DOI: 10.31038/CST.2016115
Abstract
Lysine-Specific Demethylase 2 (LSD2), is a flavin-dependent demethylase which acts on the fourth and ninth lysine residues of the histone protein H3 (H3K4 and H3K9). Its homolog, Lysine-Specific Demethylase 1 (LSD1), has been found to be an oncogene in several cancer pathways; due to the two enzymes’ similar structures, LSD2 can be considered a target for the treatment of human cancers. However, the current literature on LSD2 does not agree upon its function in cancer, i.e., whether it functions as a tumor-suppressor or an oncogene, but rather suggests that it may be integral to pathways that serve both effects in human cancer. This paper discusses five major studies on LSD2 in cancer. The first two directly involve human cancer cell lines and disagree on LSD2’s role; Katz et al. found LSD2 in breast cancer to function as an oncogene while Yang et al. studied LSD2 in lung cancer and found it to be vital to a tumor-suppressive pathway, due to its role as an E3 ligase in the autoubiquitylation of O-GlcNAc Transferase (OGT). The other two studies indicate that LSD2 may play multiple roles in human cancer cell survival; LSD2 was found to work in a feed-forward loop with the Nuclear Factor Kappa-light-chain-enhancer of activated B cells (NF-кB), a protein involved in tumor viability, but found to be underexpressed in glioblastomas, where it acts as a target of microRNA-215 (miR-215), which is vital to the survival of glioblastomas under hypoxic conditions. Finally, LSD2 serves as part of an expression pathway in human stem cell carcinomas that controls chemosensitivity and apoptosis. Ultimately, these results indicate that LSD2 is an important target for further study and may play a crucial role in understanding pathways that illuminate novel treatments for human cancer.
Key words
LSD2/KDM1B/AOF1, oncogene, tumor-suppressor, OGT, NF-кB, miR-215
Introduction
Post-translational modification (PTM) of proteins plays important functional roles in gene expression patterns and various cellular pathways. Gene expression within a cell and the maintenance of cell phenotype is highly regulated, and orchestrated by epigenetic PTM such as histone modification and DNA methylation. Carcinogenesis and tumor expression are heavily dependent on alteration of normal gene expression, and thus epigenetic modifications, such as methylation and demethylation, play an important role in tumor progression [1].
The regulation of methylation state, specifically the insertion and deletion of methyl groups on lysine residues of proteins, is carried out by lysine methyl transferases (KMTs) and lysine-specific demethylases (KDMs), respectively. While they were originally classified as histone-modifying enzymes, their role in PTM of non-histone proteins is increasingly being recognized. The KDMs are divided into two distinct categories – the flavin adenine dinucleotide (FAD) dependent amine oxidases (KDMs 1A and 1B) and the iron-and α-ketoglutarate-dependent KDMs (KDMs 2-6) – based on the mechanism of demethylation each utilizes [2]. The roles that such epigenetic enzymes play in the malignant states of human cancer cells are pressing to elucidate, as these can reveal targets for novel therapeutic cancer treatments.
LSD2/KDM1B/AOF1 is a flavin-dependent, lysine-specific demethylase that functions on the mono- and di-methylated states of histone H3K4 (H3K4me1/2) [3], and also shows demethylase activity against di-methylated H3K9 (H3K9me2) [4]. It is a closely related homolog of a more widely studied protein, LSD1/KDM1A/AOF2, which has been shown to be primarily oncogenic in the context of human cancers [5]. Demethylases have been tied to carcinogenesis as they control epigenetic regulation of processes such as cellular motility, apoptosis, and angiogenesis [1]. However, much of the histone demethylase and non-histone substrate functions of LSD2 have yet be explored, and there is a strong case for further study of the role of LSD2 in cancer.
First, LSD2 is, in both structure and function, similar to LSD1, a protein known for its role as an oncogene [5, 6]. LSD2’s location on chromosome 6, cytoband p22.3[7], an area with high concentrations of chromosomal disarrangements in many types of cancers, also indicates that it may play a role in either the promotion or suppression of tumorigenesis [8]. Additionally, LSD2 has been shown to form a complex with methyltransferases euchromatic histone-lysine N-methyltransferase 2 (EHMT2/G9a) and the histone-lysine N-methyltransferase (NSD3) [3], which have been shown to be upregulated in breast cancers [9].
