Abstract
South Asians, representing one quarter of the world’s population, have disproportionally high rates of obesity and cardiometabolic disease thus resulting an epidemic health crisis. This crisis could be the consequence of epigenetic effects exacerbated during the colonial-era famines resulting in a unique starvation-adapted physiology. Due to evolutionary mismatch in circumstances of abundance, this starvation-adapted physiology can become harmful. Evidence for this starvation adaptation in South Asians includes high body fat and unfavorable adipokines; low lean body mass; lower resting energy expenditure (compounded by lack of brown adipose tissue); greater insulin resistance and insulin response; exaggerated lipemic response to fat and sugar intake; less capacity to handle an overabundance of food; lower fat burning (oxidative capacity) and VO2max during aerobic exercise; and energy- conserving response to resistance exercise, as well as increased lipoprotein (a) levels. The Roma people, also of South Asian ancestry, may represent an interesting pre-colonial historical control. Physician and patient knowledge of this unique physiology in South Asians will promote a stronger physician- patient relationship and foster compliance with treatment.
Introduction
At approximately one-quarter of the world’s population, South Asians represent one of the largest populations globally [1]. It is widely recognized that South Asians have higher cardiometabolic risks than people of other ethnicities. South Asians have the highest abdominal and visceral fat (truncal obesity) per given BMI [2,3]. South Asians are up to four to five fold more likely to develop type II diabetes when compared to the white populations of both the United States (US) and United Kingdom (UK), respectively [4,5]. Additionally, South Asians are more than twice as likely to develop metabolic syndrome than Europids in the UK [6]. Their risk of atherosclerotic cardiovascular disease (ASCVD) is four times greater than the general US population [7,8]. Moreover, ethnic Asian-Indians were reported to have higher median coronary artery calcium scores (CAC score, one of the most important predictors for ASCVD risk) [9] than all other ethnicities studied (i.e., Caucasian, Hispanic, African-Americans and East Asians) [8]. This is a veritable health crisis, given the size of the South Asian population and their higher risk of cardiometabolic disease [7].
Some of this risk could be due to lifestyle and dietary factors. South Asians on average spend less time exercising than the general population [10-12]. Studies have shown that the intake of refined carbohydrates, saturated fats, fried foods, and processed foods is high among South Asians [13-19]. However, there is a large amount of evidence pointing to a unique physiology among South Asians that likely has a genetic basis and represents resistance or adaptation to starvation. These adaptations have been suggested to predispose South Asians to cardiometabolic disease in an environment of abundance [20-23]. Therefore, it can be argued that the susceptibility of South Asians to cardiometabolic disease may be linked to starvation and famine adaptation. Furthermore, it is proposed that the near 200- year period of recurring, severe famines that occurred under British colonial rule may have had a role in shaping the current physiology of South Asians. The current review will shed light on the connection between the emerging evidence correlating nutritional deprivation and transgenerational susceptibility to cardiometabolic disease with the culprit being the colonial famines.
The Multiple Famines and Starvation in South Asia During the British Colonial Period
Prior to British colonial rule in South Asia, from the early fourteenth to the end of the seventeenth centuries, major famines occurred once every fifty years. These famines were also limited in geographical extent [24].
During colonial rule the nature and frequency of famines changed drastically. Famines were quite prevalent in South Asia during the British colonial period (1757 to 1947). The economic historian Mike Davis recounts 31 major famines in the 190 years of British colonial rule, compared to only 17 famines in the 2000 years prior [25]. Famines accelerated in frequency as British rule consolidated and spread. This spread is demonstrated by the occurrence of twelve serious famines and four severe scarcities between 1765 and 1858 (roughly the first 90 years of British rule) [24]. This is compared to acceleration in the frequency of severe famines during the period between 1850 and 1899 (50 years during ongoing British rule) where there were 24 major famines in South Asia [26].
The severity of the famines in South Asia made them some of the worst in world history. Three of the ten deadliest famines and six of the twenty-five worst drought-associated famines that were coupled with drought occurred in colonial India [27,28]. Additionally, the geographically widespread nature of the South Asian famines during colonial times was unprecedented in South Asian history. These famines affected areas of South Asia that were previously immune to famine, such as Marwar and Bengal [24]. Thus, the famines were more frequent, widespread, affected larger numbers of people, and increased in intensity (as nationwide catastrophes) during British rule. Even when famines did not occur, undernourishment was the norm during the colonial period [29]. Food grain availability per capita, a quantifiable parameter of the degree of undernourishment, decreased due to impoverishment from 200 kg in 1900 to 157 kg on the eve of World War II and further declined to 137 kg by 1946 [30]. Given South Asians continual exposure to severe famines, it makes sense to look at how that may have had long-term effects.
How Does Famine Affect Cardiometabolic Risk in Future Generations (Epigenetics)?
It has been shown that an adverse environment for a community in the past can have a long-term negative health consequence in the present [31]. Exposure of a population to just one famine is known to increase the risk of diabetes and ASCVD via multigenerational epigenetic effects [32]. These effects and their duration over multiple generations have been observed in worm and mouse models [32,33]. In humans, this epigenetic effect of famines has also been shown to increase cardiometabolic risk. Specifically, multiple studies of different populations in Sweden, China, and the Netherlands have shown this epigenetic-induced increase in cardiometabolic risk among descendants (including the grandchildren) of famine survivors [34- 36]. One evidence of epigenetic change in Chinese famine survivors is the high incidence of DNA methylation [21,22,37].
Based on the severity and frequency of the famines in South Asia during the colonial period, we postulate that these famines had a lasting epigenetic effect on the predisposition of South Asians to cardiometabolic disease. Indeed, South Asians who develop type II diabetes have a much higher rate of DNA methylation, an established indicator of epigenetic change, than do Caucasians. This finding may explain the increased incidence of type II diabetes. The relationship of DNA methylation to type II diabetes is so strong that it has been postulated that this biomarker may be a potential future screening tool for early intervention prior to diabetes onset [38].
The Recent Overabundance of Food Availability in South Asia Causes Evolutionary Mismatch
During the last 25 years, there has been a rapid increase in prosperity, and thus, food availability, in South Asia and amongst the South Asian diaspora [39]. This increase has led to rising incidence rates of noncommunicable diseases (NCDs) such as heart disease, stroke, and type II diabetes. This implies that there may be an evolutionary mismatch of genetic traits that were once advantageous for survival during food scarcity and have since become detrimental in the current environment of abundance; this is also known as the “thrifty gene” or “thrifty phenotype” hypothesis [40].
