DOI: 10.31038/AFS.2022453
Abstract
In 2016 America’s coastal counties were home to more than 40 percent of the total population despite accounting for less than 10 percent of the country’s landmass. Large-scale changes in land use lead to proportional increases in impervious ground cover, ultimately resulting in increased input of stormwater runoff into adjacent waterways. Stormwater runoff reduces salinity and increases contaminant loads as rainwater washes pollutants, including pesticides such as bifenthrin, into receiving waters. The present study examined bifenthrin toxicity and the potential combined effect of reduced salinity for larval sheepshead minnows (Cyprinodon variegatus) and grass shrimp (Palaemon pugio). LC50 values were established in salinities of 20, 10, and 5 psu as 0.431, 0.415, 0.377 µg/L and 0.00650, 0.00640, 0.000109 µg/L for larval C. variegatus and P. pugio, respectively. Salinity did not significantly affect bifenthrin toxicity to larval C. variegatus, but mortality rates increased to 90% when larval P. pugio were exposed to 0.0015 µg/L of bifenthrin in 5 psu compared to 20 psu. Given that stormwater input is increasing as a result of increasing impervious cover, it is critical to understand how exposure to bifenthrin in low-salinity regimes affects estuarine organisms.
Keywords
Pesticides, Bifenthrin, Fish, Crustaceans, Toxicity, Salinity, Tidal creeks, Runoff
Introduction
Impervious surface, a result of urbanization, reduces rainwater infiltration and promotes runoff which accelerates input of sediments, microplastics, metals, pesticides, fertilizers, and bacteria into surface waters [1-3]. A study from 2001 found that pesticides are pervasive in waterways nationwide, with at least one pesticide found in more than 95% of streams sampled and in about 85% of fish sampled [4]. The influx of contaminants such as pesticides often result in habitat degradation and impaired ecosystem functions [5,6]. Impervious cover has also been directly linked with ecosystem effects including changes to community structure, a decline in density, as well as reduced species diversity [2,5,7,8].
Pyrethroids currently account for more than 25% of world-wide insecticide use and are widely applied to crops, turf, golf courses, lawns and home gardens in the U.S. [9,10]. Bifenthrin ((2-methyl-1, 1-biphenyl-3-yl)-methyl-3-(2-chloro-3, 3, 3-trifluoro-1-propenyl)-2,2-dimethylcycloprpanecarboxylate) is a fourth generation synthetic pyrethroid insecticide. The use of bifenthrin has increased in the last 20 years as a result of bans on pesticides such as DDT, the establishment of the Federal Clean Water Act, which mandates the reduced use of organophosphate insecticides, as well as the increased effectiveness and stability of this new insecticide [9]. However, with greater photostability and insecticidal efficacy than previous generations of pyrethroids, bifenthrin has the potential to be more toxic to non-target species [11,12].
More than one million pounds of bifenthrin was used agriculturally in the U.S. in 2016, mainly applied to corn, soy, cotton, and orchards [13]. There has also been an increasing trend in urban applications of bifenthrin [14]. Urban applications of bifenthrin in the Central Valley of California were reported at 45,000 pounds, over double that of agricultural applications at 20,000 pounds, in California in 2005 [15]. Additionally, pesticide use by acre on golf courses has been reported as equivalent as or greater than use on agricultural crops [16].
Bifenthrin is currently one of the most frequently detected contaminants in California surface waters in areas of urban and agricultural land development [17,18]. Reported surface water concentrations of bifenthrin ranged from 0.005 to 3.79 μg/L (parts per billion – ppb) and bottom and suspended sediment concentrations have been reported in the range of 1.2 to 437 ng/g (ppb) dry weight. The 96-hour LC50 (estimated concentration in which fifty percent of the test organisms die) for rainbow trout, bluegill sunfish, sheepshead minnow, and mysid was 0.15, 0.35, 17.8, and 0.00397 μg/L (ppb), respectively [19-21].
In general, pyrethroid pesticides, like bifenthrin, have octanol/water partition coefficient (log Kow) values of 5 to 7 and, therefore, partition into the organic carbon fraction of sediments. Although sediment sequestration may lead to confinement in areas of application, pyrethroids are often transported into surface waters via runoff, moving with suspended sediments and dissolved organic matter [22,23]. Additionally, these hydrophobic compounds can become stored in creek-bed sediments to later become resuspended as runoff increases turbidity [18]. Beyond entering waterways via runoff, spray drift, and release of agricultural tailwater also contribute to pyrethroid contamination [22,24,25].
The primary mechanism of pyrethroid toxicity is interference with sodium channel polarization in synaptic nerve terminals [26]. Effectively, this interaction simulates neurotransmission when there is none, causing spastic activity followed by paralysis [20,26]. Additionally, pyrethroids have been shown to inhibit ATPase enzyme production [27]. As pyrethroids impede the ATPase enzyme the critical concentration gradient built to maintain ionic balance and osmoregulation is degraded [27]. Sublethal toxicity has also been reported in organisms exposed to pyrethroids, including altered behavior, reduced growth, immune system effects, endocrine/reproductive effects, histopathological effects, as well as biochemical responses [28-30].
Beyond increased contamination, stormwater runoff as a result of increased impervious cover can also unpredictably and rapidly reduce salinity, with reported drops greater than 26 psu in less than 6 hours [31,32]. While estuarine species have mechanisms to cope with predictable environmental variability (such as tidal phases and seasonal conditions) the rapid and unpredictable effects of stormwater runoff can have serious implications for the biological communities of receiving waterways [1,33]. Numerous studies have demonstrated that reduced salinity can have vastly negative impacts on planktonic larval organisms, including decreased rates of yolk sac absorption, growth, development of feeding apparatus, respiratory tissues, and mortality [1,34-37].
