Abstract
Biotechnology can open new possibilities to recover important nutrients from the large amount of residual raw materials that are lost during the value chain from fish in the sea until it reach consumers. These resources may make up around 60% of the round weight of fish and usually comprise viscera, head, trimmings, frames, skin, and processing water. Technologies like enzyme-assisted hydrolysis and esterification, autolysis, and fermentation are all examples of biotechnologies that can upcycle valuable biomolecules. Furthermore, these biological processes can be combined with fractionation processes to tailor-make new biomolecules with valuable functional, bioactive, sensory, and nutritional properties of these perishable bioresources.
Keywords
Biotechnology, Fish side-streams, Valorization, Enzymatic hydrolysis, Co-products, Fermentation
Introduction
The Ocean provides essential food and nutrients to human health and diets; however, the resources are not utilised to their full potential. Resources get lost along the value chain from catch to consumer or result in non-food products. Simultaneously, more nutrients are needed to feed the growing population in a world with limited natural resources. Food production contributes significantly to greenhouse gas emissions, and global estimates show that 1/3 of the food is lost or wasted [1]. Consequently, about 6 % of the world’s greenhouse gas emissions come from potential food that is never eaten [2,3]. It is a global aim to reduce fish loss and waste by 50% by the year 2030 prioritizing actions like maximizing the usage of co-products and by-catch [4]. Minimizing losses and waste takes precedence in the waste hierarchy, as emphasized by Usmani et al. [5] and Teigiserova et al. [6]. Despite the significance of prevention, it may not always be adequate for maximizing the utilization of intricate and nutrient-rich bioresources. In such cases, food processing comes into play, offering the potential to repurpose, recover, or convert lost biomolecules into valuable products, aligning with a circular economy approach, as highlighted by Usmani et al. [5] and Archad et al. [7]. Biotechnology emerges as a valuable tool in the effort to upcycle these resources, reintegrating them into food value chains for human health and nutrition or other high-value applications.
This short review highlights important residual marine bioresources and a selection of potential biotechnologies to improve their utilization.
Marine Bioresources and Their Biotechnological Potential
Nearly half of the global biological production originates from the Ocean; however, it contributes only 2% of the calorie intake and 15% of the protein intake today [8]. The Ocean is assumed to provide a larger portion of our future food supply due to e.g., high pressure on agricultural land and climatic vulnerability. The global production of fish and seafood has reached around 200 million tons annually [9]. Of the 179,6 million tons of fish globally produced in 2020, 87% were used for human consumption and 12,9% for non-food purposes [10]. Not usually included in these bioresource and food loss calculations are the side-streams that comprise bioresources that were not intended for food in the first place but may contribute significantly to food and nutrition security for a growing population.
These bioresources can also be classified as residual raw materials, co-products, plus products, surplus, leftovers, or potential resources for upgrading or upcycling. Some authors also use the term by-products or waste. Since the term by-product is referred to as resources not intended for food use in the Animal By-product Regulation (Regulation (EC) No 1069/2009 [11], we choose not to use that term in our communication. Moreover, we also avoid using the term waste since it is not associated with high-value applications such as food or feed but should be composted or destroyed. Fish side streams may contain important nutrients such as proteins and lipids and their derivatives can have potential use in food, feed, or pharmaceuticals. Defining these resources as waste or by-products will complicate further use as food ingredients or other products intended for human consumption.
Seafood represents a broad and heterogeneous group of organisms comprising phytoplankton, zooplankton, microorganisms, plants, invertebrates, fish, and mammals. The global marine database reports catch data of more than 1700 different species [12], and many of these species are not being fully exploited for their potential as dietary sources. The main fish species from global fisheries are anchovies, sardines, herring, cod, tuna, salmon, and mackerel, which account for about 30% of the global wild fish catch [13]. Besides these species, many species are still undiscovered or poorly known. The same is the case for organs from commercial fish that are not usually defined as the main food products. Fish catch and fish consumption have increased. So has the generation of residual raw materials. Since more food will be sourced from the Ocean in the future, more side streams will be generated and consequently more knowledge is needed to find high-value applications for these resources.