How do the Unique Physiological Traits of South Asian Populations Point to Starvation Adaptation?
In support of the “thrifty gene” hypothesis, studies in animal models have revealed starvation adaptation (or starvation resistance) after exposure to food shortages. For example, in Drosophila, these adaptations include sequestering greater energy reserves (greater lipid accumulation) and reducing the rate at which energy reserves are used (reduction in the metabolic rate and lower activity level of the organism) [41]. Additionally, the physiological equivalence of insulin resistance has also been demonstrated in Drosophila as a sign of starvation adaptation [42]. These traits of starvation adaption appear to be transgenerational in Caenorhabditis elegans as well [33]. Further investigation is needed, but, on the basis of these animal studies and the historical famines in South Asia, it is hypothesized that South Asians may express some of these traits due to generations of starvation adaptation [43].
The Unique and Different Physiology of South Asians
As suggested above, the physiology of South Asians is different from that of other ethnicities regarding important cardiovascular and metabolic measures. The topics discussed below outline the specific physiological differences seen in South Asians and relate them to starvation/famine adaptation.
High Body Fat and Unfavorable Adipokines
It is reasonable to assume that a propensity to store body fat is conducive to surviving famine. South Asians have a form of obesity that is different from that of most populations, which is referred to as the “thin-fat phenotype.” This characterization refers to a disproportionate amount of body fat typically concentrated in the abdomen in an otherwise lean individual, who often has a normal BMI. In fact, South Asians have the highest body-fat percentages and lowest lean mass of any ethnicity in the USA [44,45]. Compared with populations of European ancestry, South Asians have a total body- fat composition that is 3-5% higher for any given BMI [46]. Notably, South Asians have especially high levels of body fat and are more prone to developing obesity [47,48].
Higher body-fat percentages in South Asians is characterized by increased secondary storage in deep and visceral fat (though superficial subcutaneous fat is reduced); ectopic fat (such as intramyocellular and intrahepatic fat deposition); adipocyte hypertrophy; and a less favorable adipokine profile, which are cytokines produced in fat cells that regulate metabolism, energy, and inflammation; this condition is referred to by some as “ethnic lipodystrophy” [44,49-52]. Of note, South Asians have been shown to have the tendency to store ectopic fat as hepatic fat compared with other ethnicities, which is associated with greater hepatic insulin resistance [53]. Regarding the adipokine profile, the levels of adiponectin, which promotes peripheral insulin sensitivity, are lower in South Asians, and the levels of resistin, another adipokine that promotes insulin resistance and obesity, are higher in South Asians [44,54,55].
Low Lean Body Mass
In addition to having higher body fat percentages, South Asians have lower lean body mass levels than do people of other ethnicities. It is hypothesized that part of the reason for this is that South Asians have higher expression of the gene encoding myostatin [50,56]. Myostatin is a protein found in skeletal muscle that inhibits its growth. This hypothesized effect would explain why a higher body fat percentage and low lean mass exists among newborns of South Asian migrants to the UK, Netherlands, and Surinam that persists for at least four to five generations [57,58]. Low lean body mass is associated with higher rates of insulin resistance and cardiovascular disease (CVD) risk [59-61].
Numerous severe famines affected South Asia throughout the 19th and first half of the 20th centuries. The effects were worsened by British colonial policy, which led to a high mortality rate from the starvation that occurred [57]. Lean mass (especially, muscles and organs) is known to consume calories at a higher rate than fat. Therefore, the intense selection pressure from the numerous severe famines of the 19th and first half of the 20th centuries would have favored low levels of lean mass in addition to a higher body fat percentage.
The low lean mass of South Asians has been postulated to be one of the evolutionary reasons for the higher incidence rates of NCDs, such as cardiometabolic disease, in this population. Previous research has implicated colonial famines as a major exacerbator of the development of low lean mass due to starvation adaptation [58]. One study suggested that South Asians have had historically low lean mass for at least 11,000 years due to a variety of factors (such as delayed transitions from foraging to agriculture and the change to a predominantly vegetarian diet) [57]. However, we believe that the epigenetic effects of the famines on the South Asian physiology during the colonial period cannot be discounted.
Three factors that support the severity of the effects of these colonial famines are the exceedingly high death toll (up to 85 million deaths); the documented decrease in life expectancy during this period; and the decrease in stature (height) compared to an increase in stature in most of the other populations worldwide) [25,58,62-66].
Lower Resting Energy Expenditure
Thermoneutral resting energy expenditure is the energy expended necessary for basic physiological functioning at rest while awake, fasting, and without needing to use energy to regulate body temperature. As a result of their lower lean mass and higher body fat at the same height, weight, and BMI compared with people of other ethnicities, South Asians have lower thermoneutral resting energy expenditure (burning less energy at rest, approximately 32% less than Caucasians) [67-69]. This is another adaptation that may have helped some South Asians survive famines. One study showed that at the cellular level, South Asians seem to have higher mitochondrial efficiency, evidenced by the higher oxidative phosphorylation capacity in this group compared with Americans of North European origin [70]. The excess ATP-produced and higher oxidative state has been shown to prevent insulin receptor deactivation, resulting in insulin resistance [71,72].
One other reason contributing to the relative lower energy expenditure at rest, besides higher percentages of body fat, amongst South Asians is the lower amount of brown adipose tissue (BAT, or brown fat) [69,73]. BAT is a type of adipose tissue that is specialized for heat generation and can be responsible for up to 20% of total energy expenditure in its activated mode [74]. In South Asians, BAT volumes have been shown to be approximately 34% less than Caucasians [69]. BAT is responsible for nonshivering thermogenesis (heat generation). For South Asians this means a reduced capacity to generate nonshivering thermogenesis or waste heat [69]. In cold-adapted populations, higher levels of BAT are protective for cardiometabolic health (including diabetes), as BAT produces uncoupled mitochondrial respiration for heat generation that burns off excess calories (nonshivering thermogenesis) [73]. The protective mechanism likely involves the use of BAT burn or its metabolism of excess calories in states of caloric overabundance [73,75].
These phenomena may explain why amongst diaspora populations of East Asians (who, like Caucasians, are a cold-adapted population with relatively elevated levels of thermogenic capacity) have a much lower predisposition to cardiometabolic disease than South Asian diaspora populations [73]. In fact, among individuals aged 20-29 years living in the US, the incidence of diabetes in South Asians is threefold higher than in Chinese, as well as two times higher when compared with individuals of European origin [53]. This finding occurs despite a similar famine laden history in both South Asia and China [53,76,77].
