Abstract
The primary barrier of eradicating HIV virus is the existence of latent reservoirs. To overcome this problem, it is urgent to identify specific and effective agents that can activate HIV latent reservoirs and bind with effective anti-retroviral therapy to eventually eliminate HIV virus. In previous study, we reported a chalcone analogue called Amt-87 that can significantly reactivate the transcription of latent HIV. However, it is of limited use due to high toxicity. Here, we have synthesized a series of structurally modified analogues of Amt-87 and evaluation for their reactivation of HIV-1 latency revealed one excellent active compound 4k, which can be taken up for further studies.
Introduction
It is more than 30 years passed since HIV was first reported in 1981 [1]. There are many drugs designed by targeting enzymes which are important for replication event during different stages of HIV-1 life cycle, preventing HIV-1 virus replication, therefore decrease the virus in the host blood to an undetectable level [2,3]. However, HAART is unable to completely eliminate the virus due to the existence of latent viral reservoirs, which are formed early after host infection and undetectable by common clinical tests [4], thus the host immune system can be invaded again upon the treatment interruption. HIV latency reservoir in the host CD4 T cell is the main obstacle to cure HIV-1 as reported recent years. Effective curative strategies aiming at eradication of HIV-1 virus are being developed [5-7]. One significant therapy which is called “shock and kill” has been proposed to achieve HIV eradication, there are two steps in this approach, using latency-reversing agents (LRAs) to reactivate the latent proviruses in the “shock” phase and then combining with the HAART to make the reactivated cells be sensitive to host immune system and cytopathogenicity response in the “kill” phase [8,9]. In this strategy, finding drugs to activate HIV latency catches much attention. Currently there are several activators have been proved, for example HMAB, SAHA, prostration JQ1 and so on, but most of these drugs are toxic to host cell and have some side effects [9]. Thus, highly efficient and specific drugs for reactivating HIV latency is important for HIV-1 eradication. Chalconoids, natural phenol compounds, have an array of biological activities including anti-inflammatory, antioxidant, antitumor and antibacterial activities and inhibit angiogenesis in vivo and in vitro [10-14]. Chalcone analogues have attracted a great deal of interest due to their synthetic and biological importance in medicinal chemistry. In our previous study, we reported the synthesis and biological evaluation of a chalcone analogue Amt-87, which can significantly reactivate the transcription of latent HIV proviruses and act synergistically with known LRAs such as prostratin and JQ1 to reverse HIV latency [15]. Unfortunately, Amt-87 is less active and requires high concentrations to reactivate latent HIV. Herein, we describe the optimization and characterization of the structure−activity relationship (SAR) of chalcone scaffold to generate a series of compounds with enhanced HIV latency reactivation activity.
Results
Synthesis of Chalcone Derivatives
Chalcone derivatives 4a-4k were prepared according to a two-step procedure (Scheme 1) previously reported. Condensation of 1-(2-hydroxy-5-methylphenyl)ethanone with 2- or 3-carboxybenzaldehyde in the presence of alkali gave carboxylic acid derivatives 3a and 3b. Treatment of 3a or 3b in CH2Cl2 with various amines in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) and N-hydroxybenzotriazole (HOBt) afforded various amides 4a-4k with yields ranged from 30% to 50% (Scheme 1).
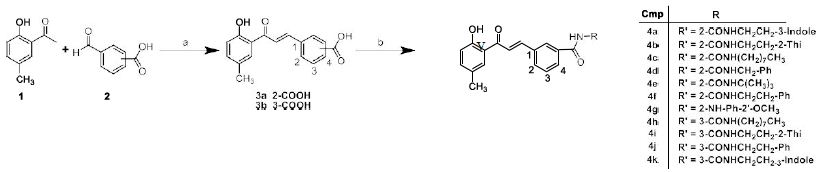
Scheme 1: Synthesis of chalcone amide derivatives 4a-4k. Reagents and conditions: (a) NaOH, EtOH, r.t., 24 h. (b) various amines, anhydrous HOBt, EDCI, DCM, 12 h.
