DOI: 10.31038/IDT.2024512
Introduction
An explicit goal of multiple sclerosis (MS) therapy is the “best possible disease control” taking into account the “best possible quality of life” of the patient, with the option of using highly effective therapeutic agents early or as early as possible in response to disease activity [1], but also a to seek proactive therapy by making use of all therapy options [2].
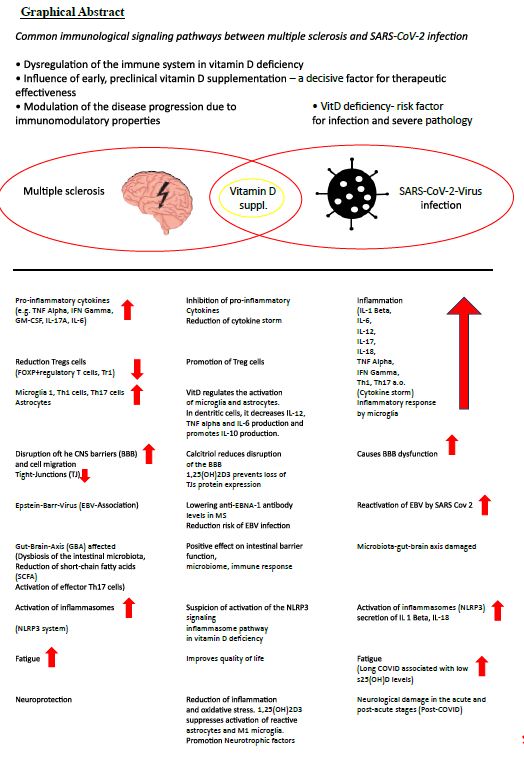
Multiple sclerosis (MS) is an inflammatory neurodegenerative disease with a suspected autoimmune origin. The disease begins earlier than current diagnostic criteria can detect. It affects the entire central nervous system and not just the white matter, as the original term-inflammatory demyelinating disorder-suggests [3]. It is characterized by a very heterogeneous course of the disease, which is represented by relapse-associated neurological deterioration, but also by an increase in disability that is independent of relapse [4]. It is generally accepted that infections in people with MS (PwMS) can have a negative impact on the course of the MS disease. This justifies that all potentially therapeutic and preventive options, especially for COVID-19, should be exploited.
MS is associated with reduced vitamin D status [5]. The molecular mechanisms in the pathogenic effect of vitamin D deficiency in MS are diverse and are orchestrated by encephalitogenic T cells with B cells, microglia, dendritic cells, interleukins (IL-1 beta, IL-6, IL-12, IL-17, TNF alpha, (tumor necrosis factor), MHCII, interferon gamma, among others) [6].
Not only is vit D deficiency associated with MS risk, but s25(OH)D levels are inversely correlated with risk of relapse, CNS lesions, and disability progression. Vitamin D suppl. reduces the number of new Gd+-enhancing or new/enlarged T2 lesions on MRI [7-10]. With MS, which is currently not curable, a high level of activity is required to prevent complications, especially infections of any kind. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an exceptionally transmissible and pathogenic coronavirus that emerged in late 2019, causing a pandemic of acute respiratory illness known as coronavirus infection 2019 (COVID-19). New omicron variants are constantly being discovered [11], for example BA.2.86 (Pirola),JN. New findings show that BA.2.86 efficiently enters lung cells and uses TMPRSS2 for entry into lung cells. The mutations S50L and K356T are for the efficient Lung cell entry of BA.2.86 is responsible. BA.2.86 has a high resistance to therapeutic antibodies and evades the antibodies induced by infection and vaccination [12,13]. COVID-19 can develop into a severe disease associated with immediate and delayed sequelae in various organs, including the central nervous system (CNS) [11].
Over the last 3 years, a complex connection between SARS-CoV-2 infection and MS has emerged [14].