Marine resources comprise essential macronutrients like proteins, peptides, and lipids and minor nutrients like carbohydrates vitamins, minerals, and antioxidants. The proximate composition of fish and shellfish is primarily water, proteins, and lipids which in the fish muscle usually make up about 98% of the total mass [14]. Such proximate data for different fish species are collected in databases such as the uFiSh [15]; however, the nutritional and chemical composition of fish varies with species, seasons, geographical locations, stages of maturity, and size, and varies particularly among different organs of the fish. The degree of processing decides what residual raw materials become available. For example, when filleting whitefish or fatty fish, half of the biomass is left as residual raw materials or waste [16-18]. Gutting makes the viscera available, de-heading adds the heads to the residual raw materials and filleting also adds the cut-offs, frames, bones, and sometimes skins. These side streams are illustrated in Figure 1 and may vary due to species, seasons, age, degree of spawning etc.
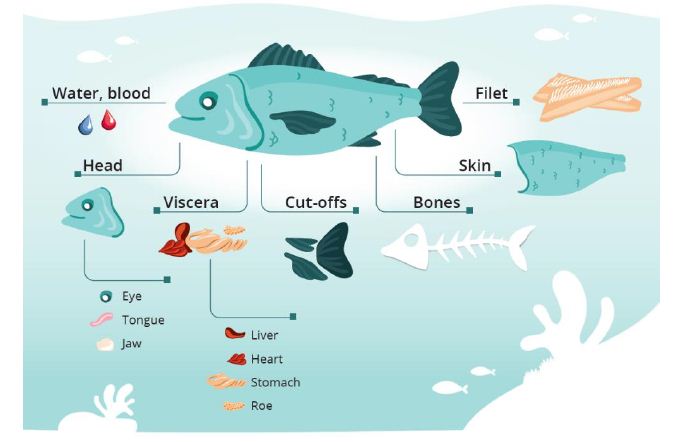
Figure 1: Illustration of residual raw materials from fish handling and processing. Copyright: E. Falch/M. Gilbu
A particularly important nutrient is the marine lipids and the long-chain omega-3 fatty acids. The lipid content and composition are reflected by the diet and are unequally distributed in the fish. For the fatty fish, these lipids are found in the muscle, cut-offs, and visceral organs, while in the lean fish species, the lipids are mainly found in the liver and visceral organs. For wild-caught fish, the gut usually demonstrates a large variation of proximate composition and lipids due to differences in feed composition and content. On the other hand, for the farmed species the composition does not vary much due to the same feed given to the fish, and usually a period with no feeding and emptying of the stomach before slaughtering. The fisheries of cod fish (Gadidae family; cod, saithe, tusk, ling, haddock) lose much biomass on the sea. We therefore calculated the potential content of long-chain n-3 fatty acids from these side-streams and found that filleting generated 2/3 as residual raw materials and a production of 10,000 kg fillet made 1000 kg lipids comprising ca 30% long-chain n-3 fatty acids [16,17]. This is a significant contribution to a healthy diet and could provide heart health to a large population since 250 mg per day of these fatty acids is the recommended level for using health claims for maintaining a healthy heart [19,20]. The lipid content in the liver varied between 45 and 60% and the lipid content in the visceral fraction varied between 2 and 9% [16,17]. So, these lost resources could contribute to health particularly these marine lipids that are part of a scarce resource from the oceans and waters. Residual raw materials from most species are generally rich in marine fatty acids, which are in high demand globally.
However, it is important to shed light on the highly perishable nature of the marine residual raw materials. It usually degrades fast due to high water content and thereby perfect conditions for microbes, autolysis by endogenous enzymes and lipid oxidation due to the high content of unsaturated lipids [21,22]. This fast biochemical degradation requires correct handling and processing [21]. Additionally, assuring consumers’ acceptance requires not only controlling and preventing unwanted reactions but also choosing the right processing to make stable and palatable products with texture and physico-chemical properties as ingredient [23]. Here, biotechnologies and fractionation can play a major role.