Chemical toxicity may intensify salinity stress, causing an increase in sublethal and lethal effects. In fact, chemical toxicity in combination with salinity stress has been attributed to decreased physiological functions, specifically in the physiological pathways that are responsible for contaminant metabolism and detoxification [38]. Salinity has also been shown to affect biotransformation rates and toxicity for several classes of chemicals [38]. It has been reported that salinity generally decreases water solubility and increases Kow values for pesticides [39]. The water solubility of bifenthrin has been reported as <1 mg/L, with reported log Kow in deionized water as 6.27 ± 0.16, and with 6.78 ± 0.04 in seawater (35 psu) [12,20]. These data suggest that salinity should be considered when bifenthrin toxicity estimates are made for estuarine organisms.
Two abundant, widely distributed, euryhaline, common estuarine test organisms, the grass shrimp, Palaemon pugio, and the sheepshead minnow, Cyprinodon variegatus, in their larval stages, were selected for the current study [40]. Grass shrimp are generalist foragers that may feed as primary or secondary consumers and play a key role in coastal nutrient cycling as detritivores [40]. Additionally, P. pugio are an important prey item for numerous commercially and recreationally important estuarine species [40,41]. Larval P. pugio are approximately 2.6 mm at hatching and subsequently undergo a multistage larval development period that can take from 11 days to several months [42]. Planktonic larvae feed on other zooplankton, phytoplankton, and detritus [40]. South Carolina grass shrimp tolerate salinities from nearly freshwater to full strength seawater, and those collected from low salinity sites were of smaller size than those from higher salinity waters. Ultimately, salinities between 20 and 25 psu are optimal for larval development [40].
C. variegatus can live in ambient salinities ranging from 0 psu to greater than 140 psu but a preference for salinities near or less than 20 psu has been documented [43,44]. They feed mostly on plant matter, algae, detritus, mosquito larvae, and smaller fish [45,46]. Sheepshead minnows serve as an important link in the food chain as a source of nutrient cycling and as prey for larger commercially and recreationally important species such as the spotted sea trout, red drum, Atlantic croaker, turtles, and wading birds [41]. Fertilized C. variegatus eggs incubate for 4 to 12 days before planktonic larvae of ~4 mm hatch [45]. Larval C. variegatus feed on other zooplankton, phytoplankton, and detritus [45,46].
To mitigate negative effects of bifenthrin on natural communities, it is imperative to examine how bifenthrin affects survival rates of important estuarine species. Standard toxicity bioassays, conducted under laboratory conditions, may not be predictive of how changing environmental conditions such as salinity may alter the chemical toxicity of these compounds. The current study sought to evaluate the impacts of acute salinity reduction in combination with bifenthrin exposure on larval estuarine species by using C. variegatus and P. pugio as model species.
As part of a SC Department of Natural Resources study examining the relationship between urbanization and salinity profiles in estuarine tidal creeks, sediment samples were collected from the same tidal creek sites and analyzed for bifenthrin contamination. The measured field concentrations are reported herein and compared to the laboratory-derived toxicity thresholds to assess relative risk to larval estuarine organisms.
Materials and methods
Animal Acquisition and Holding
Sheepshead minnows, Cyprinodon variegatus, collected from a tidal pond on the Fort Johnson campus (32° 44’ 53.60” N; 79° 54’ 4.45” W) were acclimated and maintained in laboratory conditions of recirculating filtered, aerated seawater at 25°C, 20 psu salinity, and a 16-hour light: 8-hour dark photoperiod. Brooding groups of 2-3 males and 4-6 females were placed in spawning chambers within 75-L aquaria. Adult fish were fed once daily with TetraminVR flake food. Fish eggs collected every day were placed in 20 psu aerated seawater, examined on a light box for hatching events, and hatched larvae were counted, separated, held in aerated 20 psu seawater, and fed daily with Artemia.
Adult grass shrimp, Palaemon pugio, were collected from Leadenwah Creek, Wadmalaw Island, South Carolina (32° 38’ 51.00” N; 80° 13’ 18.05” W). Shrimp were acclimated and maintained in 75-L aquaria with aerated, 25°C, 20 psu seawater, and a 16-hour light: 8-hour dark photoperiod. Adult shrimp were fed once daily with TetraminVR flake food. Gravid female shrimp were separated, held in hatching chambers containing 20 psu aerated seawater, and examined daily for hatching events. Chambers were removed after hatching event. Larvae were counted, separated, held in aerated 20 psu seawater and fed daily with Artemia.
Seawater Processing
Seawater collected from Charleston Harbor (32° 45’ 11.52” N; 79° 53’ 58.31” W) was allowed to settle, polished via a sand filtration unit, UV sterilized, and filtered again with 5 μm nominal filtration. The polished seawater was subsequently pumped through a 10 μm carbon filtration before being diluted to 20 psu with deionized water. All water used for testing was additionally pumped through a sterile 0.22-μm filter.