Daily Vitamin D Supplementation is a Prerequisite for the Suppression of Inflammatory Processes
The risk of severe infection from COVID-19 should provide additional motivation for one daily high-dose vitamin D administration [15,16]. As part of prevention, it is worth mentioning that with circulating s25(OH)D values ≥ 55 ng/mL, the SARS-CoV-2 positivity rate was significantly lower than with values below or with deficiency [17]. Current data suggest a protective role for VitD, particularly with a lower risk of intensive care unit admission and a reduced risk of death [18,19]. In addition, the occurrence of Long-Covid is an aspect of implementing this simple, effective, safe and costeffective therapy with a broad therapeutic window for the prevention and treatment of COVID-19 disease [20-22]. Although there is still no indisputable evidence that Vit D supplementation (VitD suppl.) reduces the risk of SARSCov-2 infection in healthy individuals, there is collective evidence that it benefits vulnerable individuals [23]. PwMS with comorbidities, psychiatric illnesses, hypertension, obesity (an increased BMI may correlate with a severe course of Covid-19), age > 50 years, severe disability and methylprednisolone boost therapy as well as some DMTs (disease-modifying therapies) have a higher risk of infection and an increased risk of severe COVID-19 courses [24-26]. Infections (SARS-COV-2) can increase MS symptoms (pseudo-relapses) or cause real relapses [27]. In post-COVID syndrome (Long Covid), one in eight patients presents with symptoms such as fatigue, shortness of breath, cough, joint pain, chest pain, muscle pain, headache and paresthesia in the limbs after at least 3 months. The latter can also occur in PwMS per se [28,29]. if vitamin D administration results in a lower risk of infecton,severity of illness with admission to the intensive care unit or a reduced risk of death in people at risk,Long-Covid occurs less frequently [18,19,21,22,30-33],it is not ethically justifiable to withhold high dose vitamin-D administration from pleople at risk.
Mechanisms of Action of Vitamin D in COVID-19
Barrea et al. list in detail 14 mechanisms as described by Vit D suppl. the risk of COVID-19 infection can be reduced and sufficient Covid 19 vaccination is supported [19,29,32,34-38]. Vit D and its metabolites inactivate viruses (increase in antimicrobial peptide cathelicidin, defensins), lead to reduction of the risk of cytokine storm, reduce matrix metalloproteinase-9 concentration and thereby increase the host’s metabolic tolerance to damage, reduce the risk of pneumonia and myocarditis, lead to the reduction of the concentration of pro-inflammatory cytokines, especially interleukin 6 (IL-6), which promotes the permeability of the BBB, which leads to the potentiation of CNS damage in PwMS, is serious [29,39-42].
Vit D enables neuroprotection by reducing inflammation and oxidative stress. Low 25(OH)D levels were inversely correlated with high IL-6 levels and were independent predictors of COVID-19 severity and mortality [43]. 1,25(OH)2D3 inhibits immunoglobulin synthesis, regulates B cell activity and reduces auto-Ab production. It converts B cells into plasma cells [18]. Vit D reduces the risk of infection with EBV [29].
Current studies show evidence that chronic inflammation in Long Covid-19 Infection with reactivation of the latent Epstein-Barr virus (EBV) can lead to a worsening of the health status in PwMS [44-48]. In MS, there is a high level of molecular mimicry between the EBV transcription factor EBNA-1 and the CNS protein GlialCAM (glial cell adhesion molecule of the central nervous system [49]. Bernal et al. were able to detect EBV reactivation by detecting EBV DNA and antibodies against EBV-lytic genes [50].
In 66.7% of Long Covid patients, EBV reactivation could be demonstrated by a positive titer for EBV EA-D (early antigen-diffuse)-IgG or EBV-VCA (viral capsid-antigen)-IgM) can be provided [45]. Long COVID patients with fatigue and neurocognitive disorders were with serological evidence of recent EBV reactivation (early antigen-D [EA-D] IgG positivity) or high nuclear antigen IgG levels [51].
The triad of inflammatory markers IL-1ß, IL-6 and TNF can be found in both Long Covid and MS [52-54]. Low sun exposure acts synergistically with high EBNA-1 Ab levels and was associated with an increased risk of MS [55]. There is a connection between high EBNA-1 antibody levels and low s25(OH)D levels. On the other hand, a high dose of VitD suppl. the EBNA-1 antibody levels in PwMS [56-58]. Another parallel arises from the increase in GFAP (glial fibrillary acidic protein) as a dysfunction of the astrocytes about 4 months after the start of SARS-Cov2 infection [51]. The concentration of NfL (Neurofilament light chain), GFAP and total tau in CSF in patients with COVID-19 was often elevated with neurological symptoms [59]. Elevated sNfL has already been verified in mild to moderate COVID-19 disease [60]. On the other hand, the risk of mortality increased if sNfL and sGFAP levels were already elevated upon hospital admission [61].
