Abstract
Majority Chinese esophageal cancer patients are squamous cell carcinoma (ESCC) and with metastasis at initial diagnosis. Treatment for metastatic ESCC who failed first-line chemotherapy is an unmet medical need. Targeting human epidermal growth factor receptor 2 (HER2) and vascular endothelial growth factor receptor 2 (KDR) have been approved to be effective for esophageal adenocarcinoma (EAC). We explored the clinical relevance of these molecular signaling in ESCC cohorts and collected clinical evidence on applying apatinib, a Chinese FDA-approved KDR inhibitor for late-stage gastric carcinoma, in 26 patients with chemotherapy-refractory metastatic ESCC. The clinical response rate and disease control rate of these patients to apatinib 500mg once daily regimen was 12% and 60%, respectively. The patients’ median progression-free survival time (PFS) was 3.2 months (95% CI, 2.23-4.17 months) and overall survival time (OS) was 5.3 months (95% CI, 4.46-6.14 months). The commonest grade 3-4 treatment related adverse events included leukopenia (7.7%) and anemia (7.7%). No drug-related death occurred. In conclusion, apatinib has favorable activity and acceptable safety, and could be a new treatment option for patients with chemotherapy-refractory metastatic ESCC.
Keywords
apatinib, chemotherapy-refractory, metastatic, esophageal squamous cell carcinoma
Introduction
In China, esophageal carcinoma (EC) is the third most frequent cancer and the fourth leading cause of cancer death [1]. As it’s different from western countries where esophageal adenocarcinoma (EAC) is more common, 95% of the clinical pathological type of Chinese EC is squamous cell carcinoma (ESCC)[1]. Approximately 130,000 new cases of ESCCs are diagnosed annually in China and most of them are with metastasis at initial diagnosis [1]. Even for patients undergo surgery at an early stage, more than half of ESCCs proceed recurrence or metastases within 2-3 years [2]. Treatment of ESCC has not really changed for 30-40 years and primarily consists of chemotherapy [3]. Platinum or fluorouracil-based regimens are the first-line treatment for metastatic ESCC, with a median progression-free survival (PFS) less than 6 months [4]. No chemotherapeutic regimen has been defined for those who failed first-line chemotherapy.
Several targeted therapies have been approved for the treatment of EAC by the US Food and Drug Administration (FDA), including trastuzumab for human epidermal growth factor receptor 2 (HER2)-positive esophageal or gastric adenocarcinomas, and the anti-vascular endothelial growth factor (VEGF) receptor 2 (KDR) antibody ramucirumab either as a single agent or in combination with paclitaxel in the second-line setting. To explore the scientific rationale of these targeted strategy in ESCC, we did a comprehensive analysis of the expressions of HER2, KDR and VEGF in clinical ESCC cohorts in comparing to normal esophagus tissue. The results indicate that KDR and VEGF are consistently over-expressed in multiple ESCC cohorts, while the expression of HER2 is even downregulated in ESCC, suggesting a potential anti-KDR strategy for ESCC.
Apatinib was approved by Chinese FDA in 2014 for patients with latestage gastric carcinoma through targeting KDR. It has been reported to inhibit VEGF-mediated tumor microvascular density and tumor growth in ESCC cell lines and xenograft mice [5]. Some patients with advanced EC have tried various targeted drugs as a subsequent line treatment including apatinib [6, 7]. However, most of the studies were not stratified according to tumor histology, a systematic study focusing on the efficacy and safety of apatinib for the ESCC patient with chemotherapy-refractory metastasis is barely reported. In this regard, we conducted a retrospective study and investigated the efficacy and toxicity of apatinib in treating 26 patients with chemotherapy-refractory metastatic ESCC.
Methods
Bioinformatics data mining
GSE23400 dataset including 53 ESCC tumor samples and 53 adjacent paired normal esophagus samples [8] and GSE20347 dataset including 17 ESCC micro-dissected tumor samples and 17 matched samples from adjacent normal esophagus tissue [9] were used to analyze the mRNA expression and DNA copy number of HER2, KDR and VEGF in ESCC. GSE13898 dataset including 75 EAC samples from 64 patients and 28 paired normal esophageal samples [10] and GSE36458 dataset including 112 EAC and 45 normal tissue [11] were used to analyze HER2, KDR and VEGF expression in EAC. Analyses were performed using GEO2R with default settings.
Patients
This single-institution single-arm retrospective study was approved by the Ethics Committees of Henan Cancer Hospital and carried out in accordance with the Declaration of Helsinki. All patients provided written informed consent before participating in the study. Patients with chemotherapy-refractory metastatic ESCC received apatinib as subsequent line treatment between December 2015 and December 2016 were included. ESCC was confirmed histologically, and was surgically unresectable or recurrent. Metastasis in these patients were examined in lymph nodes, liver, lung or mediastinum. Patients had to have first-line chemotherapy failure before participating in the study. Treatment failure was defined as intolerable adverse effects or disease progression during treatment. Additional enrollment criteria were as following: at least one measurable lesion as defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; Eastern Cooperative Oncology Group (ECOG) performance status of 0-2; a life expectancy of >12 weeks; and acceptable hematologic, hepatic, and renal function. Patients with uncontrolled blood pressure with medication (140/90 mmHg), or with bleeding tendency, or receiving thrombolytics or anticoagulants were excluded.