Larval Aqueous Assay
Range finder tests were conducted with both species to determine the definitive test concentrations. A 96-hour aqueous static-renewal toxicity test was conducted to determine the LC50 of bifenthrin in 20, 10, and 5 psu filtered seawater for C. variegatus and P. pugio. All testing started within 48 hours of hatching. Fish and shrimp larvae were fed Artemia prior to testing and at each 24-hour renewal during the 96-hour test. Stock solutions of bifenthrin were made using pesticide-grade acetone and the final acetone concentration in all treatments and the seawater control was 0.1%. Each species was tested using 5 nominal concentrations of bifenthrin plus a control (0.00 μg/L); 0.17, 0.25, 0.37, 0.56, 0.84 μg/L for the C. variegatus assay, and 0.0015, 0.0025, 0.0045, 0.0065, and 0.016 μg/L in the P. pugio assay; at each of the three salinities (20, 10, and 5 psu). Three replicate beakers were loaded with 400 mL of seawater dosed with bifenthrin at the target exposure concentration. Larvae (24-48 h old) were taken through a step-wise reduction in salinity. Individuals were allowed to acclimate in a glass finger bowl containing 20 psu water for 90 minutes before being transferred to 15 psu seawater. This process of 90-minute acclimation followed by a transfer into filtered seawater reduced by 5 psu was repeated until the desired exposure concentration was reached (20, 10 or 5 psu). All larvae were transferred each time regardless of salinity reduction to account for any handling stress. After salinity acclimation was complete, 10 larvae were added to each replicated beaker. Beakers were covered with clean foil and aerated through a hole in the foil using a sterile glass pipette tip. Beakers were randomly distributed in an incubator maintained at 25°C and a16-hour light: 8-hour dark photoperiod. Every 24 hours the water was renewed in the same process as above. Temperature, salinity, pH, and dissolved oxygen values were recorded, and each individual was assessed for survival and survivors were transferred to the renewed beaker.
Tidal Creek Sediment Sampling
Tidal creeks in the greater Charleston area were analyzed in ArcGIS Pro using National Land Cover Data, NOAA Coastal Change Analysis Program land use data, and digital elevation models. Four tidal creeks, Guerin Creek, Seaside Creek, Toomer Creek, and Dupont-Wappoo Creek, with similar watershed area and creek volume but representing a range of development and impervious cover within the watershed were selected. Guerin Creek is the most natural creek with less than 1% impervious cover and development, Seaside and Toomer Creek watersheds have roughly 11% impervious cover and 25.9% and 30.2% of land development respectively. Dupont-Wappoo Creek watershed is the most urbanized with about 64% of the watershed developed and around 36% impervious cover. Within each creek four sampling sites were distributed along the length of the creek, from headwaters to the mouth, with the exception of Guerin Creek, where a site in each branch of the headwaters was selected (Figure 1). In late September, bottom sediment samples were collected at each site using a pre-cleaned stainless steel 0.04 m2 Young grab. A sample was also collected from the Leadenwah Creek control site where P. pugio were collected for testing. The surficial sediment (top ~3 cm) was homogenized and placed in a pre-cleaned container and stored on ice while in the field. Samples were stored at -40°C until analytical chemistry was conducted.
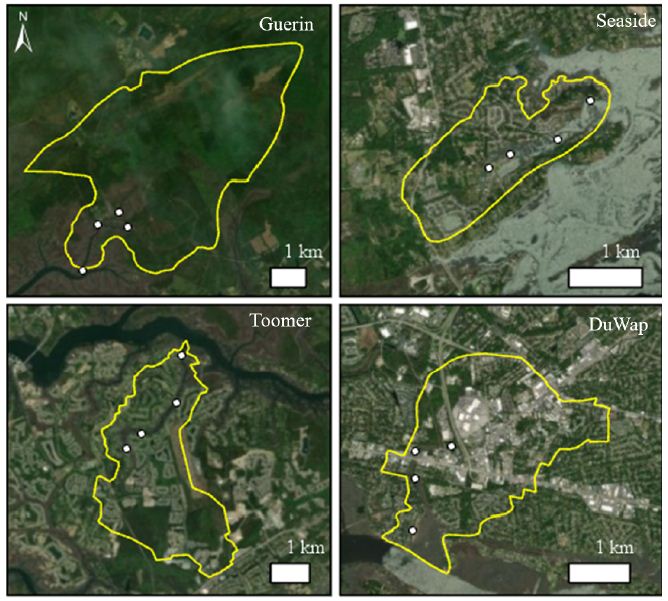
Figure 1: Satellite image of creeks sampled with watershed boundaries and collection sites marked
Analytical Chemistry
Sediment samples collected from each segment of the study site tidal creeks and one sample collected from the reference creek (Leadenwah Creek) were analyzed for a suite of pyrethroid insecticides, including bifenthrin, using accelerated solvent extraction. Sediment samples were weighed (~10 g) and mixed with anhydrous sodium sulfate in a mortar bowl to remove water from the sample. The samples (including method blanks and reference spikes) were transferred to stainless steel ASE cells, spiked with a suite of isotopically labeled internal standards, and extracted with 100% dichloromethane (DCM). Sulfur was removed from the sample extracts using coiled copper strands activated with ~10% hydrochloric acid. Residual water was removed by filtering the sample extract through additional anhydrous sodium sulfate after which the sample was then concentrated to 0.5 mL using TurboVap II Concentration Workstation (40°C, nitrogen at 14 psi). Concentrated samples were cleaned up using activated carbon and alumina solid phase extraction. Eluents were concentrated to 0.5 mL and solvent-exchanged to hexane. Samples were run on an Agilent 6890/5973 gas chromatograph mass spectrometer using a programmable temperature vaporizer inlet connected to a DB-XLB analytical column (30 m x 0.25 mm x 0.25 µm). The mass spectrometer was operated using electron impact and selected ion monitoring modes. Sample chromatograms were analyzed using MSD Chemstation software (ver. E.02.02.1431). An eight-point calibration curve (0. 5-100 ng) was run prior to running samples; r2 values for all analytes of interest were at least 0.995. The method detection limit (MDL) was calculated according to Ragland et al. [47] detectable concentrations of bifenthrin were reported in ng/g dry weight concentrations.