Current research on LSD2’s role in cancer is nascent and primarily focuses on five LSD2 interactions: LSD2 and its synergy with DNA methyltransferase (DNMT) inhibitors in breast cancer [3, 10], LSD2 and O-GlcNAc transferase (OGT) degradation in lung cancer [11], LSD2 as part of a feed-forward circuit with NF-кB [4], LSD2 as a mediator in glioblastoma tumorigenesis under hypoxic conditions [12], and LSD2 as part of an apoptosis and chemotherapy resistance pathway in cancer stem cells [13]. Additionally, available literature disagrees on the fundamental nature of LSD2’s role in human cancers – it is unknown whether it functions as an oncogene or a tumor-suppressor [10, 11]. LSD2 has been shown to be highly upregulated in breast, colorectal, thyroid, and liver cancers [7], and therefore, the study of LSD2 may lead to the discovery of both epigenetic pathways vital to oncogenes as well as potential therapeutic targets.
LSD2 is a demethylase in the KDM1 family that associates primarily with the body regions of actively transcribed genes [14]. It positively regulates gene transcription through binding chromatin in H3K36me3-enriched coding regions that are downstream of gene promoters [6]. The demethylase consists of three major domains: 1) a SWIRM domain (residues 264-372), 2) a C-terminal catalytic amine oxidase domain (AOD), (residues 372-822), and 3) an N-terminal dual zinc finger domain (residues 50-264) composed of an N-terminal zinc finger, a CW-type zinc finger, and two linker sequences [6] (Figure 1).
![Figure 1: Representation of the domains contained in LSD1 and LSD2[3, 6, 15]. A) Structural representation of LSD2 B) Structural representation of LSD1](http://researchopenworld.com/wp-content/uploads/2016/10/CST-2016-107-Figure1.png)
A) Structural representation of LSD2
B) Structural representation of LSD1
Figure 1. Representation of the domains contained in LSD1 and LSD2 [3, 6, 15].
LSD2 shares with LSD1 a <25% sequence identity, but has often been grouped with its better-known homolog [16]. The two proteins share some features, such as a SWIRM domain, plasticity of active sites, and a mechanism for catalysis (due to the similarities between their respective AOD regions). However, LSD2 has several defining characteristics distinct from those of LSD1 which merit further investigation into its unique role in cancer proliferation [6].
Structurally, LSD2 has several notable features. The first, the SWIRM domain of LSD2, has a 24% sequence identity with that of LSD1. Significantly, and unlike that of LSD1, the SWIRM domain of LSD2 packs closely to the N-terminal domain. It also lacks a C-terminal helix, which is replaced by a coiled loop that may serve as a secondary binding site for the N-terminal tail of the histone H3. LSD2 also lacks a coiled tower domain, which indicates that LSD2 cannot share LSD1’s cofactor, the corepressor of the RE1-silencing transcription factor (CoREST), or its ability to bind with histone deacetylases (HDAC’s) [6].
LSD2 forms a complex with polymerase II and the SET family histone methyl transferases, NSD3 and G9a, which maintain the methylation status of H3K36 and H3K9, respectively. As part of this complex, LSD2 cofactors with glyoxylate reductase 1 homolog (GLYR1/NPAC), a H3K36me3 reader consisting of a Pro-Trp-Trp- Pro (PWWP) domain, AT-hook motif, and a dehydrogenase domain. This interaction with GLYR1 enhances the demethylase activity of LSD2 at H3K4. The linker region of GLYR1 drives its cofactor activity regardless of the substrate used, indicating that the cofactor has a direct purpose in histone demethylation. GLYR1 has been theorized to operate on the tail of histone H3, and does not alter the shape of LSD2 or of its catalytic domain, thus having no effect on LSD2’s substrate specificity [15].
Analogous to LSD1, whose function can differ depending on its binding partners, LSD2 also serves as an activator of gene expression through its non-histone functions, for example, by being recruited to promoters of inflammatory genes in response to the NF-кB subunit c-Rel. This interaction potentiates demethylation of H3K9, leading to the expression of NF-кB in a forward feedback loop [4].
Methods and Materials
A literature search was carried out using the following search terms: LSD2, AOF1 and KDM1B. In order to be examined in depth, each paper was required to study LSD2, though LSD2 was not required to be part of the primary aim of the paper. However, the interaction between LSD2 and human cancer cells, or LSD2 and a related protein with a relevant pathway in human cancers, must be examined within each paper as one of the aims. Papers that focused primarily on LSD1/ KDM1A/AOF2 were excluded. Papers that discussed LSD2 as part of their primary aim, but did not involve human cancer cells or a known human cancer cell pathway, were also excluded. 67 total papers were found using the above search criteria, and 50 were ruled out for reasons including non-relationship to LSD2 (for example, many papers were instead about lipid-storage droplet 2 or grouped LSD2 with LSD1 as the same protein) and non-relationship to cancer (papers on stem cells, hyperinsulinemia, and other diseases were ruled out). 5 of these papers explicitly investigated LSD2 in cancer, and these are discussed in detail hereafter.