In summary, regarding energy expenditure, South Asians were substantially impacted by starvation adaptation. These adaptations would have promoted hyperefficient mitochondria and a lack of BAT. Both adaptations would have been helpful in promoting energy efficiency. Having less brown fat is thought to be a tropical adaptation, although an additional benefit may have been less energy consumption during starvation [73]. However, in times of abundance, this lack of BAT predisposes South Asians to cardiometabolic disease.
Greater Insulin Resistance/Insulin Response
Certain genetic factors involved in cardiometabolic processes including prediabetes/diabetes have also been found to be contributing factors to the unique South Asian physiology [43]. A single-nucleotide polymorphism (SNP) of the MC4R gene associated with higher levels of visceral (including hepatic) fat and insulin resistance is highly prevalent in South Asians [78]. Furthermore, at least six genetic variants associated with the premature onset of insulin-resistant diabetes have been found to be much more prevalent in South Asians than in Europeans [79]. Another study suggested that based on genetic data (polygenic risk score), South Asians had about a four times higher risk of type 2 diabetes than the European population [80]. South Asians have a higher postprandial insulin response, with a higher tendency toward insulin resistance in the overfed state (Figures 1 and 2) and there is a three- to four-fold higher prevalence of insulin resistance in lean South Asian men than in lean men of other ethnic groups [81,82].
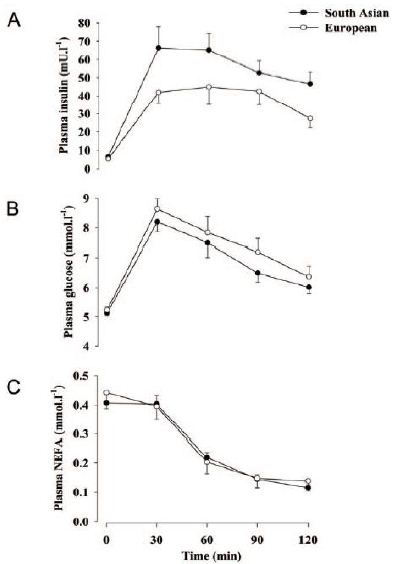
Figure 1: Plasma insulin (A), glucose (B) and nonesterified fatty acid (NEFA) (C) responses to a 75 g oral glucose load.82 Note the higher insulin response, but the equivalent serum glucose and NEFA responses, to the same glucose load in South Asians (than that in Europeans). Thus, thriftiness with glucose as seen in South Asians would be a useful starvation adaptation. [Reproduced from Hall, et al. (2010) with permission from PLOS].
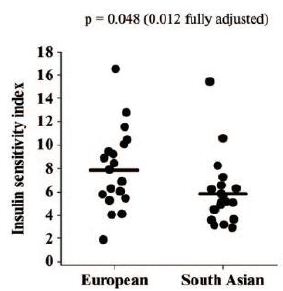
Figure 2: Comparison of European men to South Asian men shows that South Asian men have a lower insulin sensitivity index (reflecting a higher insulin response to an oral glucose load). The horizontal bars denote mean values. The P values shown are for the difference between the European and South Asian groups, either unadjusted or adjusted for age, BMI and fat mass.82 This finding suggests higher insulin resistance overall in South Asian men. Higher insulin resistance may be a useful starvation adaptation by preserving glucose. [Reproduced from Hall, et al. (2010) with permission from PLOS].
Regarding fat intake, one study showed that five days of consuming a high-fat and high-calorie diet resulted in the development of insulin resistance, manifesting as a reduced nonoxidative insulin-stimulated glucose disposal rate, in all South Asian participants (but in none of the Caucasian participants) [83]. Therefore, high fat intake produces insulin resistance more readily in South Asians. A physiological mechanism underpinning this was recently determined to be related to lipid droplet dynamics linked to perilipin-5 (PLIN5), a protein that interacts with intramyocellular lipids to transport them to mitochondria. High PLIN5 levels typically maintain insulin sensitivity in Caucasians in response to high fat intake.
South Asians have even higher levels of PLIN5 in response to high fat intake, yet despite these higher levels, toxic fat breakdown products accumulate in the muscle, resulting in insulin resistance. Lipid droplet dynamics (which involve the release of fatty acids to fuel mitochondrial oxidation) in South Asians are therefore likely impaired or compromised [84]. Even early in life insulin resistance is more prominent in South Asians as the umbilical cord blood of newborns of South Asian ancestry manifest elevated insulin levels [85,86].
It is known that insulin resistance predisposes and correlates to cardiometabolic risk. Insulin resistance can be ameliorated by exercise. However, in the setting of higher insulin resistance, a greater duration of exercise may be needed to improve insulin sensitivity [87,88]. This occurrence could explain why South Asians may need more exercise. It is known that South Asians may need up to 80% more exercise to stave off cardiometabolic disease. Specifically, to maintain the same level of cardiometabolic health as Caucasians, South Asians need to exercise much more than their Caucasian counterparts. For instance, 150 minutes of moderate intensity exercise per week is recommended for Caucasians, but the time needed for South Asians to achieve the equivalent cardiometabolic benefits of moderate intensity exercise is 232–266 minutes per week [89,90].
Insulin resistance is believed to be beneficial in the starvation- adapted individual by reducing glucose uptake by muscles [91]. More importantly, glucose would be diverted to the brain, which does not need insulin for glucose uptake; this is known as the selfish brain hypothesis [92-94]. Alternatively, it has been suggested that the benefits provided by insulin resistance during starvation may actually be by preventing the net degradation of proteins [95]. Insulin resistance also promotes lipogenesis and fat retention, both of which would be helpful in a starvation state; this would explain why South Asians develop insulin resistance at much lesser levels of body fat and why the lower BMI/waist circumference (WC) cutoffs were established (see below).
Exaggerated Lipemic Response to Excess Sugar and Fat Intake (Predisposes to Insulin Resistance as a Starvation Survival Mechanism)
Due to an elevated predisposition to insulin resistance, South Asians have demonstrated different physiological responses to sugar and fat intake. Dietary sugar intake, like fructose and glucose, in healthy South Asians with normal indices of insulin sensitivity generates a much higher lipemic response when compared with Caucasians, due to an enhanced hepatic de novo lipogenesis (DNL). There is a known negative correlation between de novo lipogenesis and insulin sensitivity [96]. Additionally, when given a high-fat diet, South Asians had greater adverse effects on their lipid profile and insulin sensitivity than Europeans/Caucasians [97]. Such an exaggerated lipemic response would be beneficial as a starvation adaptation to promote fat storage.