Reactivation of latent HIV-1 in J-Lat A2 cells and structure–activity relationship. In order to identify compounds that can efficiently reactivate latent HIV-1, the J-LatA2 latency model was used in a screen that involved flow cytometry. This cell line was generated by infecting Jurkat T cells with viral particles containing an HIV-1 vector expressing Tat and GFP under the control of the viral 5’ LTR, with an IRES (internal ribosome entry site) separating the two open reading frames [15]. All synthesized chalcone compounds were evaluated for their activation of latent HIV-1 in J-Lat A2 cells and Amt-87 was used as the positive control. The results are presented in Table 1. The structure–activity relationship (SAR) was further explored as described below. Among these structurally related chalcone derivatives, we found that 4b, 4d, 4g were more active than Amt-87 in terms of HIV latency-reversing activity. Specifically, 4g showed the most optimal properties in the latent HIV-1 reactivation activity, inducing 14.6 ± 2.1% and 20.5 ± 2.8% GFP production in J-Lat A2 cells at 25 μg/mL and 50 μg/mL, respectively. What’s more, compound 4g has no significant cytotoxicity at a concentration of 50 μg/mL. These observations may demonstrate the potential of chalcone derivatives as LRA leads.
Table 1: Summary of HIV reactivation of Chalconoids in J-Lat cells
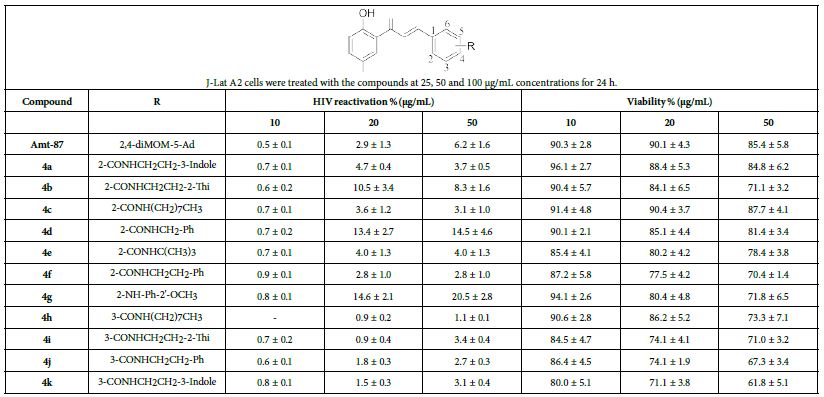
Levels of HIV-1 activation and cell viability were evaluated by FACS analysis. Data are presented as mean ± SD of at least three independent experiments performed in triplicate.
Discussion
In this study, we synthesized and biologically evaluated chalcone derivatives for their HIV latency-reversing activity using HIV-LTR based cell model. Among these new derivatives, compound 4g effectively induced GFP production in J-Lat A2 cells at 25 and 50 μg/mL, while displaying no significant cytotoxicity at these concentrations. This study provides valuable data for the future development of chalcone as effective LRAs.
Materials and Methods
Chemistry
General procedure for the synthesis of Chalcones [15]. Claisene Schmidt condensation. To a solution of the corresponding aldehyde (1 equiv) and corresponding acetophenone (1 equiv) in EtOH (3 mL for 1 mmol of acetophenone) was added NaOH (5 equiv). The reaction mixture was stirred at room temperature for 24 h and neutralized with 10% HCl solution to form yellow precipitate. The yellow precipitate was filtered and washed with appropriate amount of water. The crude product was purified by chromatography using hexane/EtOAc and recrystallized by MeOH to give the desired compounds.
(E)-2-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)Benzoic Acid (3a)
Following the general procedure for the Claisen-Schmidt condensation, 1-(2-hydroxy-5-methylphenyl)ethanone 1 (1.1 mmol, 165.2 mg) and 2-carboxybenzaldehyde (1.0 mmol, 150.1 mg) were used to give 3a as a yellow solid. Yield 45.7%. 1H NMR (600 MHz, C5D5N): δ 8.41 (d, J=8. 2 Hz, 2H), 8.19 (d, J=15.5 Hz, 1H) , 8.12 (d, J=17.2 Hz, 1H), 8.07 (d, J=1.0 Hz, 1H), 7.88 (d, J=8.2 Hz, 2H), 7.31 (dd, J=1.8, 8.4 Hz, 1H), 7.1l (d, J=8.4 Hz, 1H), 2.18 (s, 3H). 13C-NMR (150 MHz, C5D5N): δ 193.9, 168.3, 161.6, 143.9, 138.7, 137.7, 137.2, 130.5 (2C), 129.0 (2C), 128.3, 126.4 (2C), 120.5, 118.2, 20.0. ESI-HRMS (-): m/z [M-H]- calcd for C17H13O4–, 281.0819, found, 281.0816.