Data Analysis
Two-way analysis of variance with interaction was used to determine significant differences among treatments (RStudio, PBC, Boston, MA). Dunnett’s test was used to assess treatment differences from the control and to determine no observable effects concentration (NOEC) and lowest observable effects concentration (LOEC) values. Median lethal concentration (LC50) values with a 95% confidence interval were determined using nominal chemical concentrations (SAS Probit Analysis, PROC PROBIT, SAS V.9.1.3, Cary, NC). Significant differences (α=0.05) in toxicity thresholds among salinity treatments and between species was determined using the LC50 ratio test [47].
Results and discussion
Cyprinodon variegatus Aqueous Assay
Changes in salinity alone did not significantly affect C. variegatus survival (p value=0.1633) (Figure 2). Results from a two-way analysis of variance with interaction revealed that bifenthrin concentration was the only factor that significantly affected mortality (Table 1). Less than 10% mortality was observed in the 5, 10, and 20 psu control treatments. The LOEC was 0.37 μg/L bifenthrin at all salinity exposures (Table 2). The NOEC was 0.25 μg/L bifenthrin at all salinity exposures. There was 100% mortality in the highest bifenthrin treatment of 0.84 μg/L for all salinity treatments. The 96-hour LC50 values for the 20, 10, and 5 psu salinity treatments were determined to be 0.432 μg/L (95% CI=0.381-0.484); 0.415 μg/L (95% CI=0.367-0.463), and 0.377 μg/L (95% CI=0.337-0.414), respectively (Table 2). These values are in the range of a previously reported LC50 of 0.47 μg/L for larval sheepshead minnows [48]. Previously published bifenthrin LC50 values for adult sheepshead minnows include 17.8 μg/L and 19.8 μg/L [21,30,49-51]. Greater larval sensitivity is often attributed to higher surface-area-to-volume ratio, underdeveloped fat stores that could sequester lipophilic compounds, and immature immune systems and organs that are important for detoxification and elimination of toxicants [52]. The threshold of toxicity and lack of significant effect due to reduced salinity is not surprising for C. variegatus. This species has been reported as a notably hardy organism able to survive in salinities ranging from 0 to 140 psu [43,44,53].
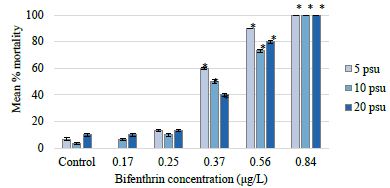
Figure 2: Cyprinodon variegatus mean percent mortality at each bifenthrin treatment and salinity exposure. For each salinity exposure, significant differences in percent mortality from the respective control (p value <0.05) are indicated with an asterisk.
Table 1: Cyprinodon variegatus two-way analysis of variance with interaction
|
Response: Mortality rate |
Sum squared |
Df |
F value |
Pr (>F) |
| Salinity |
226 |
2 |
1.906 |
0.1633 |
| Bifenthrin Dose |
77009 |
5 |
259.906 |
< 0.0001* |
| Salinity: Bifenthrin Dose |
1019 |
10 |
1.7187 |
0.1140 |
| Residuals |
2133 |
36 |
Table 2: C. variegatus median lethal concentration (LC50), 95% confidence intervals, lowest observable effects concentration (LOEC), no observable effects concentration (NOEC). There were no significant differences in LC50 values at the different test salinities (LC50 ratio test p>0.05).
|
Salinity |
LC50 (μg/L) |
95% CI (μg/L) |
LOEC (μg/L) |
NOEC (μg/L) |
|
20 psu |
0.431 |
0.381-0.484 |
0.37 |
0.27 |
|
10 psu |
0.415 |
0.367-0.463 |
0.37 |
0.27 |
|
5 psu |
0.377 |
0.337-0.414 |
0.37 |
0.27 |
Palaemon pugio Aqueous Assay
There was no mortality observed in the 5, 10, and 20 psu control treatments for the P. pugio 96-hour assay (Figure 3). However, 90% mortality was observed in the lowest bifenthrin treatment of 0.0015 μg/L in 5 psu. Results from a two-way analysis of variance with interaction revealed that salinity and bifenthrin dose both significantly affected mortality (p-values <0.0001) and there was a significant interaction between the two variables (Table 3). LOEC values were 0.0065 μg/L at 20 psu, 0.0045 μg/L at 10 psu, and 0.0015 μg/L at 5 psu. NOEC values were 0.0045 μg/L for 20 psu, 0.0025 for 10 psu, and <0.0015 at 5 psu (Table 4). There was 100% mortality in the highest bifenthrin treatment of 0.016 μg/L for all salinity treatments (Figure 3). The 96-hour LC50 values for the 20, 10, and 5 psu salinity treatments were determined to be 0.00650 μg/L (95% CI=0.00637-0.00664); 0.00646 μg/L (95% CI=0.00639-0.00652), and 0.000109 μg/L, respectively (Table 4). Due to the high mortality rates in every bifenthrin concentration tested at 5 psu, 95% confidence intervals could not be calculated. A ratio test comparing the LC50 values revealed no statistically significant difference between 20 and 10 salinity treatments (p=0.9660). Although the lack of confidence intervals in the 5 psu treatment prohibited running a ratio test, salinity clearly affected the mortality rates for larval P. pugio, with the LC50 at 20 psu calculated to be 65 times higher than at 5 psu. The 96-hour LC50 of 0.0065 μg/L in 20 psu found in this study was lower than a previously published 96-hour LC50 value of 0.013 μg/L for larval P. pugio [30] but was consistent with the LC50 of 0.0056 μg/L reported by [48]. While there was no significant difference in mortality between the 20 psu and 10 psu treatment, a marked increase in grass shrimp mortality occurred in the 5 psu treatments. In fact, there was over 90% mortality in the lowest bifenthrin concentration of 0.0015 μg/L at 5 psu compared to less than 10% mortality at the same concentration in the 20 psu salinity exposure.