Less Capacity to Handle Overabundance of Food
Despite the higher tendency toward insulin resistance and high insulin levels, South Asians have a lower beta cell reserve for insulin secretion compared to people of any other ethnicity [50]. This phenomenon may have been a response to the repeated famines South Asians endured [53]. Physiological changes associated with insulin resistance and metabolic syndrome (dysglycemia and dyslipidemia) occur at much lower body fat levels in South Asians than they do in people of other ethnicities [98]. These data support the establishment of lower BMI/ WC cutoffs for obesity by the World Health Organization (WHO) and International Diabetes Federation (IDF) for the South Asian population [99].
Possible explanations for this inability to handle a surplus of energy is called the adipose tissue overflow hypothesis [100]. The primary compartment in which fat is stored is in the superficial subcutaneous adipose tissue, where excess fat is inert metabolically. As a consequence of enduring severe famine, there would be no physiological need for a fully developed store of excess fats in the superficial subcutaneous adipose tissue. Due to the smaller capacity of subcutaneous fat storage available to South Asians, this compartment is overfilled much earlier than it is in Caucasians. Therefore, fat storage resumes in more metabolically active compartments (deep subcutaneous and visceral adipose tissues), resulting in metabolic complications such as dysglycemia and dyslipidemia at smaller BMIs than other ethnic groups, such as Caucasians.
Such a starvation-adapted body would also need a lower beta cell reserve for insulin secretion, possibly due to hypostimulation of these beta cells [101,102]. Less insulin is necessary to function in a starvation/undernourished state; however, this reduced beta cell reserve would be more easily exhausted during a state of chronic overnutrition (i.e., chronic insulin resistance) [103]. Interestingly, Chinese individuals have the second lowest level of pancreatic beta cell reserve after South Asians [50]. This finding could also be a vestige of adaptation to survival during numerous famines in China.
Lower Fat Burning (Oxidative Capacity) and VO2max during Aerobic Exercise
South Asians have a lower fatty acid oxidation capability in states of aerobic exercise, with a trend towards lower fatty oxidation in the sedentary state [82,97,104]. Normally in the exercise state, fatty acids are released through lipid droplet lipolysis to fuel mitochondrial fatty acid oxidation demands [84]. Related to this, it has been found that the fat oxidative capacity during aerobic exercise is up to 40% lower in South Asians than in Caucasians [82]. In that study, fitness and VO2max were much lower in South Asians than in Caucasians even though mitochondrial content and function were similar or higher in South Asians than in Caucasians [82]. In other words, the intramuscular expression of oxidative and lipid metabolism genes in South Asians is not reduced, but there is still a lower oxidative capacity in the muscle during aerobic exercise. This finding is thought to be another manifestation of insulin resistance in South Asians that may originate as a result of impaired lipid droplet dynamics [84]. Starvation adaptation can explain why the South Asian phenotype favors a lower capability to burn fat during exercise, as well as in sedentary and overfed states.
Starvation adaptation would also explain the accumulation of intramyocellular fat due to the mitochondrial oxidation issues described above. This accumulation allows the oxidation of fat at a lower rate, thereby conserving fat stores. Additionally, intramyocellular fat accumulation impairs insulin receptors, leading to insulin resistance, which in turn prevents the utilization of glucose by muscles [91]. A starvation-adapted body would also be more likely to become insulin resistant more readily from the intake of saturated fat to maximize the storage of calories (in the form of fat, rather than burning it in muscle).
Energy-Conserving Response to Resistance Exercise
Besides differences in fatty acid oxidation during aerobic exercise, there is also a difference in response to resistance exercise among South Asians. The physiological response to identical resistance exercise training is less favorable among South Asians than among Caucasians. Although muscle protein synthesis was equivalent, South Asians had a poorer response with regard to body fat reduction, resting carbohydrate level reduction, fat metabolism increase, VO2max increase, and upper body strength increase [104].
Regarding systolic blood pressure reduction, South Asians also had a worse response to exercise. It was hypothesized this was due to reduced bioavailability (seen in prior studies with South Asians) of nitric oxide (NO2 a potent vasodilator) in resistance endothelial vessels that supply muscle [104-106]. It would be expected there would be a less favorable muscle response to resistance exercise (in regard to VO2max, less upper body muscle strength, and less fat reduction) [104]. Instead, in the starvation-adapted individual, this muscle response would aim to conserve calories and would be blunted. Additionally, in the starvation state, there would be a decrease in blood pressure reduction in response to exercise because of reduced NO2 bioavailability [104]. A decrease in the level of NO2, which has a vasodilatory effect in muscle, would be beneficial for reducing blood flow to the muscle and the subsequent calorie expenditure by muscle to preserve calories for use by the brain (selfish brain hypothesis). This may have taken place in South Asians to conserve energy in a starvation adapted state.
Higher Lipoprotein(a) in South Asians
Vitamin C scarcity during times of famine may have led to an adaptation that predisposes South Asians to cardiovascular disease during times of prosperity. During famine in some areas of colonial India the estimated prevalence of scurvy (or acute vitamin-C deficiency) by a British colonial medical official was as high as 60- 70% [107]. To compensate for this, lipoprotein(a) or Lp(a), may have become elevated in South Asians. This is because Lp(a) is a vitamin C analog that is hypothesized to be protective against vitamin C deficiency [108]. Moreover, Lp(a) as a surrogate of vitamin C helps in wound healing and protects against the effects of scurvy, which is characterized by capillary fragility, hemorrhage, and inadequate wound healing [108]. Here is how Lp(a) and cardiovascular disease are connected. Subclinical vitamin C-deficiency can occur in modern society. This deficiency can weaken arterial walls since vitamin C is critical to maintaining the collagen framework [109]. Lp(a), in functioning as a replacement for vitamin C, tends to also bring LDL-cholesterol to weakened arterial walls and incite plaque development [109]. A study in mice that could not produce vitamin C for themselves, and were enabled to produce Lp(a), showed that mice (lower order mammals that do not usually develop atherosclerotic plaques) developed atherosclerotic plaque when exposed to Lp(a) just like humans [110].