Biotechnology for Increased Utilization
Among the common and accepted definitions of biotechnology is “the integration of natural science and organisms, cells, parts thereof, and molecular analogues for products and services” [24]. The use of enzymes and microbes (including microalgae) in combination with fish side streams all fall within the biotechnology category and may help increase the utilization of these resources. These processes are illustrated in Figure 2. The potential uses of these selected biotechnologies are further discussed below.
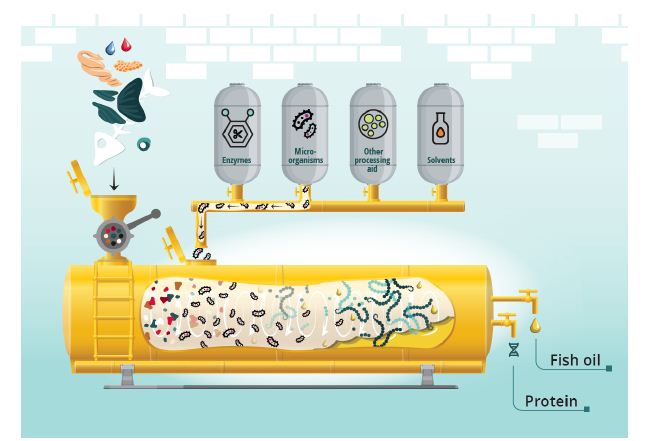
Figure 2: Illustration of biotechnological processes for utilizing residual raw materials from fish. Copyrights: E. Falch/M. Gilbu
Enzyme Assisted Bioconversion
First, enzyme-assisted bioconversion is widely used in research but not adapted to its full potential industrially. Enzymes, as natural catalysts, offer the ability to break down molecules without resorting to high temperatures or harmful chemicals. This way, they can facilitate nutrient extraction from processing side streams transforming them into diverse molecules, including peptides and lipids. A notable advantage of employing commercial enzymes as processing aids lies in their precision. Specific enzymes can selectively target specific molecules, allowing for tailored modifications based on desired properties like functionality, taste, or health effects. For instance, enzymes can hydrolyze proteins into peptides with specific characteristics or convert fatty components into specific omega-3 fatty acids. Another benefit of using hydrolytic enzymes is their role in releasing lipids from the protein-rich matrices before the centrifugal separation of lipids instead of using harsh chemical extraction or high-temperature treatment [25-27]. Given that most side-streams from fish are protein-rich, proteases can effectively hydrolyse proteins into smaller peptides, serving as a valuable pretreatment step for releasing valuable compounds. Among industrial enzymes, hydrolases, particularly proteases and lipases, are extensively employed, with proteases being among the best characterized [28]. Enzymatic hydrolysis exhibits significant potential in recovering proteins and peptides from protein-rich side streams [25,29]. The primary outcome from these processes is fish protein hydrolysates serving as both a protein source and a source of bioactive peptides. Numerous studies have highlighted the bioactive properties of fish protein hydrolysates, including antihypertensive, antimicrobial and antioxidative properties alongside preservative, functional and flavour-enhancing properties [30-32]. A well-known reported taste challenge of fish protein hydrolysates is the formation of bitter peptides [31].
While specific proteases are often required to achieve these properties, the enzymatic hydrolysis process typically benefits from complementary fractionation techniques such as membrane filtration. This combined approach holds promise for unlocking the full potential of enzyme-assisted bioconversion in industrial applications.
Enzymes can also be used to design new bioactive molecules. One example is up-concentrating marine omega-3 fatty acids (concentrates). Fish oil can be separated after thermal treatment or after enzymatic hydrolysis with proteases. With fish oil as the basis, lipases can catalyze the hydrolyzation of fatty acids or ethyl esters from the glycerol backbone, making it possible to fractionate into molecules with higher concentrations of omega-3 fatty acids. This can be conducted with either specific or non-specific lipases, meaning that the lipases can hydrolyse fatty acids randomly, on specific positions in the molecules or on specific fatty acids. After obtaining the targeted fatty acids, lipases can again be used to esterify into new acylglycerols with higher concentrations of omega-3 fatty acids [33].