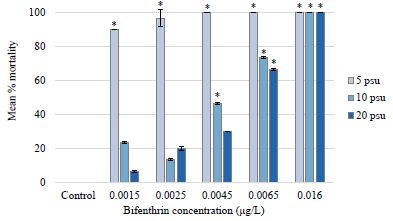
Figure 3: Palaemon pugio mean percent mortality at each bifenthrin treatment and salinity exposure. For each salinity exposure, significant differences in percent mortality from the respective control (p value <0.05) are indicated with an asterisk.
Table 3: P. pugio two-way analysis of variance with interaction
|
Response: Mortality rate |
Sum squared |
Df |
F value |
Pr (>F) |
| Salinity |
16411 |
2 |
49.233 |
<0.0001* |
| Bifenthrin Dose |
56622 |
5 |
67.947 |
<0.0001* |
| Salinity: Bifenthrin Dose |
10900 |
10 |
6.54 |
<0.0001* |
| Residuals |
6000 |
36 |
Table 4: P. pugio median lethal concentration (LC50), 95% confidence intervals, lowest observable effects concentration (LOEC), no observable effects concentration (NOEC). A ratio test comparing the LC50 values revealed no statistically significant difference between 20 and 10 salinity treatments (p=0.966). Although the lack of confidence intervals in the 5 psu treatment prohibited running a ratio test, salinity clearly affected the mortality rates for larval P. pugio, with the LC50 at 20 psu calculated to be 65 times higher than at 5 psu.
|
Salinity |
LC50 (μg/L) |
95% CI (μg/L) |
LOEC (μg/L) |
NOEC (μg/L) |
|
20 psu |
0.006500 |
0.006367-0.006635 |
0.0065 |
0.0045 |
|
10 psu |
0.006459 |
0.006395-0.006523 |
0.0045 |
0.0025 |
|
5 psu |
0.000109 |
* |
0.0015 |
<0.0015 |
*Unable to calculate confidence interval
Larval grass shrimp were two orders of magnitude more sensitive than the larval sheepshead minnows to bifenthrin, an expected result based on previously reported LC50 values for these organisms [30,48]. While salinity did not significantly affect bifenthrin toxicity in the sheepshead minnow, the grass shrimp LC50 value for bifenthrin decreased 65-fold for shrimp tested at 20 psu compared to 5 psu. These findings clearly suggest species-specific interactions, increased toxicity with reduced salinity for grass shrimp, and potential sublethal effects due to combined salinity and chemical stress.
Tidal Creek Pyrethroid Contamination
Detectable levels of bifenthrin were found only in the two watersheds; these watersheds had the highest levels of impervious cover. Dupont Wappoo Creek, within the most developed watershed sampled, returned dry mass concentrations of 16.79, 2.23, 1.39, and 2.40 ng/g bifenthrin, at collection site, 1, 2, 3, and 4, respectively, decreasing in concentration from headwater to mouth of the creek (Figure 4). These concentrations are within the range of previously measured bifenthrin concentrations from sediments sampled in an agriculturally influenced creek in California, reporting values from 1.2 ng/g to 437 ng/g [54]. Sediment from sites 3 and 4 in Toomer Creek had bifenthrin concentrations of 0.479 and 0.155 ng/g, respectively (Figure 4). No pesticides were detected in the sediment sampled from the reference site where the shrimp were collected.
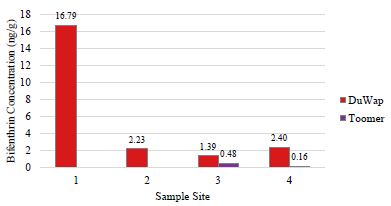
Figure 4: Bifenthrin concentrations (ng/g dry mass) measured in Charleston area tidal creek sediments. Each creek was sampled along a transect from headwater (site 1) to mouth (site 4). Bifenthrin was not detected (<MDL of 0.06-0.17 ng/g) in Guerin Creek or Seaside Creek.