Results
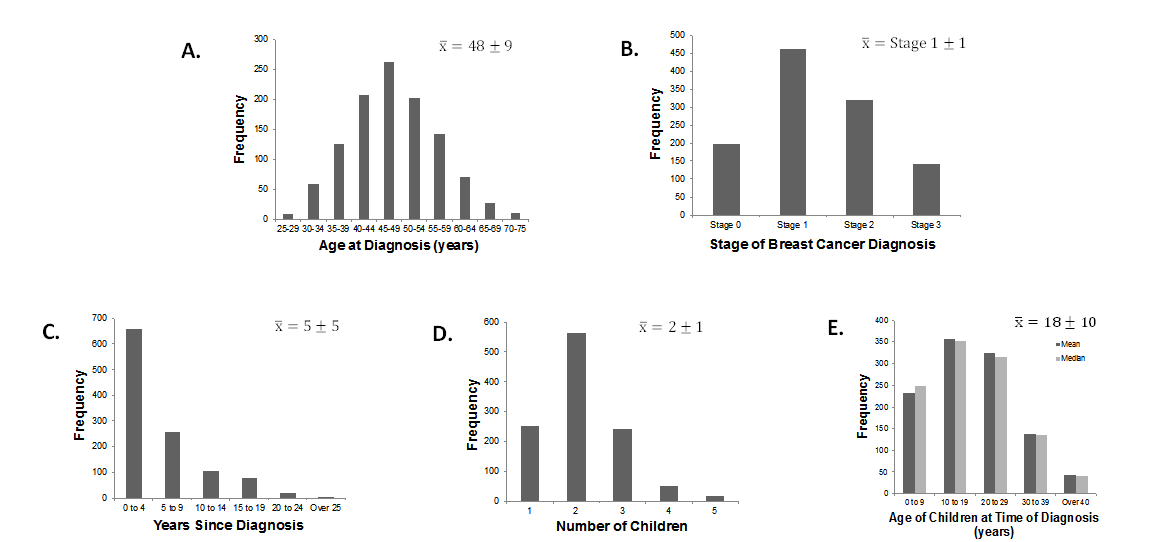
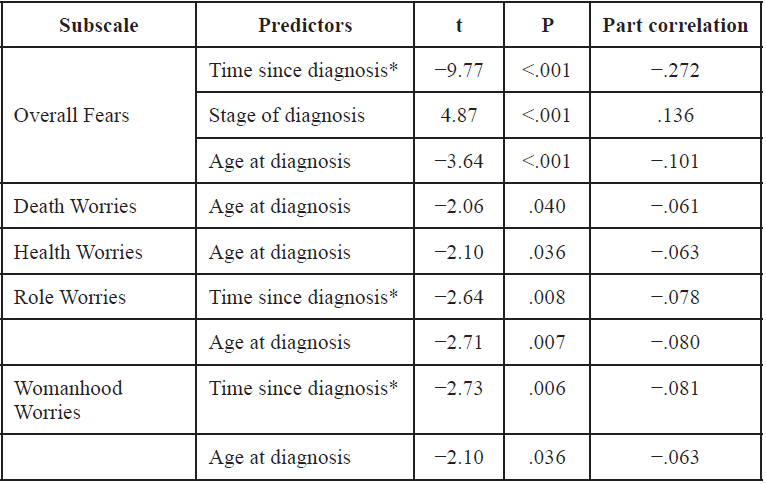
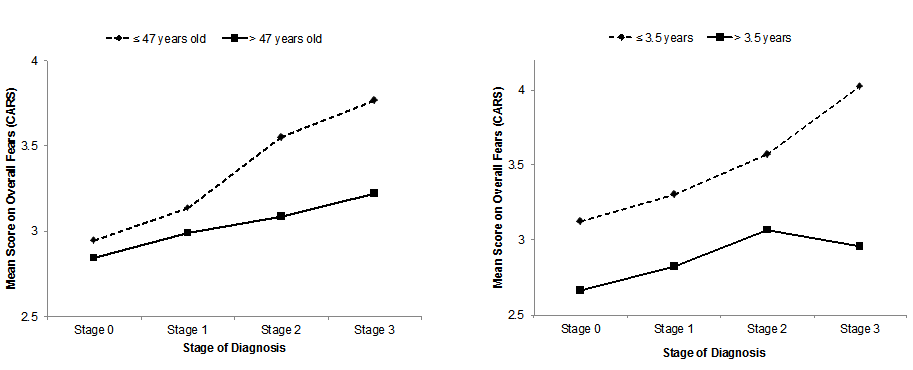
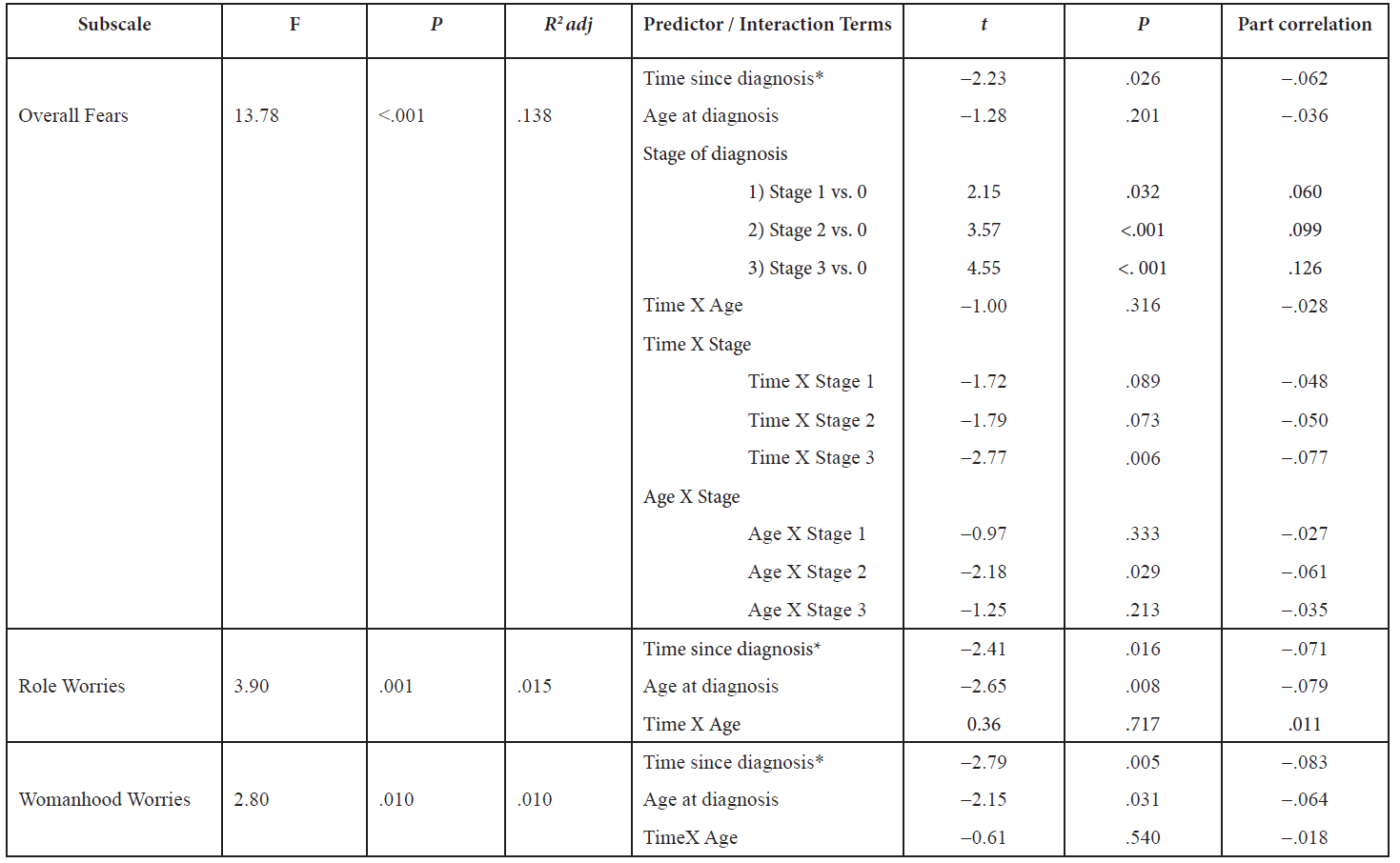
Current literature disagrees on the nature of LSD2 in carcinogenesis
Two major studies have specifically discussed the role of LSD2’s chromatin-remodeling functions in cancers. The first is a study by Katz et al. which examines the interactions of DNMTs and LSD2 in breast cancer [10]; the second by Yang et al. [11] which discusses the role of LSD2 as an E3 ligase in lung cancer. Interestingly, these primary studies differ on their categorization of the role of LSD2 in cancer: Katz denotes it oncogenic while Yang describes it as having tumor-suppressing functions.