Enzymatic hydrolysis may also be conducted using endogenous enzymes, which means those enzymes that are already present in the raw materials. In such autolysis, the conditions are adjusted to facilitate the activities of enzymes and prevent microbial growth [34]. These fish hydrolysates are generally a basis for feed and pet food. During autolysis, the biomass becomes liquid which makes it possible to separate proteins from lipids and further fractionation. The most common process is silage where formic acid is added to reduce the pH, but there are also examples of processes with no use of formic acid together with the endogenous enzymes. In Norway, recirculation companies such as Scanbio (www.scanbio.com) provide a logistic solution to the aquaculture industry and with several vessels, they move around to collect silage for further processing into feed or pet food.
While the use of industrial enzymes can help control the reaction and conduct planned reaction products, the reactions in autolysis are more challenging to control since there will be many different types of enzymes in play. One example of this is from the work with cod viscera where cholesterol unexpectable was reduced on behalf of the cholesteryl esters [35]. With no inactivation of endogenous enzymes, a range of different enzymes will be present to hydrolyse, esterify and degrade the different molecules. Consequently, this leads to an uncontrolled range of reactions and reaction products.
The examples above have focused on the enzymes in their pure form (industrial enzymes) or as a component in the raw material. Industrial enzymes are generally a result of precision fermentation where microbes act as enzyme producers. The large enzyme producer Novozymes, searches for enzymes with specific activities in nature for then using bacteria or fungi as enzyme factories (https: //www.novozymes.com/). Their strain database comprises > 50,000 microstrains. The leftovers (nutrients, water, and microorganisms) from Novozymes enzyme production are used as farm fertilizer.
Fermentation for Upcycling
Fermentation has become important for a wider range of applications and is expected to play an even more important role in future ingredients and products [36,37]. The technology is environmentally friendly with low use of energy [38,39] and no use of harmful chemicals or harsh temperatures. Fermentation has long traditions for use in fish preservation and to improve the sensory attributes of fish products [40]. Longer shelf life can directly prevent fish loss and waste, but fermentation can also offer possibilities to improve the utilization of the fish side streams [38-40]. Common microorganisms used in fermentation are certain bacteria strains, yeast, mould, and microalgae.
Biomass fermentation is usually run in bioreactors with microorganisms grown under specific conditions that facilitate controlled growth with a substrate. This is an efficient way to produce biomolecules with fast-growing cells with doubling times in hours compared to months or years for animal cells [36]. Examples of potential biomolecules produced during the fermentation of fish side streams are proteins, peptides, gelatine, oils, enzymes, antioxidants, nutrients, flavours, speciality minor nutrients, biofuel, and fertilizers [38-42]. There are also some good recent examples of the use of bacterial fermentation to improve the application of oil from fish silage [41] and the nutritional quality of fish meal [42] for use in food and feed ingredients.
Fish side streams are also a good substrate for the cultivation of microalgae to produce valuable nutrients [39,43] with applications in food, feed or as biofuel. Cultivation of microalgae is particularly suggested as an important future protein source. These side streams usually contain organic matter, nitrogen, phosphorus, and other nutrients that are beneficial for microalgae growth. However, depending on the nutrient requirements of the different microalgae strains it might be necessary to adjust with additional nutrients or dilute to get the right nutrient balance. Microalgae can utilize the nutrients present in fish side streams, promoting their development and offering a promising solution for both the management of waste and the production of microalgae biomass. There are several examples of cultivation of microalgae from fish-side streams. Venugopal and Sashidharan [43] discussed microalgae cultivation from fish by-catch and side streams as a future protein source, while Vidya et al. [44] combined processing side streams from dairy and fish as substrates for microalgae with the production of lipids and high-value pigments and Tropea et al. [45] combined fermented fish side streams with lemon peel for production of aquafeed. These are just a few examples and researchers claim that we only see the start of the full potential of using fermentation for new food ingredients [46].