(E)-3-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)Benzoic Acid (3b)
Following the general procedure for the Claisen-Schmidt condensation, 1-(2-hydroxy-5-methylphenyl)ethanone 1 (1.1 mmol, 165.2 mg) and 3-carboxybenzaldehyde (1.0 mmol, 150.1 mg) were used to give 3b as a yellow solid. Yield 50.5%. 1H NMR (600 MHz, DMSO-d6): δ 12.3 (s, 1H), 8.40 (s, 1H), 8.18 (d, J=7.9 Hz, 1H), 8.08-8.14 (m, 2H), 8.03 (d, J=7.7 Hz, 1H), 7.89 (d, J=15.6 Hz, 1H), 7.62 (t, J=7.7 Hz, 1H), 7.40 (dd, J=1.7, 8.3 Hz, 1H), 6.92 (d, J=8.3 Hz, 1H), 2.33 (s, 3H); 13C NMR (150 MHz, DMSO-d6): δ 193.9, 167.4, 160.3, 144.0, 137.8, 135.4, 133.5, 132.2, 131.8, 131.0, 130.2, 129.7, 128.5, 123.5, 120.8, 118.0, 20.4; ESI-HRMS (-): m/z [M-H]- calcd for C17H13O4–, 281.0819, found, 281.0814.
General Procedure for Synthesis of Amide Compounds 1a-1p. A Mixture of 3 (3a or 3b)
(1 equiv), HOBt (1.2 equiv) and EDCI (1.2 equiv) was dissolved in CH2Cl2, and stirred for 30 min. The mixture was then added with appropriate amine (2.0 equiv), and stirred at the room temperature for 12 h. After completion of the reaction, the mixture was concentrated in vacuum to give the crude product. The crude product was purified by column chromatography with hexane/EtOAc, and recrystallized with EtOAc to afford pure products.
(E)-N-(2-(1H-Indol-2-yl)Ethyl)-2-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)Benzamide (4a)
The title compound 4a was obtained by the reaction of compound 3a with 2-(1H-indol-2-yl)ethan-1-amine following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d): δ 12.49 (s, 1H), 8.08 (d, J=15.6 Hz, 1H), 7.57-7.59 (m, 1H), 7.52 (d, J=7.9 Hz, 1H), 7.45 (td, J=3.2, 8.9 Hz, 4H), 7.38 (dt, J=1.6, 7.5 Hz, 1H), 7.30-7.35 (m, 2H), 7.26 (dd, J=2.0, 8.4 Hz, 1H), 7.23 (d, J=8.1 Hz, 1H), 7.09 (t, J=7.6 Hz, 1H), 7.06 (d, J=2.2 Hz, 1H), 6.99-7.03 (m, 1H), 6.88 (d, J=8.4 Hz, 1H), 3.80 (d, J=6.2 Hz, 2H), 3.05 (t, J=6.5 Hz, 2H), 2.27 (s, 3H); 13C-NMR (151 MHz, CHLOROFORM-d): 192.6, 167.6, 166.7, 160.5, 141.7, 136.9, 136.6, 132.0, 131.3, 129.9, 129.2, 128.6, 127.8, 127.0, 126.7, 126.4, 121.9, 121.3, 121.2, 118.6, 118.5, 117.6, 117.4, 110.3, 64.6, 39.0, 29.5; HRMS calcd for C27H24N2O3 [M-H]- 423.1714, found 423.1718.