It would follow that starvation-adapted populations, such as South Asians, would have a high prevalence of elevated Lp(a) levels. In fact, South Asians have one of the highest prevalence (40-45%) of abnormal Lp(a) levels [111]. Lp(a) promoted survival during famine and undernourishment when life expectancy was low such as occurred in colonial India when it was as low as 20 years between 1910-1920 [62,63,66]. Lp(a) becomes problematic during times of prosperity and higher life expectancy as an elevated Lp(a) level is one of the strongest independent risk factor for premature cardiovascular disease [110,112].
A Pre-Colonial South Asian Historical Control Population
The Roma people (historically referred to as “gypsies”) in Europe emigrated from northern South Asia during the eleventh century. It is interesting to note that the Roma peoples’ rates of diabetes are similar to or significantly higher than rates of diabetes among white Caucasians [113-117]. However, reports of increased prevalence of diabetes among Roma populations are thought to occur due to predisposing factors of lower socioeconomic status, and increased smoking rates of the Roma people compared with the general population who on average have higher socioeconomic status [117- 119]. This increase contrasts sharply with the four to five times higher rate of diabetes in South Asians living in Western countries compared with white populations [4,5].
Additional evidence has found that the Roma people have no increased genetic susceptibility to diabetes compared with the general population [119]. This finding contrasts with the South Asian populations, which has a known genetic predisposition to diabetes [80].
The Roma may represent a historical control on the rate of diabetes in people of South Asian ancestry without the effect of the colonial famines. This control could potentially show how the colonial famines may have adversely impacted the South Asian rates of diabetes.
Conclusion
Taken together, the data indicate that South Asians have a unique physiology that may have evolved via epigenetic effects to ensure survival during severe and frequent famines. It is likely that these adaptations have predisposed the South Asian population to cardiometabolic diseases in an environment of abundance.
We suggest this perspective of the unique South Asian physiology based on a history of famines will help explain why this population is in a veritable health crisis of cardiometabolic disease and engender future studies. This research could be done using similar methodologies as those used in studies on the multigenerational effects of the Great Chinese Famine or the Överkalix Famine [34,35].
Physicians who are knowledgeable of this physiology will be appropriately more aggressive in diagnosing and treating South Asian patients in an ethnically sensitive fashion [120]. Physicians will also have higher empathy for and credibility with their South Asian patients that may ultimately lead to a higher compliance via a stronger physician-patient relationship [121]. South Asian patients who have a greater insight into their own bodies and history may be able to improve their vigilance and motivation to adhere to suggested lifestyle and medical therapies [122].
References
- Population, total – South Asia. The World Bank (2022) available at: https: //data. worldbank.org/indicator/SP.POP.TOTL?locations=8S
- Raji A, Seely EW, Arky RA, Simonson DC (2001) Body fat distribution and insulin resistance in healthy Asian Indians and J Clin Endocrinol Metab 86(11): 5366-5371. [crossref]
- Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, et (2007) Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 86(2): 353-359. [crossref]
- Farmaki AE et (2022) Type 2 diabetes risks and determinants in second-generation migrants and mixed ethnicity people of South Asian and African Caribbean descent in the UK. Diabetologia. 65(1): p. 113-127. [crossref]
- Gadgil MD, Anderson CAM, Kandula NR, Kanaya AM (2014) Dietary patterns in Asian Indians in the United States: an analysis of the metabolic syndrome and atherosclerosis in South Asians Living in America J Acad Nutr Diet 114(2): 238-243. [crossref]
- Tillin T, Forouhi N, Johnston DG, McKeigue PM, et (2005) Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans and white Europeans: a UK population-based cross-sectional study. Diabetologia 48(4): 649- 656. [crossref]
- Krishnan S. (2019) South Asians and Cardiovascular Disease: The Hidden Threat. American College of Cardiology. [crossref]
- Volgman AS, Palaniappan LS, Aggarwal NT, et al. (2018) Atherosclerotic Cardiovascular Disease in South Asians in the United States: Epidemiology, Risk Factors, and Treatments: A Scientific Statement from the American Heart Association. Circulation 138(1): e1-e34. [crossref]
- Greenland P, Blaha M J, Budoff M J, Erbel R, & Watson K E (2018) Coronary Calcium Score and Cardiovascular Journal of the American College of Cardiology. 72(4): 434–447. [crossref]
- Fischbacher CM, Hunt S, Alexander L (2004) How physically active are South Asians in the United Kingdom? A literature review. J Public Health (Oxf) 26(3): 250-258. [crossref]
- Ranasinghe CD, Ranasinghe P, Jayawardena R, Misra A (2013) Physical activity patterns among South-Asian adults: a systematic review. Int J Behav Nutr Phys Act 10: 116. [crossref]
- Biddle GJH, Edwardson CL, Rowlands AV, et al. (2019) Differences in objectively measured physical activity and sedentary behaviour between white Europeans and south Asians recruited from primary care: cross-sectional analysis of the PROPELS trial. BMC Public Health 19(1): 95. [crossref]
- Subhan FB, Chan CB (2019) Diet quality and risk factors for cardiovascular disease among South Asians in Applied Physiology, Nutrition, and Metabolism 44(8): 886-893. [crossref]
- Joshi P, Islam S, Pais P, et al. (2007) Risk Factors for Early Myocardial Infarction in South Asians Compared With Individuals in Other JAMA 297(3): 286-294. [crossref]