Future Perspectives
Using these food resources more effectively is crucial for food security, environmental impact reduction, and economic benefits in food production. While preventing waste is the best option in the waste hierarchy [5,6,47], food processing and biotechnology can allow the reuse, recovery, or conversion of lost biomolecules into valuable products [5,7]. There is a wide variety of potential new applications to improve the utilization of food and fish resources and new overviews of possibilities [48-50] and some studies also explore the new ingredients used in food products such as the use of microalgae in burgers [51]. This area is expected to continue to grow so nutrients in the future are not lost but becoming a part of a circular economy system.
Acknowledgement
I acknowledge all motivated and innovative students and PhDs who contribute to the better utilization of our precious global food resources and enrich our jobs as supervisors. I also acknowledge NTNU Grafisk with Mariane Gilbu for the illustrations. Funding from Norwegian Research Council grant 294539 (SUPREME), grant 303497 (OMEGA) and JPI A Healthy Diet for a Healthy Life for the new Up4Food project helps us keep on researching for the best solutions.
References
- FAO, 2015 https://www.fao.org/3/cc0461en/online/sofia/2022/world-fisheries-aquaculture.html
- Ritchie H (2020) “Food waste is responsible for 6% of global greenhouse gas emissions” Published online at OurWorldInData.org. Retrieved from: https://ourworldindata.org/food-waste-emissions’ [Online Resource]
- Poore J, Nemecek T (2018) Reducing foods environmental impacts through producers and consumers. Science 360: 987-992. [crossref]
- 2022. Blue Transformation-Roadmap 2022-2030: A vision for FAO’s work on aquatic food systems. Rome. https://doi.org/10.4060/cc0459en
- Usmani Z, Sharma M, Awasthi AK, Sharma GD, Cysneiros D, et al. (2021) Minimizing hazardous impact of food waste in a circular economy – Advances in resource recovery through green strategies. Journal of Hazardous Materials 416: 126154-126154. [crossref]
- Hamelin L, Thomsen M (2020) Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy. The Science of the Total Environment 706: 136033-136033. [crossref]
- Arshad RN, Abdul-Malek Z, Roobab U, Ranjha MMAN, Režek Jambrak A, et al. (2022) Nonthermal food processing: A step towards a circular economy to meet the sustainable development goals. Food Chemistry 16: 100516-100516. [crossref]
- European Commission, Food from the Oceans, 2017, report, European Commission, European Union (European Union) https://research-and-innovation.ec.europa.eu/knowledge-publications-tools-and-data/publications/all-publications/food-oceans_en
- Our World in Data, 2021 https://ourworldindata.org/fish-and-overfishing#discards
- FAO 2022b. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. https://doi.org/10.4060/cc0461en
- European Parliament 2009 Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by-products Regulation) (europa.eu), COMMISSION REGULATION (EU) No 142/2011, of 25 February 2011. implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive.
- FAO (2020) The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome. https://doi.org/10.4060/ca9229en
- Ritchie H, Roser M (2021) Fish and Overfishing-How are fish stocks changing across the world? How much is overfished?” 2021, Published online at OurWorldInData.org. Retrieved from: https://ourworldindata.org/fish-and-overfishing [Online Resource]
- Kolakowska A, Sun Pan B (1990) The nutritive composition of the major groups of marine food organisms. In: Seafood: Resources, Nutritional Composition and Preservation, Ed., Sikorski, Z.E., CRC Press, 29-54. Boca Raton, FL, 1990.
- FAO/INFOODS Databases FAO/INFOODS global food composition database for fish and shellfish, version 1.0-uFiSh1.0, Food and Agriculture Organization of the United Nations Rome, 2016.
- Falch E, Rustad T, Aursand M (2006) By-products from gadiform species as raw material for production of marine lipids as ingredients in food or feed. Process Biochemistry 41: 666-674.