Treatment
Patients received oral apatinib 500 mg (Hengrui Pharmaceutical Co., Ltd., Shanghai, China) in tablet form once daily. A treatment cycle was 28 days. No local radiotherapy or interventional therapy was offered during apatinib dosing. Dose reduction was allowed one time to a dose level not lower than 250mg once daily. Dose re-escalation was not permitted. Patients continued treatment until disease progression or experienced intolerable toxicity or withdrew consent from the study.
Statistical analysis
Response was assessed according to the RECIST version 1.1 as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) in patients with measurable lesions. Tumor assessments took place every 8 weeks using computed tomography. Progression free survival (PFS) was measured from the initiation of apatinib to the occurrence of progression, or death without evidence of progression. Overall survival (OS) was measured from the first day of apatinib administration to the day of death or to the final day of the follow-up period. Survival curves for OS and PFS were estimated using the Kaplan-Meier method. Toxicities were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Safety analysis set consisted of all patients who received at least one dose of apatinib and completed the required safety data collection. Primary end points were safety and PFS. The last follow-up was performed on May 1, 2017.
Results
Gene expression of HER2, KDR and VEGF in EC cohorts
There are much less clinical annotated gene profiling datasets of EC than other common cancers. We chose four most comprehensive datasets (GSE23400, GSE20347, GSE13898 and GSE36458) with relative large sample size to evaluate the gene expression of HER2, KDR and VEGF in ESCC and EAC cohorts. As shown in Figure 1, EAC cohorts have consistently increased mRNA expression and DNA copy number of HER2 (P=0.038 and 8.51E-04, respectively), while only have over-expressed KDR (P=0.007) but not VEGF (P=0.194). In contrast, ESCC cohorts have consistently over-expressed KDR and VEGF (P=0.019 and 1.44E-07, respectively) but even downregulated mRNA and DNA copy number of HER2 (Log2 fold change = -1.037, and -2.164, respectively) (Figure 2). These results not only provided scientific supports to the anti-HER2 treatment strategy for EAC, but also may indicate the potential of anti-KDR strategy for ESCC.
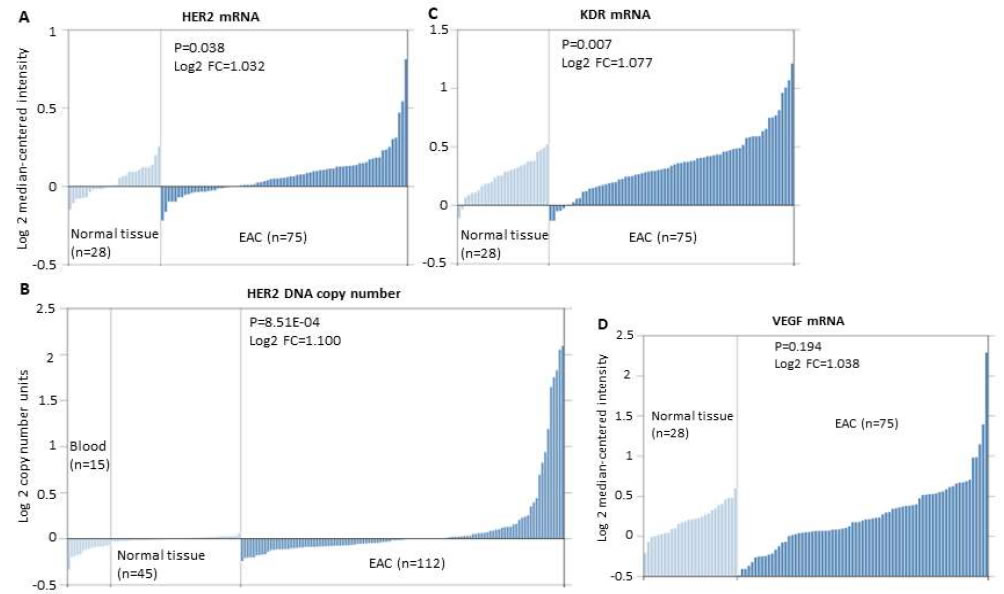
Figure 1.Expression of HER2, KDR and VEGF mRNA or DNA copy number in EAC vs. normal esophageal tissue in the GSE13898 (10) and GSE36458 (11) datasets. A. HER2 mRNA expression in EAC tissue (n=75) is significantly higher than that in the normal esophageal tissue (n=28). Log2 FC=1.032, P=0.038. B. HER2 DNA copy number in EAC tissue (n=112) is significantly higher than that in the normal esophageal tissue (n=45). Log2 FC=1.100, P=8.51E-04. C. KDR mRNA expression in EAC tissue (n=75) is significantly higher than that in the normal esophageal tissue (n=28). Log2 FC=1.077, P=0.007. D. VEGF mRNA expression in EAC tissue (n=75) and normal esophageal tissue (n=28). Log2 FC=1.038, P=0.194.