Because there are no effective drugs that block EBV reactivation in Long Covid [62], there are multiple arguments for Vit D suppl. High-dose Vit D-Suppl (14,000 IU/day) for 48 weeks or 20,000 IU/week for 48 weeks selectively reduced anti-EBNA-1 antibody levels in PwMS (RRMS) [58,63].
Several mechanisms are under discussion:
- VitD could induce better clearance of EBV infected B cells,
- Vit D could directly target and impair viral replication in EBV-infected cells,
- Produce better control of inflammation in general,
- In an EBV-mediated inflammatory cascade, 1,25(OH)2D3 could suppress the activation of reactive astrocytes
- It is likely that at high s25(OH)D levels, the VitD receptor EBNA 2 (Epstein-Barr virus nuclear antigen 2) is displaced upon DNA binding [58,63-68].
Early Start of Therapy is a Crucial Factor
The early start of therapy with VitD suppl. is crucial for influencing influenza and COVID-19 infections [69]. The Corona-19 mortality risk correlates inversely with the VitD status and a mortality rate close to zero could theoretically be achieved at over 50 ng/mL s25(OH)D [70]. The importance of Vit-D metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment of infections (COVID-19) is increasingly being considered in clinical practice as part of a multitherapeutic approach [34,71,72].
Dosage Suggestions for Vitamin D Supplementation
Currently, there are no consensus guidelines suggesting an appropriate concentration of serum 25(OH)D to prevent COVID-19 or reduce its morbidity and mortality. It is becoming increasingly clear to start with a “loading dose” with high VitD doses over a few days and then continue with a “maintenance dose”, although various variants have been put up for discussion.
For example, one study used a weekly or fortnightly dose totaling 100,000-200,000 IU for 8 weeks (1800 or 3600 IU/day) [73].
To obtain 75 nmol/l s25(OH)D values, the following equation was described:
Dose (IU) = 40 x (75-serum 25(OH)D(3) [nmo/L] x body weight [73].
Over 30 ng/mL s25(OH)D values were also achieved with a single oral dose of 200,000-600,000 IU [38,74].
An s25(OH)D level of 40-60 ng/ml could be achieved by dosing up to 6,000 IU/day over several weeks [75,76]. A daily VitD intake of 10,000 IU/day for 4 weeks would lead to a faster optimal s25(OH)D level in the “status nascendi” of an infection [19].
Another dosage regimen was recommended: cholecalciferol 0.532 mg on day 1 and continued with 0.266 mg on days 3,7,14,21,28 (1 IU vitamin D3 = 0.025 µg vitamin D3 = 65.0 nmol Vitamin D3 [77]. The pharmacokinetic properties of calcifediol allow rapid absorption within hours, facilitating the immediate availability of 25(OH)D2 in target tissues.
This drastically reduced the need for intensive care unit admission and the mortality rate [77]. A key mechanism of 1,25(OH)D2 is its effect on Vit D receptors (VDR) on the adaptive immune system. The activity of TH1 and TH17 cells is reduced and the T regulator (Treg) cells are induced. This results in a reduced production of proinflammatory cytokines (IL-6, IL-8, IL-12, IL17, TNF alpha) and the cytokine storm is weakened [77,78].
The further daily VitD dose will depend on the s25(OH) values. The “maintenance dose” depends on the genetic polymorphism of the enzymes involved in VitD metabolism. Because interindividual differences in the organism’s response to Vit D, particularly in PwMS, are established, one of many explanations for the controversy surrounding the clinical results of Vit D suppl. [6,78,79].
An example of this individual reaction to a Vit D suppl. with 3,200 IU daily for 5 months showed a strong response to peripheral blood mononuclear cells in 60% of healthy individuals, while only a mild to moderate response was recorded in 40% despite reaching 25(OH)D values of 60-90 ng/ML was [80].