- Misra A, Khurana L, Isharwal S, Bhardwaj S (2009) South Asian diets and insulin resistance. Br J Nutr. Feb 2009;101(4): 465-73. [crossref]
- Misra A, Singhal N, Khurana L (2010) Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: role of dietary fats and oils. J Am Coll Nutr 29(3 Suppl): 289s-301s. [crossref]
- Gadgil MD, Anderson CAM, Kandula NR, Kanaya AM (2014) Dietary patterns in Asian Indians in the United States: an analysis of the metabolic syndrome and atherosclerosis in South Asians Living in America J Acad Nutr Diet 114(2): 238-243. [crossref]
- Martyn-Nemeth P, Quinn L, Menon U, Shrestha S, Patel C, Shah G (2017) Dietary Profiles of First-Generation South Asian Indian Adolescents in the United States. J Immigr Minor Health 19(2): 309-317. [crossref]
- Bhavadharini B, Mohan V, Dehghan M, et (2020) White Rice Intake and Incident Diabetes: A Study of 132,373 Participants in 21 Countries. Diabetes Care 43(11): 2643-2650. [crossref]
- Roseboom TJ, van der Meulen JHP, Osmond C, et al. (2000) Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 84(6): [crossref]
- Srichaikul K, Hegele RA, Jenkins DJA (2022) Great Chinese Famine and the Effects on Cardiometabolic Health for Future Generations. Hypertension 79(3): 532-535. [crossref]
- Wang Z, Song J, Li C, et (2020) DNA methylation of the INSR gene as a mediator of the association between prenatal exposure to famine and adulthood waist circumference. Scientific Reports 10(1): 12212. [crossref]
- Li J, Yang Q, An R, et (2022) Famine and Trajectories of Body Mass Index, Waist Circumference, and Blood Pressure in Two Generations: Results From the CHNS From 1993-2015. Hypertension 79(3): 518-531. [crossref]
- Nand B (2011) Nature and Causes of Famines in Colonial India Brahma Nand AAS- ICAS Special Joint Conference 2011 Association for Asian Studies International Convention of Asia Scholars. [crossref]
- Davis M (2001) Late Victorian Holocausts: El Nino Famine and the Making of the Third Verso Books. [crossref]
- Sourabh NC, Myllynaus Timo. (2015) Famines in Late Nineteenth-Century India: Politics, Culture, and Environmental Justice. Rachel Carson Center Virtual Exhibitions. 9: 48-50 [crossref]
- Bird (2020) The Worst Droughts And Famines In History. [crossref]
- Dodd (2020) The Deadliest Famines In History. [crossref]
- Muraleedharan VR (1994) Diet, Disease and Death in Colonial South Economic and Political Weekly 29: 55-63. [crossref]
- Sreevatsan A. (2018) British Raj siphoned out $45 trillion from India: Utsa Patnaik. [crossref]
- Motairek I, Lee Eun K, Janus S, et (2022) Historical Neighborhood Redlining and Contemporary Cardiometabolic Risk. Journal of the American College of Cardiology 80: 171-175. [crossref]
- Hanafi MY, Saleh MM, Saad MI, Abdelkhalek TM, Kamel MA (2016) Transgenerational effects of obesity and malnourishment on diabetes risk in F2 generation. Molecular and Cellular Biochemistry 412: 269-280. [crossref]
- Rechavi O, Houri-Ze’evi L, Anava S, et (2014) Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell 158: 277-287. [crossref]
- Bygren LO, Tinghög P, Carstensen J, et (2014) Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet 15: 12. [crossref]
- Li J, Liu S, Li S, et al. (2017) Prenatal exposure to famine and the development of hyperglycemia and type 2 diabetes in adulthood across consecutive generations: a population-based cohort study of families in Suihua, The American Journal of Clinical Nutrition 105: 221-227. [crossref]
- Tobi Elmar W, Slieker Roderick C, Luijk R, et al. (2018) DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in Science Advances 4: eaao4364. [crossref]
- Wang Z, Song J, Li Y, Dong B, Zou Z, Ma J (2019) Early-Life Exposure to the Chinese Famine Is Associated with Higher Methylation Level in the INSR Gene in Later Adulthood. Scientific Reports 9(1): 3354. [crossref]
- Chambers JC, Loh M, Lehne B, et al. (2015) Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control Lancet Diabetes Endocrinol 3: 526-534. [crossref]
- Bishwajit G (2015) Nutrition transition in South Asia: the emergence of non- communicable chronic diseases F1000Res 4:8. [crossref]
- Venniyoor A (2020) PTEN: A Thrifty Gene That Causes Disease in Times of Plenty? Hypothesis and Theory Frontiers in Nutrition Jun 5; 7:81. [crossref]
- Rion S, Kawecki TJ (2007) Evolutionary biology of starvation resistance: what we have learned from J Evol Biol. Sep 20: 1655-64. [crossref]
- Honegger B, Galic M, Köhler K, et (2008) Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol 7: 10. [crossref]
- Venugopal (2016) Increased prevalence of type 2 diabetes in south asian population – a genetic perspective. Journal of Diabetes, Metabolic Disorders & Control. 3(3): p. 57-58. [Crossref]
- Shah AD, Kandula NR, Lin F, et al. (2016) Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: results from the MASALA and MESA Int J Obes (Lond) 40: 639-645. [crossref]
- Kurpad AV, Varadharajan KS, Aeberli I (2011) The thin-fat phenotype and global metabolic disease Curr Opin Clin Nutr Metab Care 14: 542-547. [crossref]
- Deurenberg P, Deurenberg-Yap M, Guricci S (2002) Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 3: 141-146. [crossref]
- Misra A, Khurana L (2009) The metabolic syndrome in South Asians: epidemiology, determinants, and Metab Syndr Relat Disord 7: 497-514. [crossref]
- Misra A, Vikram NK (2004) Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and Nutrition 20: 482-91. [crossref]
- Gujral UP, Narayan KMV, Kandula NR, Liu K, Kanaya AM (2020) Incidence of diabetes and prediabetes and predictors of glycemic change among South Asians in the USA: the MASALA study BMJ Open Diabetes Res Care. [crossref]
- Narayan KMV, Kanaya AM (2020) Why are South Asians prone to type 2 diabetes? A hypothesis based on underexplored Diabetologia 63: 1103-1109. [crossref]
- Forouhi NG, Jenkinson G, Thomas EL, et (1999) Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia 42: 932-935. [crossref]
- Anand SS, Tarnopolsky MA, Rashid S, et (2011) Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS One 6: e22112. [crossref]