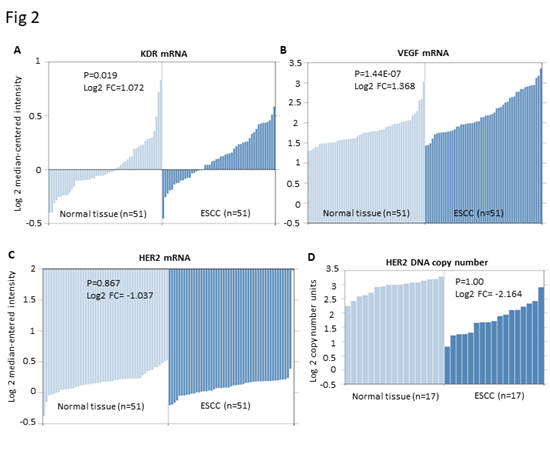
Figure 2.Expression of HER2, KDR and VEGF mRNA or DNA copy number in ESCC vs. normal esophageal tissue in the GSE23400 (8) and GSE20347 (9) datasets. A. KDR mRNA expression in ESCC tissue (n=51) is significantly higher than that in the normal esophageal tissue (n=51). Log2 FC=1.072, P=0.019. B. VEGF mRNA expression in ESCC tissue (n=51) is significantly higher than that in the normal esophageal tissue (n=51). Log2 FC=1.368, P=1.44E-07. C. HER2 mRNA expression in ESCC tissue (n=51) and normal esophageal tissue (n=51). Log2 FC= -1.037, P=0.867. D. HER2 DNA copy number in ESCC tissue (n=51) and normal esophageal tissue (n=51). Log2 FC= -2.164, P=1.00.
Patient characteristics
Between December 2015 and December 2016, 26 patients were enrolled in this study, and 5 patients were excluded because of ineligibility. The median age was 64 years (range: 48–75 years). Nineteen patients (73.1%) had good performance status, with an ECOG score of 0 or 1. Eight patients had esophagectomy and 18 patients had un-resectable primary ESCC. All patients had metastasis and the most common metastatic sites were lymph nodes (80.8%), liver (46.2%), lung (38.5%) and mediastinum (34.6%). All patients were failed to prior treatment: 17 patients received first-line chemotherapy and the other 9 patients received second-line chemotherapy. The baseline characteristics are summarized in Table 1.
Table 1.Baseline Patient Characteristics
|
N=26 |
||
|
Characteristic |
No. |
% |
|
Sex |
||
|
Male |
17 |
65.4 |
|
Female |
9 |
34.6 |
|
Age, years |
||
|
Range |
48-75 |
|
|
Median |
64 |
|
|
ECOG Performance status |
||
|
0 |
8 |
30.8 |
|
1 |
11 |
42.3 |
|
2 |
7 |
26.9 |
|
Prior chemotherapy |
||
|
First line |
17 |
65.4 |
|
Second line |
9 |
34.6 |
|
Third line |
0 |
0 |
|
Metastases |
||
|
Lymph nodes |
21 |
80.8 |
|
Mediastinum |
9 |
34.6 |
|
Lung |
10 |
38.5 |
|
Liver |
12 |
46.2 |
|
Bone |
4 |
15.4 |
|
Others |
5 |
19.2 |
|
Esophagectomy |
||
|
Yes |
8 |
30.8 |
|
NO |
18 |
69.2 |
|
Radiotherapy |
||
|
Yes |
12 |
46.2 |
|
NO |
14 |
53.8 |
|
Abbreviations: ECOG: Eastern Cooperative Oncology Group |
||
Toxicity
A total of 26 patients received at least one dose of apatinib and were included in safety analyses. All grade adverse events are listed in Table 2.
Table 2.Adverse Events graded based on CTCAE 4.0
|
Apatinib(N=26) |
||||
|
Adverse Events |
Grade 1/2, n(%) |
Grade 3, n(%) |
Grade 4, n (%) |
|
|
Hematologic |
||||
|
Leukopenia |
8(30.8) |
1(3.8) |
1(3.8) | |
|
Neutropenia |
6(23.1) |
1(3.8) |
− |
|
|
Thrombocytopenia |
4(15.4) |
− |
− |
|
|
Anemia |
10(38.5) |
2(7.7) |
− |
|
|
Non-hematologic |
||||
|
Proteinuria |
3(11.5) |
− |
− |
|
|
Hypertension |
6(23.1) |
1(3.8) |
− |
|
|
Bleeding |
2(7.7) |
− |
− |
|
|
Esophageal fistula |
− |
1(3.8) |
− |
|
|
Fatigue |
6(23.1) |
1(3.8) |
− |
|
|
Appetite loss |
9(34.6) |
− |
− |
|
|
Nausea |
7(26.9) |
− |
− |
|
|
Vomiting |
5(19.2) |
− |
− |
|
|
Abdominal pain |
4(15.4) |
− |
− |
|
|
Diarrhea |
6(23.1) |
− |
− |
|
|
Hand-foot skin reaction |
3(11.5) |
− |
− |
|
|
Hypoproteinemia |
3(11.5) |
− |
||
|
Hypocalcemia |
2(7.7) |
1(3.8) |
− |
|
|
Liver function |
||||
|
Hyperbilirubinemia |
4(15.4) |
− |
− |
|
|
Elavatedtrasaminase |
5(19.2) |
− |
− |
|
|
Renal disorder |
||||
|
Creatinine clearance decrease |
2(7.7) |
− |
− |
|
|
Hypothyroidism |
4(15.4) |
1(3.8) |
− |
|
|
Others |
||||
|
Heart failure |
− |
1(3.8) |
− |
|
|
Abbreviations: CTCAE, Cancer Institute’s Common Terminology Criteria for Adverse Events |
||||
The most common adverse events are grade 1 to 2 and manageable. For seven patients, apatinib was reduced to 250mg daily. The main reasons for dose reduction were appetite loss, fatigue, hypertension, hypothyroidism and renal function impairment. The most common grade 3 side effects included hypertension (3.8%), fatigue (3.8%), esophageal fistula (3.8%), hypocalcaemia (3.8%), hypothyroidism (3.8%) and heart failure (3.8%). Two patients withdrew the study because of grade 3 esophageal fistula and acute heart failure. Except for 1 patient who had grade 4 leukopenia, no other grade 4 toxicities were observed. One patient died of injuries after receiving 24 days of apatinib treatment. No drug-related death was observed.