LSD2 in breast cancer
In Katz et al.’s investigation of LSD2 in breast cancer, short hairpin RNA (shRNA) was used to produce up to 90% LSD2-knockdown (KD) in MDA-MB-231 breast cancer cells, with no impact on LSD1 expression. A 2D colony-formation assay in MDA-MB-231 and MCF7 cells showed that LSD2-KD led to a 25-50% decrease in colony formation, demonstrating that LSD2 promotes the survival of breast cancer cells, and may have an oncogenic role in breast cancer.
A 30% reduction in acetyl H3K9, a marker of active transcription, was also observed, demonstrating that LSD2-KD cells have lower global levels of DNA methylation. This study also found that nuclear protein lysates from LSD2-KD cells demonstrated lower demethylase activity than those of a scramble-cell control line. Additionally, the expression levels of several DNMTs did not change substantially in LSD2-KD cells, suggesting that the reduced DNA methylation seen in LSD2-KD does not result from the downregulation of protein expression of DNMT’s, but rather the blockade of DNMT activity through LSD2-KD. Thus LSD2-KD and decrease in DNMT activity are closely related in breast cancer.
LSD2 and DNMT inhibition
Katz et al. also examined the synergy between decitabine (DAC), a DNMT inhibitor, and LSD2 inhibition. They found that LSD2-KD cells had a higher sensitivity to DAC, as evidenced by lower IC- 50 values of DAC in LSD2-KD cells. DAC-treated cells exhibited re-expression of the progesterone receptor (PRA) gene (which is usually silenced in breast cancer) as well as increased global protein expression. A combination of LSD2 knockdown, DAC treatment, and tranylcypromine, another DNMT inhibitor, limited growth of MDA-MB-231 cancer cells, due to higher levels of cell death by apoptosis. Thus this combination of DNMT inhibition and LSD2-KD may have therapeutic merit as it induces apoptosis and results in the re-expression of silenced candidate genes in breast cancer cells.
LSD2 as an E3 ligase regulates OGT in a non-histone-dependent manner
The second major study that examines the function of LSD2 in cancer was conducted by Yang et al. in 2015. The study investigated the non-histone demethylase functions of the enzyme in an effort to elucidate the less-examined functions of histone demethylases as a whole. Their first major finding was that LSD2 demonstrates E3 ligase activity through autoubiquitylation of OGT, likely due to the zinc-finger domains specific to the structure of LSD2. Using shRNA, two mutants were produced. One, with the mutation of zinc-chelating residues (C53A/C58A/C92/C95A, LSD24CA) produced cells with E3 ubiquitin ligase activity, but without LSD2 histone demethylase activity. The second had a mutation of two surface residues E71A/R72A of LSD2 (LSD2ER-AA) which largely decreased its E3 ligase activity in an in vitro ubiquitylation assay. The zinc finger domains were needed for both functions, as their mutation resulted in a decrease in both demethylase and E3 ubiquitin ligase activity. However, each function was shown to be independent from the other.
LSD2’s ability to selectively demonstrate E3 ubiquitin ligase activity on OGT indicates that a part of its role in cancer may be through this pathway. OGT, an enzyme involved in O-GlcNAcylation, a process vital to cell growth, and has been shown to encourage the growth of tumor cells [17]. OGT is regulated by LSD2 at a protein level through a ubiquitin-dependent pathway, rather than through regulation of the transcription of OGT, as demonstrated through similar mRNA and expression levels of OGT in human embryonic kidney 293 cells (HEK-293).
shRNA mutants of A549 cells without LSD2 (chosen for their nearly undetectable expression of LSD2 in lab tests) grew larger colonies in soft agar assays, indicating that in lung cancer, LSD2 may selectively inhibit cell growth. The downregulation of OGT in cells in vivo had a similar effect, and the degradation of OGT was shown to be dependent only on LSD2’s E3 ligase activity, indicating that LSD2’s tumor-suppressor properties may be independent of its histone demethylase capabilities and instead dependent upon its E3 ubiquitin ligase activity. Through expression tests, oncogenes involved in the regulation of the cell cycle as well as in some cellular signaling cascades were found to be suppressed by LSD2’s E3 ligase activity. The histone demethylase capabilities of LSD2 were found instead to regulate various functions such as the Wnt receptor signaling pathway, cellular responses to stimuli, cell adhesion, and cellular immune response.