- Ke C, Narayan KMV, Chan JCN, Jha P, Shah BR (2022) Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nature Reviews Endocrinology 18: 413-432. [crossref]
- Nguyen TMD (2020) Adiponectin: Role in Physiology and Pathophysiology. Int J Prev Med 11: 136-136. [crossref]
- Forny-Germano L, De Felice FG, Vieira MNdN (2019) The Role of Leptin and Adiponectin in Obesity-Associated Cognitive Decline and Alzheimer’s Disease. Review. Frontiers in Neuroscience Jan 14; 12: [crossref]
- Metspalu M, Romero Irene G, Yunusbayev B, et al. (2011) Shared and Unique Components of Human Population Structure and Genome-Wide Signals of Positive Selection in South Asia. The American Journal of Human Genetics 89(6): 731-744. [crossref]
- Pomeroy E, Mushrif-Tripathy V, Cole TJ, Wells JCK, Stock JT (2019) Ancient origins of low lean mass among South Asians and implications for modern type 2 diabetes susceptibility. Scientific Reports 9: 10515 [crossref]
- Wells JC, Pomeroy E, Walimbe SR, Popkin BM, Yajnik CS (2016) The Elevated Susceptibility to Diabetes in India: An Evolutionary Front Public Health 4: 145. [crossref]
- Khazem S, Itani L, Kreidieh D, et al. (2018) Reduced Lean Body Mass and Cardiometabolic Diseases in Adult Males with Overweight and Obesity: A Pilot Int J Environ Res Public Health 15: 2754. [crossref]
- Nomura K, Eto M, Ogawa S, et (2020) Association between low muscle mass and metabolic syndrome in elderly Japanese women. PLOS ONE 15: e0243242. [crossref]
- Srikanthan P, Karlamangla AS (2011) Relative Muscle Mass Is Inversely Associated with Insulin Resistance and Prediabetes. Findings from The Third National Health and Nutrition Examination Survey. The Journal of Clinical Endocrinology & Metabolism 96: 2898-2903. [crossref]
- Meena HK. (2016) Railway and Famines in British India. Silpakorn University Journal of Social Sciences, Humanities, and 16(1):1-18. [crossref]
- Klein (1973) Death in India, 1871-1921. The Journal of Asian Studies. 32(4):639- 659. [crossref]
- Brennan L, Donald MJ, Shlomowitz R (1997) Towards an anthropometric history of Indians under British Res Econ Hist. Emerald Group Publishing Ltd. 17:185-246. [crossref]
- Baten J, Blum M (2012) An anthropometric history of the World, 1810-1980: did migration and globalization influence country trends? J Anthropol Sci 90: 221-224. [crossref]
- O’Neill (2022) Life Expectancy in India 1800-2020. [crossref]
- Wulan SN, Raza Q, Prasmita HS, et (2021) Energy Metabolism in Relation to Diet and Physical Activity: A South Asian Perspective. Nutrients 13: 3776. [crossref]
- Soares MJ, Piers LS, O’Dea K, Shetty PS (1998) No evidence for an ethnic influence on basal metabolism: an examination of data from India and Br J Nutr. Apr 79: 333-41. [crossref]
- Bakker LE, Boon MR, van der Linden RA, et (2014) Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: a prospective, case-controlled observational study. Lancet Diabetes Endocrinol 2: 210-217. [crossref]
- Nair KS, Bigelow ML, Asmann YW, et al. (2008) Asian Indians Have Enhanced Skeletal Muscle Mitochondrial Capacity to Produce ATP in Association With Severe Insulin Resistance. Diabetes 57: 1166-1175. [crossref]
- Fisher-Wellman KH and Neufer (2012) Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol Metab. 23(3): p. 142-53. [crossref]
- Conley KE (2016) Mitochondria to motion: optimizing oxidative phosphorylation to improve exercise J Exp Biol 243-249. [crossref]
- Sellayah D (2018) The Impact of Early Human Migration on Brown Adipose Tissue Evolution and Its Relevance to the Modern Obesity Journal of the Endocrine Society 3: 372-386. [crossref]
- Marlatt KL, Ravussin E (2017) Brown Adipose Tissue: an Update on Recent Curr Obes Rep 6(4): 389-396. [crossref]
- Becher T, Palanisamy S, Kramer DJ, et (2021) Brown adipose tissue is associated with cardiometabolic health. Nature Medicine 27: 58-65. [crossref]
- Roy T (2016) Were Indian Famines ‘Natural’ Or ‘Manmade’? : London School of Economics. [Crossref]
- Li LM (2007) Fighting Famine in North China. Stanford University Press 284. [crossref]
- Chambers JC, Elliott P, Zabaneh D, et al. (2008) Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet 40: 716-718. [crossref]
- Kooner JS, Saleheen D, Sim X, et al. (2011) Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nature genetics 43: 984-989. [crossref]
- Loh M, Zhang W, Ng HK, et al. (2022) Identification of genetic effects underlying type 2 diabetes in South Asian and European populations. Communications Biology 5: 329. [crossref]
- Gujral UP, Pradeepa R, Weber MB, Narayan KMV, Mohan V (2013) Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Annals of the New York Academy of Sciences 1281: 51-63. [crossref]
- Hall LML, Moran CN, Milne GR, et al. (2010) Fat Oxidation, Fitness and Skeletal Muscle Expression of Oxidative/Lipid Metabolism Genes in South Asians: Implications for Insulin Resistance? PLOS ONE 5:e14197. [crossref]
- Bakker LE, van Schinkel LD, Guigas B, et (2014) A 5-day high-fat, high-calorie diet impairs insulin sensitivity in healthy, young South Asian men but not in Caucasian men. Diabetes 63: 248-258. [crossref]
- Gemmink A, Bakker LEH, Guigas B, et (2017) Lipid droplet dynamics and insulin sensitivity upon a 5-day high-fat diet in Caucasians and South Asians. Scientific Reports 7: 42393. [crossref]
- Yajnik CS, Lubree HG, Rege SS, et al. (2002) Adiposity and hyperinsulinemia in Indians are present at J Clin Endocrinol Metab 87: 5575-5580. [crossref]
- Karamali NS, Ariens GA, Kanhai HH, de Groot CJ, Tamsma JT, Middelkoop BJ (2015) Thin-fat insulin-resistant phenotype also present in South Asian neonates born in the J Dev Orig Health Dis 6: 47-52. [crossref]
- Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, & Kraus (2004) Effect of the volume and intensity of exercise training on insulin sensitivity. Journal of applied physiology. 96(1): 101–106. [crossref]
- Iaccarino G, Franco D, Sorriento D, et al. (2021) Modulation of Insulin Sensitivity by Exercise Training: Implications for Cardiovascular Prevention. of Cardiovasc. Trans. Res. 14, 256–270. [crossref]