- Falch E, Rustad T, Jónsdóttir R, Shaw NB, Dumay J, et al. (2006) Geographical and seasonal differences in lipid composition and relative weight of by-products from gadiform species. Journal of Food Composition and Analysis 19: 727-736.
- FAO FISHERIES TECHNICAL PAPER 309, Yield and nutritional value of the commercially more important fish species, 1989. Rome. ISBN 92-5-102870-2 https://www.fao.org/3/T0219E/T0219E01.html
- Scientific Opinion on the substantiation of health claims related to EPA, DHA, DPA and maintenance of normal blood pressure (ID 502), maintenance of normal HDL‐cholesterol concentrations (ID 515), maintenance of normal (fasting) blood concentrations of triglycerides (ID 517), maintenance of normal LDL‐cholesterol concentrations (ID 528, 698) and maintenance of joints (ID 503, 505, 507, 511, 518, 524, 526, 535, 537) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1263.
- Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461.
- Falch E, Sandbakk M, Aursand M (2007) On-board handling of marine by-products to prevent microbial spoilage, enzymatic reactions and lipid oxidation, Chapter In book: Maximising the Value of Marine By-Products, Ed. F. Shahidi, Woodhead publishing, Cambridge, UK, 47-64.
- Meidell LS, Carvajal AK, Rustad T, Falch E (2023) Upgrading Marine Oils from Cod (Gadus morhua) On-Board the Deep-Sea Vessels-From Waste to Value. Foods 12: 1659. [crossref]
- Falch E (2023) Physico-Chemical Properties and Nutrition of Marine Lipids. Foods 12: 4078. [crossref]
- Compendium of Chemical Terminology. Oxford, UK: Blackwell Scientific Publications; 2014. DOI: 10.1351/goldbook.B00666. Wikipedia refers to the use of this definition by the Europe Federation of biotechnology: https://en.wikipedia.org/wiki/Biotechnology downloaded 13. December 2023.
- Araujo Sica P, Costa C, Márquez MC (2021) Enzymatic Hydrolysis of Fish Waste as an Alternative to Produce High Value-Added Products. Waste and Biomass Valorization 12: 847-855.
- Slizyte R, Daukšas E, Falch E, Storrø I, Rustad T (2005) Characteristics of protein fractions generated from hydrolysed cod (Gadus morhua) by-products. Process Biochemistry 40: 2021-2033.
- Liu Y, Ramakrishnan VV, Dave D (2020) Lipid class and fatty acid composition of oil extracted from Atlantic salmon by-products under different optimization parameters of enzymatic hydrolysis. Biocatalysis and Agricultural Biotechnology 30: 101866.
- Guerald F (2016) Enzymatic methods for marine by-products recovery in Book: Enzymes in food and beverage processing (Chapter 6) 2016: 107-143.
- Mora L, Toldrá F (2023) Advanced enzymatic hydrolysis of food proteins for the production of bioactive peptides. Current Opinion in Food Science 49.
- Ucak I, Afreen M, Montesano D, Carrillo C, Tomasevic I, Simal-Gandara J, et al. (2021) Functional and Bioactive Properties of Peptides Derived from Marine Side Streams. Drugs 19: 71. [crossref]
- Välimaa AL, Mäkinen S, Mattila P, Marnila P, Pihlanto A, et al. (2019) Fish and fish side streams are valuable sources of high-value components. Food Quality and Safety 3: 209-226.
- Aspevik T, Samuelsen TA, Gaarder MØ, Oterhals Å (2023) Sensory Properties and Chemical Composition of Fish Solubles Obtained from Upcycling of Fish Filleting Side Streams. Journal of Aquatic Food Product Technology 32.
- Ciriminnaa R, Meneguzzob F, Delisia R, Pagliaroa M (2017) Enhancing and improving the extraction of omega-3 from fish oil. Sustainable Chemistry and Pharmacy 5: 54-59.