Evaluation of Risk
Given the toxic effect of bifenthrin on aquatic organisms, and the lack of studies examining the interaction between salinity and bifenthrin, ecological impacts of bifenthrin have likely been underestimated. This is the first ecotoxicology study to examine salinity impacts on bifenthrin toxicity in larvae of estuarine ecosystems. This study, among others, demonstrated that the larval stages of two important estuarine species, C. variegatus and P. pugio, are sensitive to acute bifenthrin exposure. Field measurements of aquatic bifenthrin concentrations reported in California exceed previously and currently reported LC50 values [10,15,55]. Current estimated environmental concentrations for bifenthrin range from 0.005 μg/L to 19.5 μg/L in water samples and 0.155 ng/g to 437 ng/g in sediment samples, and concentration may fluctuate based on localized application patterns and impervious ground cover [55,56]. The LC50 values determined for larval C. variegatus and P. pugio at the standard test salinity of 20 psu were 40x and 2500x lower, respectively, than the maximum sediment concentration measured in this study. Compared to the reported surface water concentrations that range from 0.01 to 3.79 μg/L for bifenthrin, the LC50 value determined for larval P. pugio at the standard test salinity of 20 psu was below published concentrations, and the LC50 value determined for larval C. variegatus at the standard test salinity of 20 psu was below three published values [21,57-60]. This indicates significant risk to larval fish and shrimp from bifenthrin at environmentally relevant concentrations. The additional decrease in toxicity thresholds established for grass shrimp at lower salinities further increases their risk for bifenthrin-related mortality during storm water runoff events.
Conclusion
The present study found that bifenthrin was toxic to larval C. variegatus, and larval P. pugio with laboratory 96-hour aqueous LC50 values, in the standard testing salinity of 20 psu, of 0.431 μg/L and 0.0065 μg/L, respectively. Also noting a statically significant increase in mortality was observed in all bifenthrin concentrations in 5 psu for larval P. pugio. However, salinity did not significantly affect toxicity of bifenthrin to C. variegatus. These findings suggest that the toxicity of bifenthrin and the influence of combined salinity stress may vary significantly by species and life stage.
Additionally, bifenthrin concentrations ranging from 0.155 to 0.479 ng/g (dry wt.) were measured in South Carolina tidal creek sediments. Bifenthrin concentrations were highest in sediments with the highest level of anthropogenic development near the creek.
Further it must be considered that salinity drops greater than 26 psu in 24 hours have been recorded in South Carolina tidal creeks after rain events. This freshwater inundation induces salinity stress on the organisms in the receiving waters and reduces salinity to potentially lethal ranges for larval P. pugio. Simultaneously, the salinization of fresh inland waters, as a result of anthropogenic pressures, has been increasingly reported [61-64]. The present study substantiates the need to take salinity into account when performing toxicity assays and conducting pesticide risk assessments.
Acknowledgements
The authors wish to thank South Carolina Sea Grant and SC Department of Natural Resources for graduate research funding and providing tidal creek project data through SC DNR Project #R/CG-4. They also wish to thank Craig Plante and William Roumillat for experimental advice; Pete Key and Cameron Collins for lab assistance; Blaine West for animal collection; Ed Wirth and Brian Shaddrix for assistance with chemical analysis. Fish bioassay protocols were approved by the College of Charleston Institutional Animal Care and Use Committee (IACUC-2019–014). The NOAA, National Ocean Service (NOS) does not approve, recommend, or endorse any proprietary product or material mentioned in this publication.
Statements and Declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing Interests
The authors report there are no competing interests to declare.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Breanne Y. Hanson, Katy W. Chung and Emily C. Pisarski. The first draft of the manuscript was written by Breanne Y. Hanson and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Katy W. Chung) upon reasonable request.
References
- Alderdice DF, Velsen FPJ (1971) Some Effects of Salinity and Temperautre on Early Development of Pacific Herring (Clupea pallasi). J Fish Res Board Canada 28: 1545-1562.
- Anderson G (1985) Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (Gulf of Mexico)–grass shrimp.
- Amweg EL, Weston DP, Ureda NM (2005) Use and Toxicity of Pyrethroid Pesticides in the Central Valley, California, USA. Environ Toxicol Chem 24: 966-972. [crossref]
- Arnold CL, Gibbons CL (1996) Impervious Surface Coverage: The Emergence of a Key Environmental Indicator. J Am Plan Assoc 62: 243-258.
- Bacey J, Spurlock F, Starner K, Feng H, Hsu J, et al. (2005) Residues and Toxicity of Esfenvalerate and Permethrin in Water and Sediment, in Tributaries of the Sacramento and San Joaquin Rivers, California, USA. Bull Environ Contam Toxicol 74: 864-871. [crossref]
- Beggel S, Werner I, Connon RE, Geist JP (2010) Sublethal Toxicity of Commercial Insecticide Formulations and Their Active Ingredients to Larval Fathead Minnow (Pimephales promelas). Sci Total Environ 408: 3169-3175. [crossref]
- Bilkovic D, Roggero M (2008) Effects of Coastal Development On Nearshore Estuarine Nekton Communities. Mar Ecol Prog Ser 358: 27-39.
- Blood ER, Vernberg FJ (1992) Characterization of the Physical, Chemical and Biological Conditions and Trends in Winyah Bay and North Inlet Estuaries: 1970-1985. South Carolina Sea Grant Consort.
- Budd R, Bondarenko S, Haver D, Kabashima J, Gan J (2007) Occurrence and Bioavailability of Pyrethroids in a Mixed Land Use Watershed. J Environ Qual 36: 1006-1012. [crossref]
- Clark JM, Matsumura F (1982) Two Different Types of Inhibitory Effects of Pyrethroids on Nerve Ca– and Ca + Mg-Atpase Activity in the Squid, Loligo pealei. Pestic Biochem Physiol 18: 180-190.
- Cuomo A (1991) Toxic Fairways: Risking Groundwater Contamination From Pesticides On Long Island Golf Courses, Office of the Attorney General, Environmental Protection Bureau: New York, NY.