(E)-2-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)-N-(2-(Thiophen-2-yl)Ethyl)Benzamide (4b)
The title compound 4b was obtained by the reaction of compound 3a with 2-thiopheneethanamine following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d): δ 12.47 (s, 1 H), 8.08 (d, J=15.41 Hz, 1H), 7.67 (d, J=7.70 Hz, 1H), 7.58 (s, 1H), 7.46 (d, J=15.41 Hz, 1H), 7.37-7.42 (m, 2H), 7.34 (t, J=7.4 Hz, 1H), 7.22-7.25 (m, 1H), 7.04 (d, J=5.0 Hz, 1H), 6.85 (d, J=8.4 Hz, 1H), 6.81-6.83 (m, 1H), 6.79 (d, J=3.1 Hz, 1H), 5.98 (br. s., 1H), 3.68 (q, J=6.4 Hz, 2H), 3.10 (t, J=6.6 Hz, 2H), 2.26 (s, 3H); 13C-NMR (151 MHz, CHLOROFORM-d): 193.5, 168.6, 161.5, 142.6, 141.0, 137.6, 137.6, 133.3, 130.4, 130.2, 129.6, 128.0, 127.7, 127.5, 127.1, 125.6, 124.1, 123.0, 119.6, 118.4, 41.5, 29.7, 20.6; HRMS calcd for C23H21NO3S [M-H]- 390.1169, found 390.1171.
(E)-2-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)-N-Octylbenzamide (4c)
The title compound 4c was obtained by the reaction of compound 3a with octan-1-amine following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d): 12.47 (s, 1H), 8.09 (s, 1H), 7.69 (d, J=7.7 Hz, 1H), 7.59-7.61 (m, 1H), 7.50 (d, J=15.4 Hz, 1H), 7.45 (dd, J=1.3, 7.5 Hz, 1H), 7.42 (dt, J=1.2, 7.6 Hz, 1H), 7.36-7.39 (m, 1H), 7.24 (dd, J=2.0, 8.4 Hz, 1H), 7.19 (s, 1H), 6.86 (d, J=8.4 Hz, 1H), 3.38-3.42 (m, 2H), 2.27 (s, 3H), 1.14-1.33 (m, 12H), 0.80 (t, J=7.1 Hz, 3H); 13C-NMR (151 MHz, CHLOROFORM-d): 192.4, 167.6, 160.5, 141.6, 137.0, 136.6, 132.1, 129.2, 129.2, 128.5, 126.9, 126.8, 126.6, 122.0, 118.5, 117.3, 39.3, 30.8, 28.6, 28.2, 28.2, 26.0, 21.6, 19.6, 13.1; HRMS calcd for C25H31NO3 [M-H]- 392.2231, found 392.2233.
(E)-N-Benzyl-2-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)Benzamide (4d)
The title compound 4d was obtained by the reaction of compound 3a with benzylamine following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d): 12.48 (s, 1H), 8.11 (d, J=15.6 Hz, 1H), 7.65 (d, J=7.7 Hz, 1H), 7.55 (s, 1H), 7.42-7.45 (m, 2H), 7.39 (t, J=7.4 Hz, 1H), 7.31-7.34 (m, 1H), 7.21-7.25 (m, 3H), 7.16-7.19 (m, 1H), 6.83 (d, J=8.4 Hz, 1H), 6.21 (br. s., 1H), 4.54 (d, J=5.5 Hz, 2H), 2.24 (s, 3H), 1.18 (br. s., 1H); 13C-NMR (151 MHz, CHLOROFORM-d): 193.5, 168.5, 161.5, 142.6, 137.7, 137.6, 137.5, 133.2, 130.4, 130.2, 129.6, 128.9, 128.0, 128.0, 127.8, 127.7, 127.6, 123.0, 119.5, 118.3, 44.3, 20.6; HRMS calcd for C24H21NO3 [M-H]- 370.1449, found 370.1448.
(E)-N-(Tert-Butyl)-2-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)Benzamide (4e)
The title compound 4e was obtained by the reaction of compound 3a with 2-methylpropan-2-amine following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d): 12.48 (s, 1H), 8.06 (d, J=15.4 Hz, 1H), 7.64 (d, J=7.5 Hz, 1H), 7.58-7.60 (m, 1H), 7.47 (d, J=15.5 Hz, 1H), 7.32-7.41 (m, 3H), 7.22 (dd, J=2.0, 8.4 Hz, 1H), 6.83 (d, J=8.6 Hz, 1H), 5.63 (br. s., 1H), 2.26 (s, 3H), 1.41 (s, 9H); 13C-NMR (151 MHz, CHLOROFORM-d): 192.5, 167.2, 160.5, 141.7, 137.9, 136.5, 131.7, 129.2, 128.9, 128.5, 126.9, 126.7, 126.6, 121.8, 18.5, 117.3, 51.3, 28.7, 27.8, 19.5, 13.1; HRMS calcd for C21H23NO3 [M-H]- 336.1605, found 336.1607.