LSD2 is recruited by c-Rel to promoters of inflammatory genes to demethylate H3K9me2 leading to expression of NF-кB-driven gene expression
The interactions of LSD2/KDM1B/AOF1 and NF-кB demonstrate one role of LSD2 in gene activation by demethylation of H3K9me2, and its implications in cancer. While this study by van Essen et al. does not directly involve human cancer cells, NF-кB has been shown to serve a role in many human cancers [9]. LSD2 was found to serve as part of a feed-forward circuit involving the subunits of NF-кB, p65 and c-Rel, and the gene promoters for interleukin-II and Mdc. LSD2 was first found to have H3K9 demethylase activity in addition to H3K4 activity, though to a lesser extent. Its action against dimethyl H3K9 regulates these two genes, interleukin-II and Mdc, which are both targets of NF- кB. van Essen et al. demonstrated that in response to LPS stimulation, LSD2 is actively recruited by c-Rel to target promoters through H3K9 demethylation. Without stimulation, this demethylation occurs when a weak presence of c-Rel, while insufficient to drive transcription on its own, recruits LSD2 to the promoter. Through inhibiting each portion of this pathway, the study demonstrates that through a feed-forward loop, weak c-Rel values result in H3K9 demethylation by LSD2, recruitment of NF-кB with both of its subunits, and activation of interleukin-II and Mdc expression.
LSD2 and microRNA
Another study conducted by Hu et al. describes the interactions between microRNA (miR), glioma-initiating cells (GICs), and LSD2 in glioblastoma (GBM). In this study, miR-215 was found, using a screen, to induce hypoxia in GICs, as well as to mediate their responses under hypoxic conditions. miR-215 has been shown to act differently in different types of cancers, but in GBM has been shown to have tumorigenic capabilities. Hu found that attenuating miR-215 using inhibitors reduced growth rate and the ability to form neurospheres in GBM in both in vitro and in vivo assays. LSD2 is a target of miR- 215, and when it was inhibited with miR-215, GBM tissues showed a significant return of tumor growth. Hu has demonstrated that the miR-215-LSD2 pathway helps to adapt GICs to hypoxic conditions. Paired with the observation that LSD2 is under-expressed in GBM patients and tissues, Hu’s work may indicate that LSD2 serves a tumor-suppressive role in this pathway [12].
LSD2 and head and neck squamous cell carcinoma (HNSCC)
Bourguignon et al. studied cancer stem cell signaling pathways in the context of HNSCC [13]. They demonstrate that LSD1, LSD2, and DNMT1 are downregulated by the Oct4-Sox2-Nanog signaling pathway, which results in gene expression patterns that allow cancer stem cells to be self-renewing and resist apoptosis. The Oct4-Sox4- Nanog signaling pathway is activated by microRNA-302 (miR-302). Inhibition of miR-302 caused the upregulation of LSD1 and LSD2, a decrease in global DNA demethylation, apoptosis, and increased sensitivity to chemotherapy. This suggests that in this pathway in HNSCC, LSD2 serves a tumor-suppressive role. Table 1 summarizes the nature of current literature on LSD2 in cancer.
Table 1. Summary of the functions of LSD2 in cancer
| Research Group | Connection to LSD2 | Primary Results |
| Katz et al. | LSD2 in Breast Cancer | LSD2 functions as an oncogene. LSD2 knockdown leads to a 25% percent decrease in colony formation and increases sensitivity to the DNMT inhibitor DAC. |
| Yang et al. | LSD2 as an E3 Ubiquitin Ligase | LSD2 functions as a tumor-suppressor. LSD2 works as an E3 ubiquitin ligase to regulate OGT, which encourages the growth of tumor cells. |
| van Essen et al. | LSD2 and NF-кB | LSD2 serves as part of a feed-forward circuit, operating on H3K9 and regulating two target genes of NF-кB. LSD2 is recruited by and helps to recruit c-Rel, a subunit of NF-кB. |
| Hu et al. | LSD2 and miR-215 | LSD2 is targeted by miR-215, which controls GIC responses in GBM under hypoxic conditions. Inhibiting LSD2 along with miR-215 results in an increase in tumor growth. |
| Bourguignon et al. | LSD2 and miR-302 | LSD2 is inhibited by miR-302 in the Oct4-Sox2-Nanog signaling pathway in cancer stem cells. LSD2 upregulation via miR-302 inhibition results in increased apoptosis and chemosensitivity. |
Discussion
LSD2/KDM1B/AOF1 has been investigated in cancer primarily in breast, lung, GBM, and HNSCC cell lines. However, the current literature disagrees on the role of LSD2 in cancer as well as the pathways in which its oncogenic or tumor-suppressive properties are found. We found that in most work on histone demethylases in cancer, LSD2 has been grouped with LSD1 as a variant and is less researched in comparison to its homolog [8, 16, 18]. Further research on LSD2/ KDM1B/AOF1 is necessary in order to determine and elaborate on its role in human cancers. However, there are many important implications of available research discussing LSD2.