- DeLorenzo ME, Eckmann CA, Chung KW, Key PB, Fulton MH (2016) Effects of Salinity on Oil Dispersant Toxicity in the Grass Shrimp, Palaemonetes pugio. Ecotoxicol Environ Saf 134: 256-263. [crossref]
- DeLorenzo ME, Serrano L, Chung KW, Hoguet J, Key PB (2006) Effects of the Insecticide Permethrin on Three Life Stages of the Grass Shrimp, Palaemonetes pugio. Ecotoxicol Environ Saf 64: 122-127.
- Dunson WA, Paradise CJ, Dunson DB (1998) Inhibitory Effect of Low Salinity on Growth and Reproduction of the Estuarine Sheepshead Minnow, Cyprinodon variegatus. Copeia 1998:235.
- Fecko A (1999) Environmental Fate of Bifenthrin. Environmental Monitoring and Pest Management Branch, Department of Pesticide Regulation: Sacramento, CA.
- Floyd W (1977) The Effects of Temperature and Salinity on the Larval Development of the Grass Shrimp Palaemonetes pugio Reared in the Laboratory. Virgina J Sci 28.
- Gan J (2008) Synthetic pyrethroids : occurrence and behavior in aquatic environments. American Chemical Society: Washington, DC.
- Gilliom RJ (2001) Pesticides in the Hydrologic System – What Do We Know and What’s Next? Hydrol Process 15: 3197-3201.
- Haney DC (1999) Osmoregulation in the Sheepshead Minnow, Cyprinodon variegatus: Influence of a Fluctuating Salinity Regime. Estuaries 22: 1071-1077.
- Harper HE, Pennington PL, Hoguet J, Fulton MH (2008) Lethal and Sublethal Effects of the Pyrethroid, Bifenthrin, on Grass Shrimp (Palaemonetes pugio) and Sheepshead Minnow (Cyprinodon variegatus). J Environ Sci Heal – Part B 43: 476-483. [crossref]
- Herkovits J, Cardellini P, Pavanati C, Perez-Coll CS (1997) Susceptibility of Early Life Stages of Xenopus laevis to Cadmium. Environ Toxicol Chem 16: 312-316.
- Hildebrand SF (1919) Notes on the Life History of the Minnows Gambusia affinis and Cyprinodon variegatus. Bureau of Fisheries: Washington, DC.
- Hladik ML, Kuivila KM (2008) Occurrence of Pyrethroids in Bed and Suspended Sediments in California. J Am Chem Soc 46: 55-71.
- Holland AF, Sanger DM, Gawle CP, Lerberg SB, Santiago S, et al. (2004) Linkages Between Tidal Creek Ecosystems and the Landscape and Demographic Attributes of Their Watersheds. J Exp Mar Bio Ecol 298: 151-178.
- Hunt JW, Anderson BS, Phillips BM, Tjeerdema RS, Richard N, et al. (2006) Spatial Relationships Between Water Quality and Pesticide Application Rates in Agricultural Watersheds. Environ Monit Assess 121: 245-262. [crossref]
- Hutchinson TH, Solbe J, Kloepper-Sams PJ (1998) Analysis of the ECETOC Aquatic Toxicity (EAT) Database. III – Comparative Toxicity Of Chemical Substances To Different Life Stages Of Aquatic Organisms. Chemosphere 36: 129-142.
- Mohammed A (2013) Why are Early Life Stages of Aquatic Organisms more Sensitive to Toxicants than Adults? New Insights into Toxicity and Drug Testing.
- Johnson W (1974) Morphological Variation and Local Distribution of Cyprinodon variegatus in Florida. Retrosp Theses Diss 191.
- Kelley K, Starner K (2004) Preliminary Results For Study 219: Monitoring Surface Waters and Sediments of the Salinas and San Joaquin River Basins for Synthetic Pyrethroid Pesticides, Sacramento, CA, 2004.
- Key P, Chung K, Collins C, DeLorenzo M (2020) Toxicity of Common Environmental Contaminants on Two Estuarine Species Following Multi-Stressor Impacts, Gulf of Mexico Oil Spill and Ecosystem Science Conference, Tampa, FL. February 3-6, 2020.
- Kilby JD (1955) The Fishes of Two Gulf Coastal Marsh Areas of Florida. Tulane Study Zool 2: 175-247.
- Krebs JM, McIvor CC, Bell SS (2014) Nekton Community Structure Varies in Response to Coastal Urbanization Near Mangrove Tidal Tributaries. Estuaries and Coasts 37: 815-831.
- Kuivila KM, Hladik ML, Ingersoll CG, Kemble NE, Moran PW, et al. (2012) Occurrence and potential sources of pyrethroid insecticides in stream sediments from seven U.S. metropolitan areas. Environ Sci Technol 46: 4297-4303. [crossref]
- Laskowski DA (2002) Physical and Chemical Properties of Pyrethroids. Rev Environ Contam Toxicol 174:49–170. [crossref]
- LeBlanc LA, Orlando JL, Kuivila KM (2004) Pesticide Concentrations in Water and in Suspended and Bottom Sediments in the New and Alamo Rivers, Salton Sea Watershed, California, April 2003: S. Geological Survey Data Series 104.
- Lerberg SB, Holland AF, Sanger DM (2000) Responses of Tidal Creek Macrobenthic Communities to the Effects of Watershed. Estuaries 23: 838-853.
- Lund A, Narahashi T (1981) Modification of Sodium Channel Kinetics by the Insecticide Tetramethrin in Crayfish Giant Axons. Neurotoxicology 2: 213-229. [crosssref]
- Meertens P (2018) Monitoring Pyrethroid Pesticides in Sediment and Stormwater Runoff in the Gabilan Creek Watershed, Monterey Co., Calif. Central Coast Regional Water Quality Control Board: San Luis Obispo, CA.