(E)-2-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)-N-Phenethylbenzamide (4f)
The title compound 4f was obtained by the reaction of compound 3a with 2-phenylethan-1-amine following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d) 12.48 (s, 1H), 8.09 (d, J=15.41 Hz, 1H), 7.68 (d, J=7.70 Hz, 1H), 7.59 (s, 1H), 7.47 (d, J=15.59 Hz, 1H), 7.41 (br. s., 1H), 7.33-7.36 (m, 2H), 7.25 (d, J=8.25 Hz, 1H), 7.18-7.22 (m, 3H), 7.15-7.17 (m, 2H), 7.12 (d, J=7.15 Hz, 1H), 6.87 (d, J=8.44 Hz, 1H), 3.67-3.72 (m, 2H), 2.89 (t, J=6.79 Hz, 2H), 2.27 (s, 3H);13C NMR (151 MHz, CHLOROFORM-d) 192.5, 167.6, 160.5, 141.5, 137.5, 136.7, 136.6, 132.2, 129.3, 129.2, 128.5, 127.7, 127.7, 126.9, 126.7, 126.5, 125.6, 122.0, 118.5, 117.4, 40.1, 34.5, 19.6;HRMS calcd for C25H23NO3 [M-H]- 384.1605, found 384.1603.
(E)-3-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)-N-(2-Methoxyphenyl)Benzamide (4g)
The title compound 4g was obtained by the reaction of compound 3b with 2-methoxyaniline following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d): 12.49 (br. s., 1H), 8.49 (br. s., 1H), 8.42 (d, J=7.0 Hz, 1H), 8.12 (br. s., 1H), 7.75-7.83 (m, 2H), 7.68 (d, J=7.3 Hz, 1H), 7.61-7.65 (m, 1H), 7.59 (br. s., 1H), 7.42-7.45 (m, 1H), 7.18-7.22 (m, 1H), 7.00 (dt, J=1.7, 7.8 Hz, 1H), 6.90-6.94 (m, 1H), 6.81-6.84 (m, 2H), 3.83 (s, 3H), 2.25 (s, 3H); 13C-NMR (151 MHz, CHLOROFORM-d): 192.3, 163.4, 160.5, 147.2, 142.8, 136.7, 135.0, 134.3, 130.9, 128.4, 127.5, 127.1, 126.4, 126.1, 123.2, 120.4, 120.1, 118.9, 118.5, 117.3, 109.0, 54.8, 19.5; HRMS calcd for C24H21NO4 [M+Na]+ 410.1363, found 410.1358.
(E)-3-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)-N-Octylbenzamide (4h)
The title compound 4h was obtained by the reaction of compound 3b with octan-1-amine following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d): 12.51 (s, 1H), 8.05 (s, 1H), 7.77 (d, J=15.4 Hz, 1H), 7.68 (d, J=7.7 Hz, 1H), 7.61-7.65 (m, 2H), 7.59 (s, 1H), 7.38 (t, J=7.6 Hz, 1H), 7.22 (d, J=8.4 Hz, 1H), 6.83 (d, J=8.4 Hz, 1H), 6.41-6.49 (m, 1H), 3.39 (q, J=6.2 Hz, 2H), 2.26 (s, 3H), 1.24-1.31 (m, 3H), 1.17-1.24 (m, 9H), 0.78-0.81 (m, 3H); 13C-NMR (151 MHz, CHLOROFORM-d): 193.4, 166.9, 161.5, 144.0, 137.7, 135.7, 135.1, 131.7, 129.4, 129.2, 128.6, 128.1, 126.9, 121.3, 119.6, 118.3, 40.3, 31.8, 29.7, 29.3, 29.2, 27.1, 22.6, 20.6, 14.1; HRMS calcd for C25H31NO3 [M+Na]+ 416.2196, found 416.2193.