Treatment efficacy
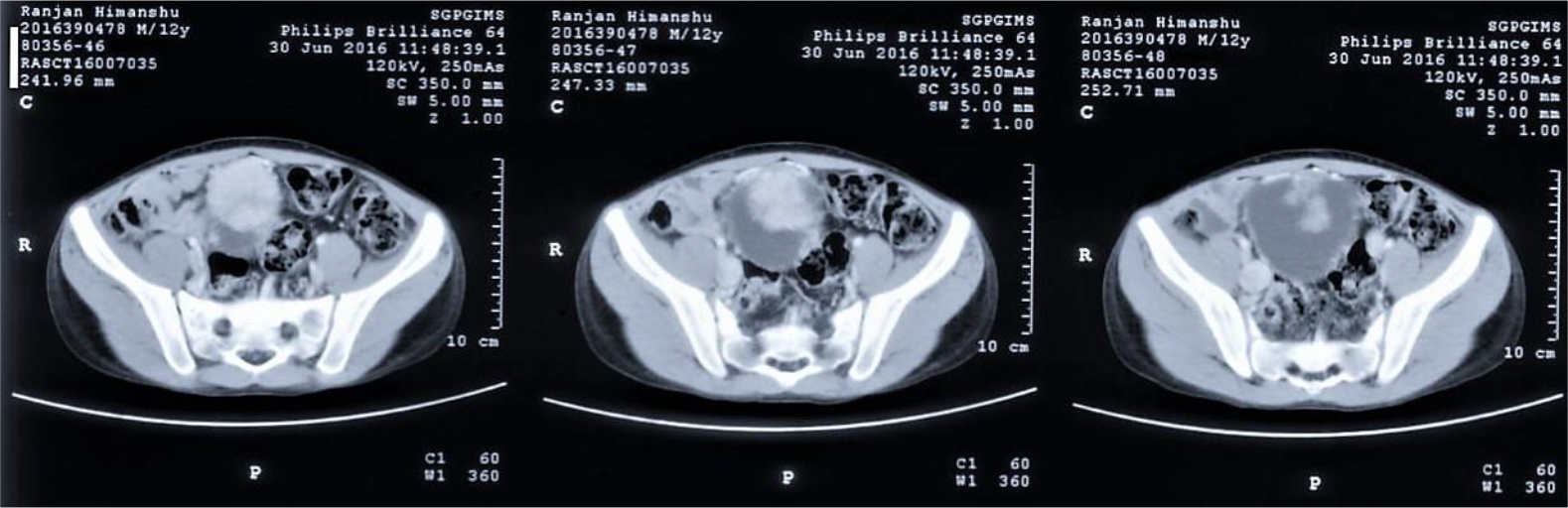
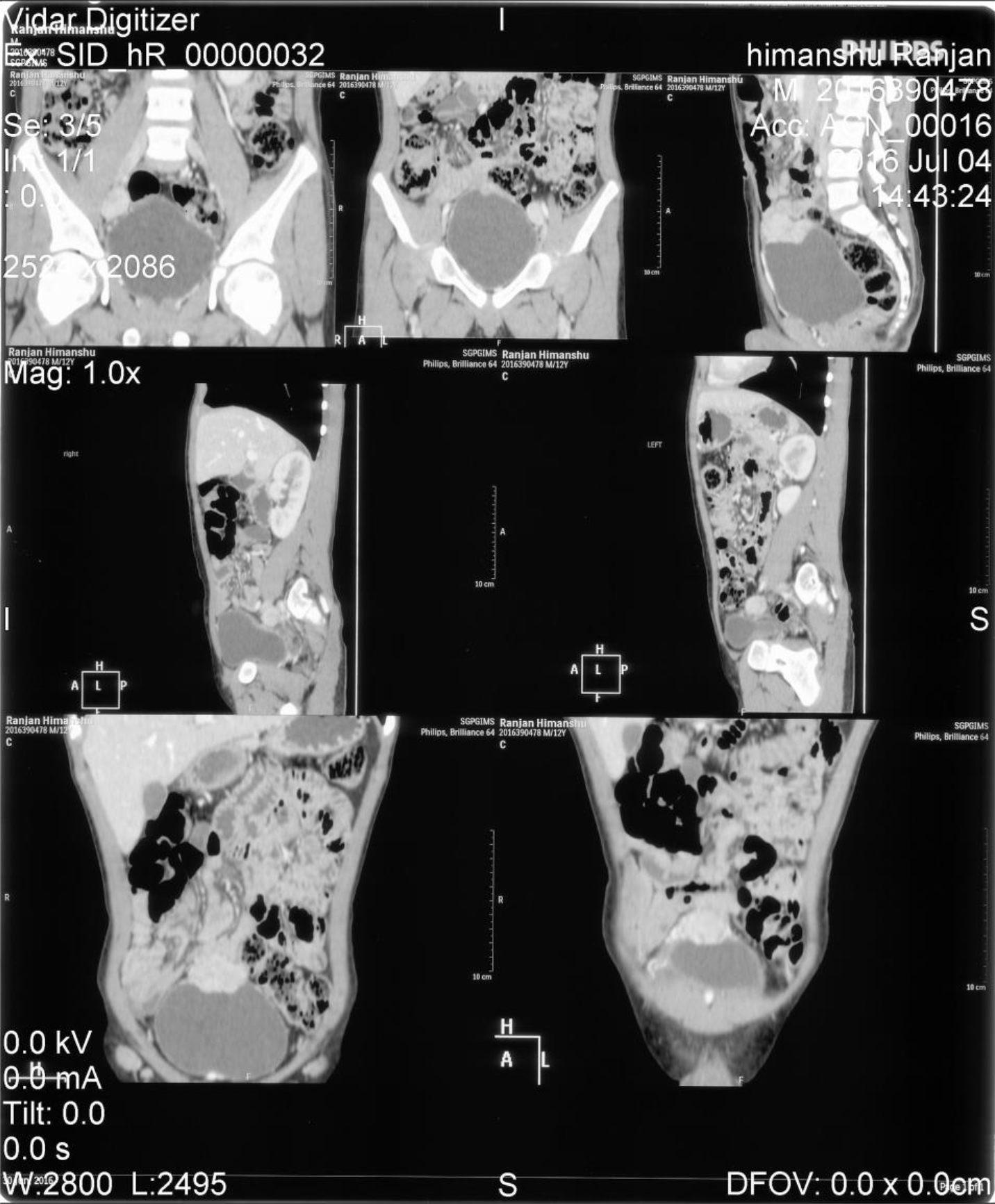
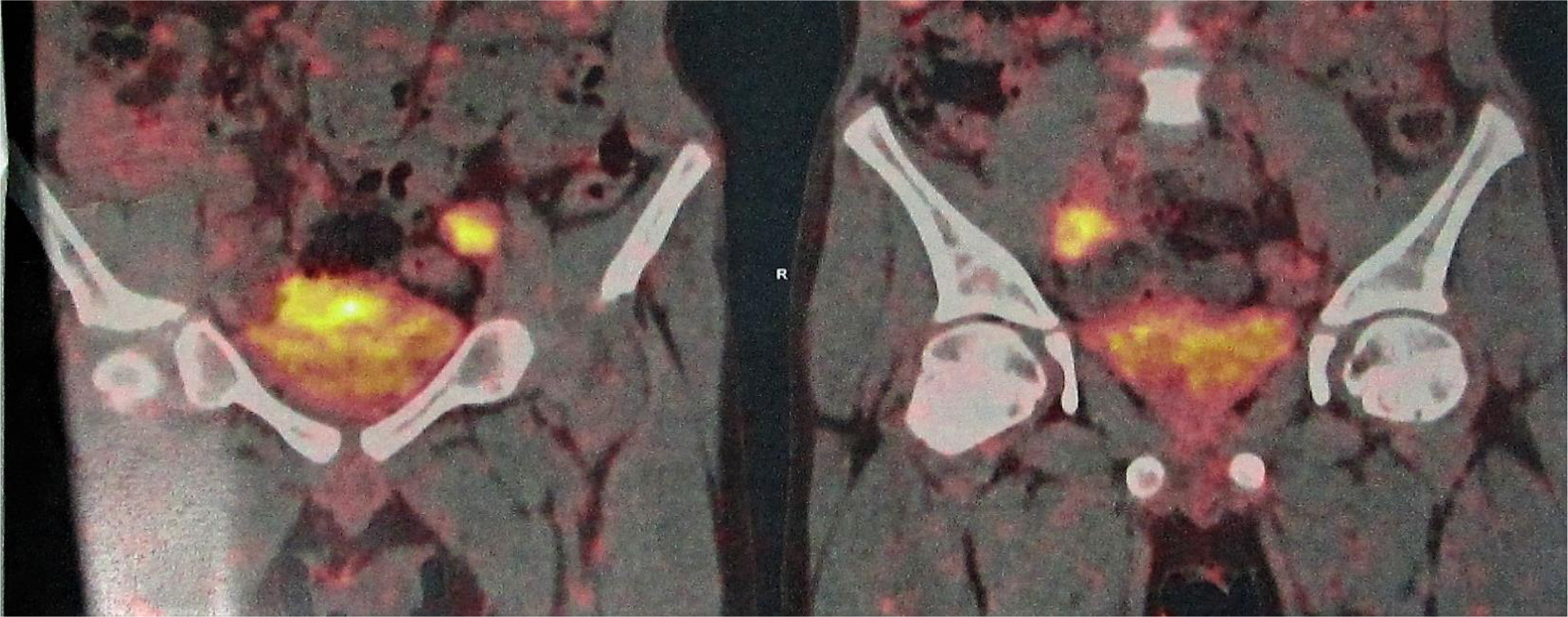
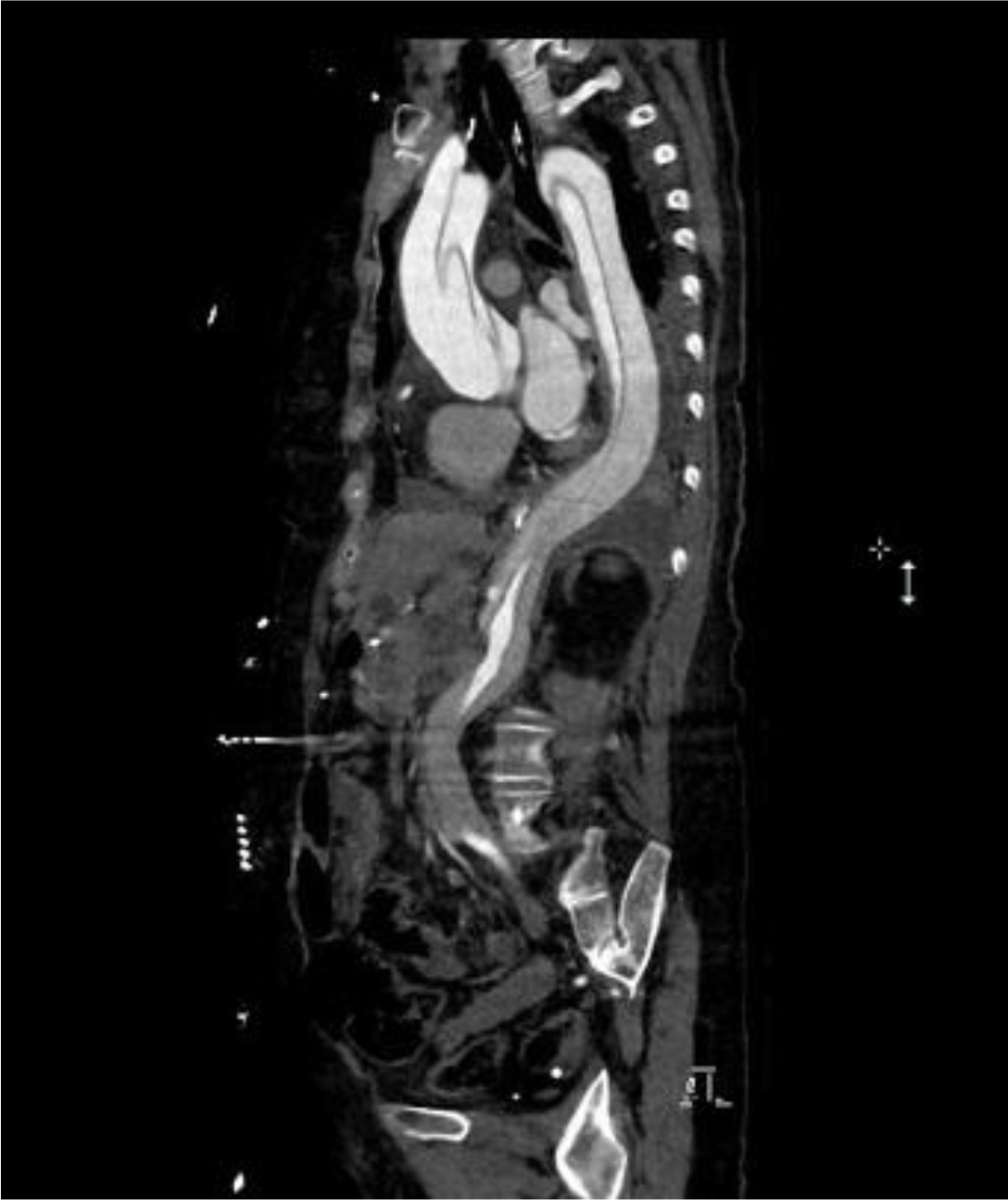
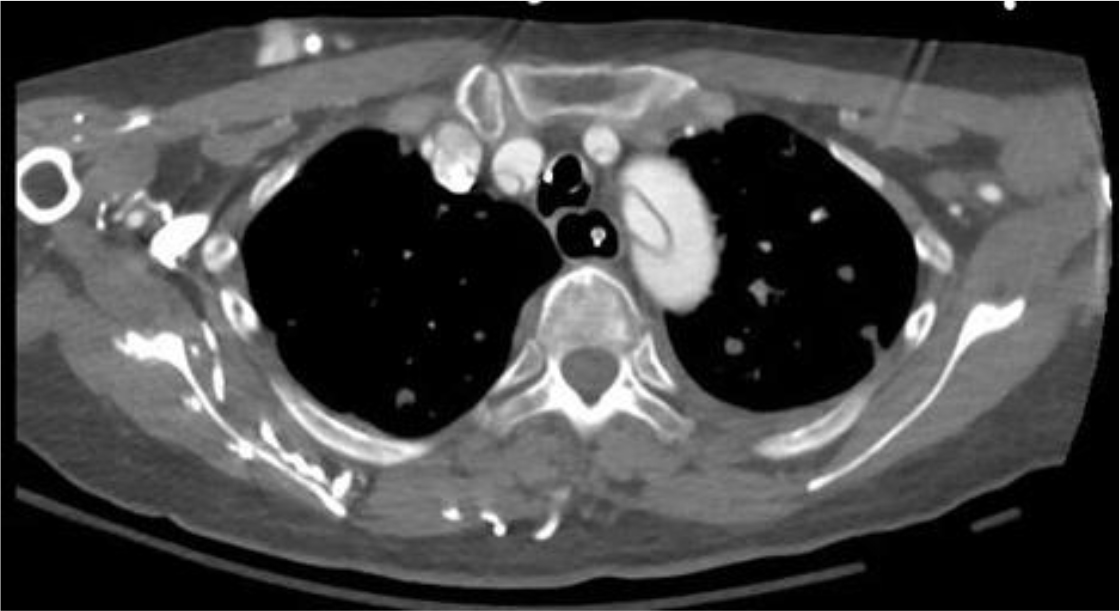
Of the 25 evaluable patients, PR was observed in 3 patients (12%) and SD was observed in 12 patients (48%). No patients met CR. Ten patients (40%) experienced disease progression. The overall response rate (ORR) and disease control rate (DCR) was 12% and 60%, respectively (Table 3). The median PFS of all 26 patients was 3.2 months (95% CI, 2.23-4.17 months) (Figure 3) and OS was 5.3 months (95% CI, 4.46-6.14 months) (Figure 4). We noted that the metastatic tumors were well responsive to the apatinib treatment in the 15 patients with PR or SD. As shown in Figure 5, metastatic tumors in lung and lymph nodes of one patient were ~50% reduced after one cycle apatinib treatment; in another patient apatinib killed ~80% liver metastatic tumors. Even in the patients experienced tumor progression for their primary ESCCs, 8 of the 10 patients showed mild shrinkage or no-change for the metastatic tumors after the apatinib treatment.