LSD2 as an oncogene
If LSD2 functions as an oncogene as observed by Katz et al., LSD2 can be considered a potential therapeutic target for the development of new compounds that inhibit its activity. LSD2-specific compounds are as yet uncommon, and many LSD1-targeting compounds also have been shown to also target LSD2 due to the similarities in their catalytic domains [6]. This necessitates further research on known LSD1-specific therapeutic compounds to determine LSD2 specificity, as well as the development of compounds that effectively target LSD2 alone.
LSD2 as a tumor-suppressor
Conversely, LSD2 may have tumor-suppressive functions. This would also have important implications in the development of targeted therapies for histone demethylases. Due to the similarities of structure between LSD2 and LSD1, LSD1-targeted therapies must be reexamined for specificity to LSD2, and modified to ensure that they will not impede tumor-suppressive activity of LSD2. Additionally, LSD2’s tumor-suppressive qualities must be further researched – they may elucidate oncogenes or tumor-promoting pathways that may occur in the absence or dysregulation of LSD2. Furthermore, the LSD2 mechanism can provide models for novel approaches to cancer therapies.
LSD2 has complex non-histone functions that serve multiple roles in carcinogenesis
LSD2’s roles in the OGT pathway in lung cancer, the miR-215 pathway in GBM, and the Oct4-Sox2-Nanog pathway in HNSCC are all tumor-suppressive. However, the summary of LSD2’s functions as examined in this review indicate that LSD2 may serve both roles: that its non-histone functions work to suppress tumors, but other functions including some histone demethylase activity of the protein may promote tumor growth. In this case, further research must be conducted to determine whether each function is independent and can be isolated, in order to create effective therapies that utilize LSD2’s tumor-suppressive functions while targeting its oncogenic ones.
LSD2 may be connected to proteins that are independently involved in cancer pathways
LSD2’s interactions with NF-кB are significant in that NF-кB has been increasingly identified as having a vital role in tumor cell survival. Activation of this protein is common in states of inflammation and malignancy in carcinoma. To a certain extent, the proinflammatory process can reduce tumorigenicity via immune surveillance; however, chronic inflammatory states can work to the advantage of transformed cells by promoting immune escape and prosurvival pathways in cancer [19]. Thus NF-кB works to activate survival genes in cancer cells [20]. LSD2, as part of a feed-forward circuit that controls activation of NF-кB and its target inflammatory genes, and as a previously identified target for oncogenesis, may serve as part of a cell pathway that promotes survival in tumor cells. Beyond this specific case, this interaction between LSD2 and NF-кB indicates that avenues for the exploration of the role of LSD2 in cancer can be found in other such possibly oncogenic or tumor-suppressive pathways or proteins and their connection to LSD2.
Conclusion
The conflicting nature of current literature on LSD2 in cancer, namely the uncertainty of its role as either a tumor-suppressor or an oncogene, presents a challenge, and suggests that LSD2’s function in cancer may be more complicated than previously believed. Therapies targeting LSD2 modeled after research on LSD1 may not prove effective because of the fundamental differences in function between the two proteins. LSD2 provides a method of exploring the complex cellular interactions that create tumors, and thus research involving this demethylase must be expanded. Ultimately, the study of LSD2 may serve an important role in elucidating epigenetic mechanisms behind oncogenesis as well as in illuminating paths to potential therapeutic cancer treatments.
Future Work
Future work on LSD2 may include the use of proteomic studies to categorize and analyze its functions and how they may operate in tumorigenesis. Induced overexpression and knockdown studies in a panel of cancer cell lines as well as in normal cells may provide more insight into the biological and oncological roles of LSD2.
Acknowledgements
The authors would like to acknowledge the Huntsman Cancer Institute, and more specifically the Center for Investigational Therapeutics, for the opportunity to work with LSD2 and for the materials and support required to carry out this review.
Competing Interests
We have no competing interests to declare.