(E)-3-(3-(2-Hydroxyphenyl)-3-Oxoprop-1-en-1-yl)-N-(2-(Thiophen-2-yl)Ethyl)Benzamide (4i)
The title compound 4i was obtained by the reaction of compound 3b with 3-thiopheneethanamine following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d): 12.49 (br. s., 1H), 7.99 (br. s., 1H), 7.70-7.76 (m, 1H), 7.62 (t, J=8.5 Hz, 2H), 7.55-7.59 (m, 2H), 7.33-7.37 (m, 1H), 7.21 (dd, J=1.9, 8.3 Hz, 1H), 7.07-7.09 (m, 1H), 6.87 (dd, J=3.3, 5.0 Hz, 1H), 6.81-6.83 (m, 1H), 6.79 (d, J=2.8 Hz, 1H), 6.63 (br. s., 1H), 6.56 (br. s., 1H), 3.64-3.68 (m, 2H), 3.07-3.10 (m, 2H), 2.25 (s, 3H); 13C NMR (151 MHz, CHLOROFORM-d): 193.4, 167.0, 161.5, 143.9, 141.1, 137.8, 135.4, 135.1, 131.9, 129.4, 129.3, 128.7, 128.1, 127.2, 126.8, 125.5, 124.1, 121.3, 119.6, 118.3, 41.5, 29.8, 20.6; HRMS calcd for C23H21NO3S [M+Na]+ 414.1134, found 414.1129.
(E)-3-(3-(2-Hydroxyphenyl)-3-Oxoprop-1-en-1-yl)-N-Phenethylbenzamide (4j)
The title compound 4j was obtained by the reaction of compound 3b with 2-phenylethan-1-amine following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d): 12.49 (br. s., 1H), 7.96 (s, 1H), 7.69-7.75 (m, 1H), 7.57-7.60 (m, 2H), 7.53-7.57 (m, 2H), 7.31-7.35 (m, 1H), 7.22-7.25 (m, 1H), 7.19-7.22 (m, 2H), 7.14 (d, J=7.3 Hz, 3H), 6.80-6.82 (m, 1H), 6.49 (br. s., 1H), 6.44 (br. s., 1H), 3.64 (q, J=6.8 Hz, 2H), 2.86 (t, J=6.9 Hz, 2H), 2.25 (s, 3H); 13C-NMR (151 MHz, CHLOROFORM-d): 192.3, 165.9, 160.5, 142.9, 137.8, 136.7, 134.4, 134.0, 130.8, 128.4, 128.2, 127.8, 127.7, 127.6, 127.1, 125.7, 125.6, 120.2, 118.5, 117.3, 40.3, 34.6, 19.5; HRMS calcd for C25H23NO3 [M+Na]+ 408.1570, found 408.1565.
(E)-N-(2-(1H-Indol-3-yl)Ethyl)-3-(3-(2-Hydroxy-5-Methylphenyl)-3-Oxoprop-1-en-1-yl)Benzamide (4k)
The title compound 4k was obtained by the reaction of compound 3b with 2-(1H-indol-3-yl)ethan-1-amine following the general procedure. Yellow solid, yield 57.5%, mp 182-183°C. 1H-NMR (600 MHz, CHLOROFORM-d): 12.49 (s, 1H), 8.08 (d, J=15.6 Hz, 1H), 7.57-7.59 (m, 1H), 7.52 (d, J=7.9 Hz, 1H), 7.45 (td, J=3.2, 8.9 Hz, 4H), 7.38 (dt, J=1.6, 7.5 Hz, 1H), 7.30-7.35 (m, 2H), 7.26 (dd, J=2.0, 8.4 Hz, 1H), 7.23(d, J=8.1 Hz, 1H), 7.09 (t, J=7.6 Hz, 1H), 7.06 (d, J=2.2 Hz, 1H), 6.99-7.03 (m, 1H), 6.88 (d, J=8.4 Hz, 1H), 3.80 (d, J=6.2 Hz, 2H), 3.05 (t, J=6.5 Hz, 2H), 2.27 (s, 3H); 13C NMR (151 MHz, CHLOROFORM-d): 192.6, 167.6, 166.7, 160.5, 141.7, 136.9, 136.6, 132.0, 131.3, 129.9, 129.2, 128.6, 127.8, 127.0, 126.7, 126.4, 121.9, 121.3, 121.2, 118.6, 118.5, 117.6, 117.4, 110.3, 64.6, 39.0, 29.5; HRMS calcd for C27H24N2O3 [M+Na]+ 447.1679, found 447.1674.