Table 3. Analysis of clinical efficacy
|
Apatinib |
||
|
Efficacy |
Number of patients (N=25) |
% |
|
Complete response |
0 |
0 |
|
Partial response |
3 |
12 |
|
Stable disease |
12 |
48 |
|
Disease progression |
10 |
40 |
|
Disease control rate (%) |
60 | |
|
Overall response rate (%) |
12 | |
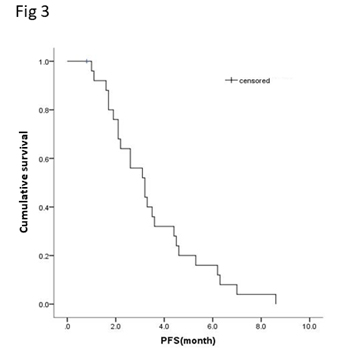
Figure 3.Kaplan-Meier curve of progression free survival (PFS) of patients with apatinib treatment.
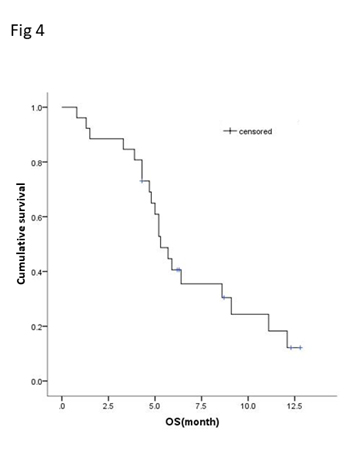
Figure 4.Kaplan-Meier curve of overall survival (OS) of patients with apatinib treatment.
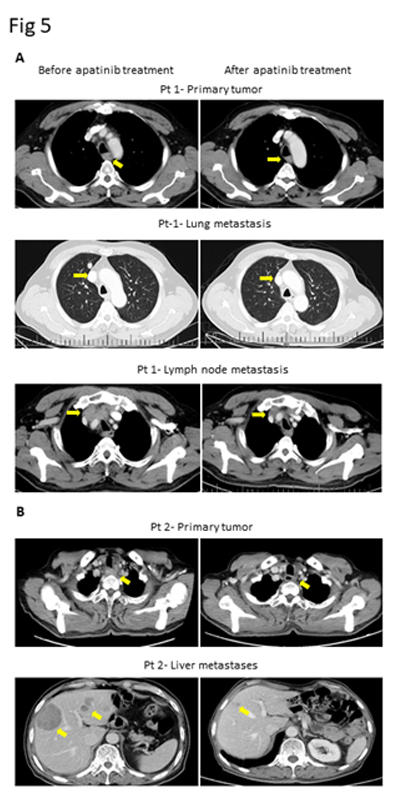
Figure 5.CT images of two ESCC patients with lung and lymph node metastasis (Pt 1, A) and liver metastasis (Pt 2, B).
Discussion
Prognosis of patients with metastatic ESCC after progression on first-line chemotherapy is poor, further cytotoxic chemotherapy provides little benefits and with significant treatment-related toxicity.
Therefore, it’s always under an urgent need to discover novel targeted therapies, which are more effective and less toxic than traditional chemotherapy options. It’s known that EAC and ESCC have completely different molecular biological characteristics. Although there have been several approved targeted therapy for EAC, whether they are beneficial to ESCC patients need exploration. In the current study, we provided both scientific and clinical evidence on apatinib through targeting the VEGF-KDR signaling in chemotherapy-refractory metastatic ESCC patients and with well-tolerated side effects.
Data from our study suggests a promising beneficial effect of apatinib in managing the metastatic ESCC patients after failed to chemotherapy. Overall, we observed a profound inhibition on metastatic tumor growth in the cohort, including tumor shrinkage at metastatic sites in all 15 patients with PR or SD, and effective controlling of tumor growth at metastatic sites in 8 of the 10 patients experienced tumor progression for their primary ESCCs. In comparing with EAC, metastatic ESCC patients have higher activations of VEGFKDR signaling in tumor tissue and blood [12], and tumor lesions at metastatic sites are more VEGF-KDR dependently angiogenic [13]. Apatinib has been shown in several tumor metastasis models in inhibiting angiogenesis and has superior anti-tumor effects [14-16]. Ideally, we would perform a tissue correlative study to compare the expression of KDR in the before- vs post-treated tumor samples. However, in these advanced and heavily-treated patients, multiple re-sampling of tumor tissue is almost impossible, and we didn’t have chance to obtain any tissue from the post-treated tumors. Anti-angiogenesis agents usually do not directly induce tumor cell cytotoxicity. Thus a prospective study in combining apatinib with switched chemotherapy is planned in our hospital.