- Celis-Morales CA, Ghouri N, Bailey MES, Sattar N, Gill JMR (2013) Should Physical Activity Recommendations Be Ethnicity-Specific? Evidence from a Cross-Sectional Study of South Asian and European PLOS ONE 8: e82568. [crossref]
- Iliodromiti S, Ghouri N, Celis-Morales CA, Sattar N, Lumsden MA, Gill JMR (2016) Should Physical Activity Recommendations for South Asian Adults Be Ethnicity- Specific? Evidence from a Cross-Sectional Study of South Asian and White European Men and PloS one 11(8): e0160024-e0160024. [crossref]
- Mansell PI and Macdonald IA. (1990) The effect of starvation on insulin-induced glucose disposal and thermogenesis in humans. Metabolism. 39(5): 502-510. [crossref]
- Hearst A(2011) YOU DON’T LOOK DIABETIC: DIABETES IN NON-OBESE SOUTH ASIANS–IS THERE A MOLECULAR OR GENETIC BASIS FOR INCREASED INSULIN RESISTANCE? Clinical Correlations. [crossref]
- Watve MG, Yajnik CS (2007) Evolutionary origins of insulin resistance: a behavioral switch BMC Evol Biol 7: 61-61. [crossref]
- Wang P, Mariman E (2008) Insulin resistance in an energy-centered Physiology & behavior 94: 198-205. [crossref]
- Soeters MR, Soeters PB (2012) The evolutionary benefit of insulin resistance. Clin Nutr 31(6): 1002-1007. [crossref]
- Hudgins LC, Hugo JL, Enayat S, Parker TS, Artis AS, Levine DM (2017) Young, healthy South Asians have enhanced lipogenic sensitivity to dietary Clin Endocrinol (Oxf) 86: 361-366. [crossref]
- Wulan SN, Schrauwen-Hinderling VB, Westerterp KR, Plasqui G (2020) Substrate utilization and metabolic profile in response to overfeeding with a high-fat diet in South Asian and white men: a sedentary lifestyle Int J Obes (Lond) 44: 136-146. [crossref]
- Patel SA, et al, (2016) Is the “South Asian Phenotype” Unique to South Asians?: Comparing Cardiometabolic Risk Factors in the CARRS and NHANES Glob Heart. 11(1): p. 89-96.e3. [crossref]
- Caleyachetty R, et al. (2021) Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort Lancet Diabetes Endocrinol. 9(7): p. 419-426. [crossref]
- Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof (2007) Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 36(1):220-196. [crossref]
- Cerf M E. (2013). Beta cell dysfunction and insulin resistance. Frontiers in endocrinology. 4:37. [crossref]
- Wysham C & Shubrook (2020) Beta-cell failure in type 2 diabetes: mechanisms, markers, and clinical implications. Postgraduate medicine. 132(8): 676–686. [crossref]
- Boland BB, Rhodes CJ, & Grimsby JS. (2017) The dynamic plasticity of insulin production in β-cells. Molecular 6(9): 958–973. [crossref]
- Alkhayl FFA, Ismail AD, Celis-Morales C, et (2022) Muscle protein synthesis and muscle/metabolic responses to resistance exercise training in South Asian and White European men. Sci Rep 12: 2469. [crossref]
- Cubbon RM, Murgatroyd SR, Ferguson C, et al. (2010) Human exercise-induced circulating progenitor cell mobilization is nitric oxide-dependent and is blunted in South Asian Arterioscler Thromb Vasc Biol 30: 878-84. [crossref]
- Murphy C, Kanaganayagam GS, Jiang B, et al. (2007) Vascular Dysfunction and Reduced Circulating Endothelial Progenitor Cells in Young Healthy UK South Asian Men. Arteriosclerosis, Thrombosis, and Vascular Biology 27: 936-942. [crossref]
- Whitcombe E (1993) Famine Economic and Political Weekly 28: 1169-1179.
- Rath M, Pauling L (1990) Hypothesis: lipoprotein(a) is a surrogate for Proc Natl Acad Sci U S A 87: 6204-6207. [crossref]
- Ivanov V, et (2020) Vitamin C inhibits the calcification process in human vascular smooth muscle cells. Am J Cardiovasc Dis. 10(2): p. 108-116. [crossref]
- Cha J, Niedzwiecki A and Rath M. (2015) Hypoascorbemia induces atherosclerosis and vascular deposition of lipoprotein(a) in transgenic mice. American journal of cardiovascular disease. 5(1): 53-62. [crossref]
- Enas E, Kannan S (2005) How to Beat the Heart Disease Epidemic Among South Asians: A Prevention and Management Guide for Asian Indians and Their Doctors. Advanced Heart Lipid Clinic 351. [crossref]
- Enas EA, Varkey B, Dharmarajan TS, Pare G, Bahl VK (2019) Lipoprotein(a): An independent, genetic, and causal factor for cardiovascular disease and acute myocardial Indian Heart J 71: 99-112. [crossref]
- Nunes MA, Kučerová K, Lukáč O, Kvapil M, Brož J (2018) Prevalence of Diabetes Mellitus among Roma Populations-A Systematic Review. Int J Environ Res Public Health Nov; 15(11): 2607. [crossref]
- Beljić Zivković T, Marjanović M, Prgomelja S, et al. (2010) Screening for diabetes among Roma people living in Croat Med J 51: 144-150. [crossref]
- Hubáček JA, Šedová L, Olišarová V, Adámková V, Tóthová V (2020) Different prevalence of T2DM risk alleles in Roma population in comparison with the majority Czech Molecular Genetics & Genomic Medicine 8: e1361. [crossref]
- Weiss E, Japie C, Balahura AM, Bartos D, Badila E (2018) Cardiovascular risk factors in a Roma sample population from Rom J Intern Med 56: 193-202. [crossref]
- Vozarova de Courten B, de Courten M, Hanson RL, et al. (2003) Higher prevalence of type 2 diabetes, metabolic syndrome and cardiovascular diseases in gypsies than in non-gypsies in Diabetes Res Clin Pract 62: 95-103. [crossref]
- Hajioff S, McKee M (2000) The health of the Roma people: a review of the published literature. Journal of Epidemiology and Community Health 54: 864-869. [crossref]
- Werissa NA, Piko P, Fiatal S, Kosa Z, Sandor J, Adany R (2019) SNP-Based Genetic Risk Score Modeling Suggests No Increased Genetic Susceptibility of the Roma Population to Type 2 Diabetes Genes (Basel) Nov 19; 10(11): 942. [crossref]
- Kim EJ, et (2018) Racial and Ethnic Disparities in Diagnosis of Chronic Medical Conditions in the USA. J Gen Intern Med. 33(7): p. 1116-1123. [crossref]
- Martin LR, Williams SL, Haskard KB, Dimatteo MR (2005) The challenge of patient adherence. Ther Clin Risk Manag 1: 189-199. [crossref]
- Jin J, Sklar GE, Min Sen Oh V, Chuen Li S (2008) Factors affecting therapeutic compliance: A review from the patient’s Ther Clin Risk Manag 4: 269-286. [crossref]