- Mohammed A, Halfhide T, Elias-Samlalsingh N (2009) Comparative Sensitivity Of Three Life Stages Of The Tropical Mysid, Metamysidopsis Insularis To Six Toxicants. Toxicol Environ Chem 91: 1331-1337.
- Oros DR, Werner, I (2005) Pyrethroid Insecticides: An Analysis of Use Patterns, Distributions, Potential Toxicity and Fate in the Sacramento-San Joaquin Delta and Central Valley, Oakland, CA.
- Overstreet RM, Heard, RW (1982) Food Contents of Six Commercial Fishes From Mississippi Sound. Gulf Res Reports 7: 137-149.
- Pattillo ME, Czapla TE, Nelson DM, Monaco ME (1997) Distribution And Abundance Of Fishes And Invertebrates In Gulf Of Mexico Estuaries, Volume II: Species Life History Summaries. ELMR Rep. No. 11, Silver Spring, MD.
- Ragland JM, Liebert D,Wirth E (2014) Using Procedural Blanks To Generate Analyte-Specific Limits Of Detection For Persistent Organic Pollutants Based On GC-MS. Analysis Anal Chem 86: 7696-7704.
- Ramos S, Amorim E, Elliott M, Cabral H, Bordalo AA (2012) Early Life Stages of Fishes as Indicators of Estuarine Ecosystem Health. Ecol Indic 19: 172-183.
- Richmond CE, Woodin SA (1999) Effect of Salinity Reduction on Oxygen Consumption by Larval Estuarine Invertebrates. Mar Biol 134: 259-267.
- Richmond C, Woodin S (1996) Short-Term Fluctuations in Salinity: Effects on Planktonic Invertebrate Larvae. Mar Ecol Prog Ser 133: 167-177.
- Santos RVS, Ramos S, Bonecker ACT (2017) Can We Assess the Ecological Status of Estuaries Based on Larval Fish Assemblages? Mar Pollut Bull 124: 367-375.
- Saranjampour P, Vebrosky EN, Armbrust KL (2017) Salinity Impacts on Water Solubility and n-Octanol/Water Partition Coefficients of Selected Pesticides and Oil Constituents. Environ Toxicol Chem 36: 2274-2280. [crossref]
- Siepmann S, Holm S (2000) Hazard Assessment of the Synthetic Pyrethroid Insecticides Bifenthrin, Cypermethrin, Esfenvalerate, and Permethrin to Aquatic System Organisms in the Sacramento-San Joaquin River System; California Department of Fish and Game Pesticide Investigations Unit: Rancho Cordova, CA.
- Smith Jr S, Cooper CM, Lizotte RE, Shields FD (2006) Storm pesticide concentrations in Little Topashaw Creek, USA. Int J Ecol Environ Sci 32: 173-182.
- Spurlock F, Lee M (2008) Synthetic Pyrethroids. American Chemical Society: Washington, DC 991: 3-25.
- Szöcs E, Coring E, Bäthe, J, Schäfer, RB (2014) Effects of Anthropogenic Salinization on Biological Traits and Community Composition of Stream Macroinvertebrates. Sci Total Environ 468-469: 943-949.
- TDC Environmental (2007) In Pesticides in Urban Surface Water. Urban Pesticide Use Trends Annual Report 2007; San Mateo, CA.
- Timpano AJ, Zipper CE, Soucek DJ, Schoenholtz, SH (2018) Seasonal Pattern of Anthropogenic Salinization in Temperate Forested Headwater Streams. Water Res 133: 8-18.
- Tweel A, Sanger D, Blair A, Montie E, Turner A, Leffler J (2015) Collaborative Research to Prioritize and Model the Runoff Volume Sensitivities of Tidal Headwaters. National Estuarine Research Reserve System Science Collaborative: Beaufort, SC.
- United States Geological Survey (2018) In Estimated Annual Agricultural Pesticide Use, Washington, DC.
- United States Environmental Protection Agency. (1988) Bifenthrin Technical Fact Sheet; USEPA: Washington, DC.
- United States Environmental Protection Agency (2009) In Pesticides – Revised Reregistration Eligibility Decision (RED) for Permethrin. Washington, DC.
- Ware GW (1991) In Fundamentals of Pesticides: A Self Instruction Guide. Third edition. Thompson Publications: Fresno, CA.
- Washburn T, Sanger, D (2011) Land Use Effects on Macrobenthic Communities in Southeastern United States Tidal Creeks. Environ Monit Assess Sep 180: 177-188. [crossref]
- Weston DP, Zhang M, Lydy MJ (2008) Identifying the Cause and Source of Sediment Toxicity in an Agriculture‐Influenced Creek. Environ Toxicol Chem 27: [crossref]
- Weston DP, You J, Lydy MJ (2004) Distribution and Toxicity of Sediment-Associated Pesticides in Agriculture-Dominated Water Bodies of California’s Central Valley. Sci. Technol 38: 2752-2759.
- Wheeler MW, Park RM, Bailer AJ (2006) Comparing Median Lethal Concentration Values Using Confidence Interval Overlap or Ratio Tests. Environ Toxicol Chem 25: 1441-1444. [crossref]
- Williams WD (2001) In Anthropogenic Salinisation of Inland Waters, in Saline Lakes, Springer: Netherlands 329-337.