Funding Information
The funding for this manuscript’s production and publication was provided by the Center for Investigational Therapeutics, Huntsman Cancer Institute, University of Utah and Salarius Pharmaceuticals.
References
- Kisseljova NP, Kisseljov FL (2005) DNA demethylation and carcinogenesis. Biochemistry (Mosc) 70: 743-752. [crossref]
- Kang MK, Mehrazarin S, Park NH, Wang CY (2016) Epigenetic gene regulation by histone demethylases: Emerging role in oncogenesis and inflammation. Oral Dis . [crossref]
- Rui Fang, Andrew J. Barbera, Yufei Xu, Michael Rutenberg, et al. (2010) Human LSD2/KDM1b/AOF1 Regulates Gene Transcription by Modulating Intragenic H3K4me2 Methylation. Molecular Cell 39:222.
- Van Essen D, Zhu Y, Saccani S (2010) A Feed-Forward Circuit Controlling Inducible NF-?B Target Gene Activation by Promoter Histone Demethylation. Molecular Cell 39:750-60.
- Wang Y, Zhu Y, Wang Q, Hu H, Li Z2, et al. (2016) The histone demethylase LSD1 is a novel oncogene and therapeutic target in oral cancer. Cancer Lett 374: 12-21. [crossref]
- Burg JM, Link JE, Morgan BS, Heller FJ, et al. (2015) KDM1 Class Flavin-Dependent Protein Lysine Demethylases. Biopolymers 104:213-46.
- KDM1B: Knut and Alice Wallenberg Foundation 7: 28.
- Mino K, Nishimura S, Ninomiya S, Tujii H, et al. (2014) Regulation of tissue factor pathway inhibitor-2 (TFPI-2) expression by lysine-specific demethylase 1 and 2 (LSD1 and LSD2). Bioscience, biotechnology, and biochemistry 78:1010-7.
- Angrand PO, Apiou F, Stewart AF, Dutrillaux B, et al. (2001) NSD3, a new SET domain-containing gene, maps to 8p12 and is amplified in human breast cancer cell lines. Genomics 74:79-88.
- Katz TA, Vasilatos SN, Harrington E, Oesterreich S, et al. (2014) Inhibition of histone demethylase, LSD2 (KDM1B), attenuates DNA methylation and increases sensitivity to DNMT inhibitor-induced apoptosis in breast cancer cells. Breast cancer research and treatment 146:99-108.
- Yang Y, Yin X, Yang H, Xu Y (2015) Histone demethylase LSD2 acts as an E3 ubiquitin ligase and inhibits cancer cell growth through promoting proteasomal degradation of OGT. Mol Cell 58:47-59.
- Hu J, Sun T, Wang H, Chen Z2, Wang S3, et al. (2016) MiR-215 Is Induced Post-transcriptionally via HIF-Drosha Complex and Mediates Glioma-Initiating Cell Adaptation to Hypoxia by Targeting KDM1B. Cancer Cell 29: 49-60. [crossref]
- Bourguignon LY, Wong G, Earle C, Chen L (2012) Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. The Journal of biological chemistry 287:32800-24.
- Chen F, Yang H, Dong Z, Fang J, Wang P, et al. (2013) Structural insight into substrate recognition by histone demethylase LSD2/KDM1b. Cell Res 23: 306-309. [crossref]
- Fang R, Chen F, Dong Z, Hu D, Barbera AJ, et al. (2013) LSD2/KDM1B and its cofactor NPAC/GLYR1 endow a structural and molecular model for regulation of H3K4 demethylation. Mol Cell 49: 558-570. [crossref]
- Kakizawa T, Mizukami T, Itoh Y, Hasegawa M, et al. (2016) Evaluation of phenylcyclopropylamine compounds by enzymatic assay of lysine-specific demethylase 2 in the presence of NPAC peptide. Bioorganic & medicinal chemistry letters 26:1193-5.
- de Queiroz RM, Carvalho E2, Dias WB1 (2014) O-GlcNAcylation: The Sweet Side of the Cancer. Front Oncol 4: 132. [crossref]
- Binda C, Valente S, Romanenghi M, Pilotto S, et al. (2010) Biochemical, Structural, and Biological Evaluation of Tranylcypromine Derivatives as Inhibitors of Histone Demethylases LSD1 and LSD2. Journal of the American Chemical Society 132:6827-33.
- Hoesel B, Schmid JA (2013) The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 12: 86. [crossref]
- DiDonato JA, Mercurio F, Karin M (2012) NF-κB and the link between inflammation and cancer. Immunol Rev 246: 379-400. [crossref]