Biochemistry
Reagents and Cell Lines
All the cell line used in this study were either purchased from American Type Culture Collection (ATCC, Manassas, VA) or described as previously [16].
Flow Cytometry Screening
The Flow cytometry screening assay was performed in Jurkat-based cells line(J-LatA2), containing LTR-Tat-IRES-GFP-LTR [15]. After incubation with Amt-87 as indicated or 0.1% dimeththyl sulfoxide (DMSO) as negative control, J-Lat A2 were harvested and washed twice with 1× phosphate buffered saline (PBS) and analyzed the expression level of GFP by flow cytometer (Epics Altra, BECKMAN COULTER). The changes of GFP expression level can indirectly reflect the transcription level of HIV.
Notes
The authors declare no competing financial interest.
References
- Siegal FP, Lopez c, Hammer GS, Brown AE, Kornfeld SJ, et al. (1981) Severe Acquired Immunodeficiency in Male-Homosexuals, Manifested by Chronic Perianal Ulcerative Herpes-Simplex Lesions. New England Journal of Medicine 305: 1439-1444. [crossref]
- DD HO, Neumann AU, Perelson AS, Chen W, Leonard JM, et al. (1995) Rapid Turnover of Plasma Virions and Cd4 Lymphocytes in Hiv-1 Infection. Nature, 373: 123-126. [crossref]
- Wei XP, Ghosh SK, Taylor ME, Johnson VA, Emini EA, et al. (1995) Viral Dynamics in Human-Immunodeficiency-Virus Type-1 Infection. Nature 373: 117-122. [crossref]
- Eisele E, Siliciano RF (2012) Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 37: 377-388. [crossref]
- Deeks SG, Brigitte A, Ben B, Monsef B, Scott C, Nicolas C, et al. (2012) Towards an HIV cure: a global scientific strategy. Nature Reviews Immunology 12: 607-614. [crossref]
- Mohammadi P, Julia dI, Miguel M, Raquel M, István B, et al. (2014) Dynamics of HIV latency and reactivation in a primary CD4+ T cell model. PLoS Pathog. 10. [crossref]
- Archin NM, Margolis DM (2014) Emerging strategies to deplete the HIV reservoir. Curr Opin Infect Dis 27: 29-35. [crossref]
- Karn J (2011) The molecular biology of HIV latency: breaking and restoring the Tat-dependent transcriptional circuit. Current Opinion in Hiv and Aids 6: 4-11. [crossref]
- Barton KM, Burch BD, Soriano-SN, Margolis MD et al. (2013) Prospects for Treatment of Latent HIV. Clinical Pharmacology & Therapeutics 93: 46-56. [crossref]
- Nowakowska Z (2007) A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 42: . 125-37.
- Boumendjel, A, Julien B, Pierre-AC, Edwige N, Madeleine B, et al. (2008) Antimitotic and antiproliferative activities of chalcones: forward structure-activity relationship. J Med Chem 51: 2307-2310. [crossref]
- Ouyang, Y. Juanjuan L, Xinyue C, Xiaoyu F, Sun S, et al. (2021) Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 11. [crossref]
- 1Sharma R, Rakesh K, Rishi K, Sukriti K, Anukriti K, et al. (2015) A Review on Mechanisms of Anti Tumor Activity of Chalcones. Anticancer Agents Med Chem. 16: 200-211. [crossref]
- Elkanzi NAA, Hajer H, Ruba AA, Wassila D, Fatin MZ, et al. (2022) Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 7: 27769-27786. [crossref]
- Wu J, Ming TA, Rui S, Hui-ru W, Diao Y, et al. (2017) A chalcone derivative reactivates latent HIV-1 transcription through activating P-TEFb and promoting Tat-SEC interaction on viral promoter. Sci Rep 7.
- Li ZC, Jia G, Yuntao W, Qiang Z (2013) The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Research 41: 277-287. [crossref]