We also observed a median PFS of 3.2 months and OS of 5.3 months in the cohort, and a 12% clinical response rate and 60% disease control rate, which is consistent with a recent study of apatinib in advanced ESCC patients [7]. Other anti-VEGFR inhibitors including sorafenib and sunitinib have been explored in advanced EC patients [17, 18], suggested their ability to stabilize chemotherapy-refractory disease. However, these studies were not exclusive to ESCC and 80% of the enrolled populations were EAC, thus the results were not stratified according to histology. In addition, we examined similar or less toxicities of apatinib than sorafenib or sunitinib in the study cohort. Grade 3/4 toxicities of single sorafenib in patients with advanced EC included a relative high percentage of rash/hand-foot reaction, even causing treatment discontinuation [17]. Combination of sunitinib with paclitaxel in advanced EC patients demonstrated more serious toxicities than single apatinib or sorafenib [18]. Grade 3/4 toxicities included high percentages of leukopenia/neutropenia (25%) and anemia (18%). Several grade 5 toxicities were even observed including upper gastrointestinal hemorrhage and esophageal fistula [18]. Our study suggests a favorable safety profile of apatinib in comparison with these anti-angiogenic agents for chemotherapy refractory metastatic ESCC patients.
We strongly agree that successes of targeted therapies depend on biomarker-driven patient selection. There has been no conclusion on biomarkers for all anti-angiogenic drugs. A biomarker study of apatinib in patients with breast cancer showed that high expression of phosphorylated KDR in tumor tissue could predict treatment efficacy [19]. For metastatic tumors, circulating VEGF might be more revealing than tissue analyses. A clinical correlative study in further investigating circulating VEGF and tissue KDR expression as potential biomarkers to predict the efficacy of apatinib in metastatic ESCC patients is undergoing in our hospital.
In conclusion, apatinib has demonstrated favorable activity and acceptable safety, and could be a new treatment option for patients with chemotherapy refractory metastatic ESCC. For further study, apatinib 500 mg once daily is the recommended dose. The prospective phase II trial including circulating biomarkers to predict treatment response is ongoing (NCT03170310).
Acknowledgement
This work was supported by National Natural Science Foundation of China (grant number 81201954).
References
- Chen W, Zheng R, Baade PD, et al. (2016) Cancer statistics in China, 2015. CA Cancer J Clin66: 115-132, [crossref]
- Su XD, Zhang DK, Zhang X, Lin P, Long H and Rong TH (2014) Prognostic factors in patients with recurrence after complete resection of esophageal squamous cell carcinoma. J Thorac Dis 6: 949-957, [crossref]
- Chen M, Shen M, Lin Y, et al.(2018) Adjuvant chemotherapy does not benefit patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Radiat Oncol 13: 150, [crossref]
- Hamamoto Y and Kitagawa Y: (2014) [Current perspective of treatment for advanced esophageal squamous cell carcinoma]. Nihon Shokakibyo Gakkai Zasshi 111: 253-259,
- Chi Y, Wang F, Meng X, Shan Z, Sun Y and Fan Q: (2019) Apatinib inhibits tumor progression and promotes antitumor efficacy of cytotoxic drugs in esophageal squamous cell carcinoma. Journal of Clinical Oncology 37: e15554-e15554,
- Li J, Jia Y, Gao Y, et al. (2019) Clinical efficacy and survival analysis of apatinib combined with docetaxel in advanced esophageal cancer. Onco Targets Ther 12: 2577-2583, [crossref]
- Li J and Wang L (2017) Efficacy and safety of apatinib treatment for advanced esophageal squamous cell carcinoma. Onco Targets Ther 10: 3965-3969, [crossref]
- Su H, Hu N, Yang HH, et al.(2011) Global gene expression profiling and validation in esophageal squamous cell carcinoma and its association with clinical phenotypes. Clin Cancer Res 17: 2955-2966, [crossref]
- Hu N, Clifford RJ, Yang HH, et al.(2010) Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genomics 11: 576, [crossref]
- Kim SM, Park YY, Park ES, et al.(2010) Prognostic biomarkers for esophageal adenocarcinoma identified by analysis of tumor transcriptome. PLoS One 5: e15074, [crossref]
- Dulak AM, Schumacher SE, van Lieshout J, et al.(2012) Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res 72: 4383-4393, [crossref]
- Dreikhausen L, Blank S, Sisic L, et al.(2015) Association of angiogenic factors with prognosis in esophageal cancer. BMC Cancer 15: 121, [crossref]
- Goel HL and Mercurio AM (2013) VEGF targets the tumour cell. Nat Rev Cancer 13: 871-882, [crossref]
- Wu S, Zhou J, Guo J, Hua Z, Li J and Wang Z (2019) Apatinib inhibits tumor growth and angiogenesis in PNET models. Endocr Connect 8: 8-19, [crossref]
- Zhang J, Liu P, Zhang Z, et al.(2019) Apatinib-loaded nanoparticles inhibit tumor growth and angiogenesis in a model of melanoma. Biochem Biophys Res Commun[crossref]
- Liang Q, Kong L, Du Y, Zhu X and Tian J (2019) Antitumorigenic and antiangiogenic efficacy of apatinib in liver cancer evaluated by multimodality molecular imaging. Exp Mol Med 51: 76, [crossref]
- Janjigian YY, Vakiani E, Ku GY, et al.(2015) Phase II Trial of Sorafenib in Patients with Chemotherapy Refractory Metastatic Esophageal and Gastroesophageal (GE) Junction Cancer. PLoS One 10: e0134731, [crossref]
- Schmitt JM, Sommers SR, Fisher W, et al.(2012) Sunitinib plus paclitaxel in patients with advanced esophageal cancer: a phase II study from the Hoosier Oncology Group. J Thorac Oncol 7: 760-763, [crossref]
- Fan M, Zhang J, Wang Z, et al.(2014) Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGF-dependency of advanced breast cancer in anti-angiogenic therapy. Breast Cancer Res Treat 143: 141-151, [c rossref]