-90 TB targets,
- Stop TB Partnership 90 (90) 90 The tuberculosis report for heads of state and governments. Geneva, Switzerland,
- World Health Organization, “Eritrea Tuberculosis profile 2018,” Geneva, Switzerland, 2018.
- Yafet Assessment of the Prevalence of Pulmonary Tuberculosis Patients at Nakfa Hospital from 2014-2019, Eritrea. [crossref]
- World Health Organization (WHO), Global tuberculosis report 2017, Geneva,
- Berihun, T. Nguse, G. Gebretekle (2018) Prevalence of Tuberculosis and Treatment Outcomes of Patients with Tuberculosis among Inmates in Debrebirhan Prison, North Shoa Ethiopia. Ethiop J Health Sci 28(3): 347-354. [crossref]
- The National Guidelines for Tuberculosis and Leprosy Control, the national TB control program, communicable disease control division, Ministry of Health, Eritrea, December
- GETIE, B. Alemnew (2020) Tuberculosis Treatment Outcomes and Associated Factors among Patients Treated at Woldia General Hospital in Northeast Ethiopia: An Institution-Based Cross-Sectional Study. Infect Drug Resist 13: 3423-3429. [crossref]
- Budgell EP, Evans D, Schnippel K, et al. (2016) Outcomes of treatment of drug- susceptible tuberculosis at public sector primary healthcare clinics in Johannesburg, South Africa: a retrospective cohort S Afr Med J 106: 1002-1009. [crossref]
- Tefera F, Dejene T, Tewelde T (2016) Treatment Outcomes of Tuberculosis Patients at Debre Berhan Hospital, Amhara Region, Northern Ethiopia. Ethiop J Health Sci 26(1): 65-72. [crossref]
- Zenebe T, Tefera E (2016) Tuberculosis treatment outcome and associated factors among smearpositive pulmonary tuberculosis patients in Afar, Eastern Ethiopia: a retrospective Braz J Infect Dis 20(6): 635-636. [crossref]
- Jacobson KB, Moll AP, Friedland GH, et (2015) Successful tuberculosis treatment outcomes among HIV/TB coinfected patients Down-Referred from a district hospital to primary health clinics in rural South Africa. PLoS One 10: e0127024. [crossref]
- Worku S, Derbie A, Mekonnen D, et al. (2018) Treatment outcomes of tuberculosis patients under directly observed treatment short-course, Debre Tabor General Hospital, Northwest Ethiopia: nine-year retrospective Infect Dis Poverty 7: 16. [crossref]
- Getnet F, Sileshi H, Seifu W, et al. (2017) Do retreatment tuberculosis patients need special treatment response follow-up beyond the standard regimen? Finding of five- year retrospective study in pastoralist BMC Infect Dis 17: 762. [crossref]
- Nakanwagi-Mukwaya A, Reid AJ, Fujiwara PI, et al. (2013) Characteristics and treatment outcomes of tuberculosis retreatment cases in three regional hospitals, Uganda. Public Health Action 3: 149-155. [crossref]
- Takarinda KC, Harries AD, Srinath S, et (2011) Treatment outcomes of new adult tuberculosis patients in relation to HIV status in Zimbabwe. Public Health Action 1: 34-39. [crossref]
- Ukwaja KN, Ifebunandu NA, Osakwe PC, et al. (2013) Tuberculosis treatment outcome and its determinants in a tertiary care setting in south-eastern Niger Postgrad Med J 20: 125-129. [crossref]
- Shin SS, Pasechnikov D, Gelmanova IY (2006) Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis 10(4): 402-408. [crossref]