- Meidell LS, Slizyte R, Mozuraityte R, Carvajal AK, Rustad T, Falch E, et al. (2023) Silage for upcycling oil from saithe (Pollachius virens) viscera – Effect of raw material freshness on the oil quality. Heliyon 6. [crossref]
- Falch E, Størseth TR, Aursand M (2006) Multi-component analysis of marine lipids in fish gonads with emphasis on phospholipids using high resolution NMR spectroscopy. Phys. Lipids 144. [crossref]
- The Good Food Institute, State of the Industry report. Fermentation: An Introduction to a Pillar of the Alternative Protein Industry, 67 pp. 2020. Downloaded from web 13. December 2023.
- Teng TS, Chin YI, Chai KF, Chen WN (2021) Fermentation for future food systems: Precision fermentation can complement the scope and applications of traditional fermentation. EMBO Rep 22. [crossref]
- Marti-Quijal FJ, Remize F, Meca G, Ferrer E, Ruiz M-J, et al. (2020) Fermentation in fish and by-products processing: an overview of current research and future prospects. Current Opinion in Food Science 31: 9-16.
- Zhang J, Akyol C, Meers E (2023) Nutrient recovery and recycling from fishery waste and by-products. Journal of Environmental Management 348: 119266.
- Chan SXY, Fitri N, Mio Asni NS, Sayuti NH, Azlan UK, et al. (2023) A Comprehensive Review with Future, Insights on the Processing and Safety, of Fermented Fish and the Associated Changes. Foods 12: 558. [crossref]
- Ozyurt GB, Özkütük AS, boğa M, Durmus M, Kyley E (2016) Biotransformation of Seafood Processing Wastes Fermented with Natural Lactic Acid Bacteria; The Quality of Fermented Products and Their Use in Animal Feeding. Turkish Journal of Fisheries and Aquatic Sciences 17.
- Natalia QP, Cristina RT, Germán BE, (2022) Lipolytic Effect of Staphylococcus warneri for Obtaining High-Quality Fishmeal from Fish Waste Fermentation. Waste and Biomass Valorization 13: 2519-2530.
- Venugopal V, Sasidharan A (2022) Functional proteins through green refining of seafood side streams. Frontiers in Nutrition (Lausanne) 9: 974447-974447. [crossref]
- Vidya Nayana K, Sreelakshmi M, Keerthi KV, Mohan KS, Sudhakar MP, et al. (2023) A sustainable cultivation of microalgae using dairy and fish wastes for enhanced biomass and bio-product production. Biomass Conversion and Biorefinery 13: 6859-6873.
- Tropea A, Potortì AG, Lo Turco V, Russo E, Vadalà R, et al. (2021) Aquafeed Production from Fermented Fish Waste and Lemon Peel. Fermentation 7: 272.
- Augustin MA, Hartley CJ, Maloney G, Tyndall S (2023) Innovation in precision fermentation for food ingredients. Critical Reviews in Food Science and Nutrition. [crossref]
- Teigiserova DA, Hamelin L, Thomsen M (2020) Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy. Science of The Total Environment 706: 136033. [crossref]
- Venugopal V, Abhilash Sasidharan A, Rustad T (2023) Green Chemistry to Valorize Seafood Side Streams: An Ecofriendly Roadmap toward Sustainability. Agric. Food Chem 46: 17494-17509. [crossref]
- Siddiqui SA, Schulte H, Pleissner D, Schönfelder S, Kvangarsnes K, et al. (2023) Transformation of Seafood Side-Streams and Residuals into Valuable Products. Foods 12: 422. [crossref]
- Aspevik T, Oterhals Åge, Rønning SB, Altintzoglou T, Wubshet SG, et al. (2017) Valorization of Proteins from Co-and By-Products from the Fish and Meat Industry. Topics in Current Chemistry 375. [crossref]
- Atitallah AB, Barkallah M, Hentati F, Dammak M, Hlima HB, et al. (2019) Physicochemical, textural, antioxidant and sensory characteristics of microalgae-fortified canned fish burgers prepared from minced flesh of common barbel (Barbus barbus). Food Bioscience 30: 100417.