Up to Date 2024
Clinical Manifestation of a SARS-Cov-2 Infection in PwMS as Ulcerative Colitis – A Novum
Another challenge in the diagnostic diagnosis of gastroenterological symptoms is exclusively COVID-19-induced colitis (enteropathic infection) without pulmonary manifestation or as the first manifestation of COVID-19 disease [81-87]. In the ileum and colon, there is extensive expression of the angiotensin converting enzyme 2 (ACE2) on the enterocytes, to which the SARS-CoV-2 corona virus binds, penetrates the cells of the intestinal epithelium and causes the inflammation or aggravates existing one chronic inflammatory bowel disease (IBD) [84,87-90]. Molecular mimicry between SARS-CoV-2 and human proteins (enteric epitopes) promotes gut-associated autoimmune diseases [91].
SARS-CoV-2 as an autoimmunogenic virus is seen in association with another 10 autoimmune diseases and multidisciplinary management can be beneficial in long-COVID [92-94]. Vit D deficiency can promote autoimmune dysregulation [95].
PwMS are Predisposed to Comorbid Autoimmune Diseases
PwMS have a tendency to be polyautoimmune [96-98] and hundreds of common genetic susceptibility loci for autoimmune diseases have been identified [99,100]. Up to 18% of PwMS suffer from additional comorbid autoimmune disorders.
Inflammatory bowel diseases (IBD) [ulcerative colitis, Crohn’s disease]) are among the most common autoimmune diseases accompanying MS [101]. About beneficial and adverse effects of DMTs and comorbid autoimmune diseases details in [102]. Knowledge of the tendency towards polyautoimmunity is the key to the precise interpretation of symptoms, even in contrast to treatment-related undesirable side effects of anti-CD20 therapy.
Disease-Modifying Therapies (DMT) Can Increase the Risk of Infection
Long-term observation has shown an increased incidence of respiratory tract infections, urinary tract infections and SARS-CoV-2 during therapy with monoclonal anti-CD20 antibodies in MS (ocrelizumab, ofatumumab, ublituximab, rituximab) [103-105]. The predominant depletion of CD20+ B cells, but also CD20+ T cells and the effect on CD8 T cells by ocrelizumab as well as the additional reduction in immunoglobulins (IgG, IgA, IgM) explains the increased risk of infection [103,106].
Discussion
The connection between Vit D and COVID-19 has been critically examined in over 120 clinical studies, including 41 RCTs, and a strong connection between Vit D and clinical outcomes in Covid-19 has been proven.
Several mechanisms have been discussed:
- Affects 1,25(OH)2D3 antimicrobial peptides (cathelicidin), tigth junction proteins and adherenc junction proteins (ZO-1, occludin, claudin-10, ß-catenin, VE-cadherin) [107].
- 1,25(OH)2 D3 suppresses the activity of TH 1 and TH 17 cells and induces Treg cells. As a result, there is a reduced production of proinflammatory cytokines (IL-6, IL-8, IL-12, IL-17, TNF alpha) and a weakening of a cytokine storm [79].
- Vit D plays an important role in controlling the renin-angiotensin-aldosterone system. Details in [78].
Furthermore, genetic polymorphisms of the Vit D metabolism pathway and nongenetic reasons could explain the controversies surrounding the clinical results of Vit D supplementation [78,79,108]. If the physiological basis for the use of Vit D to improve the health of the general population has already been found with Vit D daily doses of 5000-7000 IU/day [109], it is biologically plausible to use a Vit D suppl. to be carried out preventively in the event of impaired immune homeostasis in PwMS to improve immune function. The daily dose of Vit D is crucial for the therapeutic success of broad gene expression. A daily dose of 10,000 IU leads to genomic changes that were several times higher than with 4000 IU/day [80].
Through the immunomodulatory effect of 25(OH)D and its anti-inflammatory mechanisms, immune-mediated colitis caused by anti-CD20 antibody therapy or ulcerative colitis caused by SARS-CoV-2 could be suppressed or alleviated. This form of manifestation of COVID-19 disease is particularly important in vulnerable people (PwMS) receive attention. [81-94].
Calcitriol may play a supportive role in neuroprotection particularly in PwMS by attenuating neuroinflammation and protecting the endothelial integrity of the blood-brain barrier (BBB) [110,111]. The steep learning curve in assessing clinical symptoms in LONG COVID-19 reveals new manifestations of autoimmune diseases, particularly after severe SARS-CoV-2 infections. In addition to the risk of rheumatic diseases, the occurrence of Crohn’s disease and ulcerative colitis must be taken into account in long-term care [112-116] and is a challenge in the future. Comorbidities affect PwMS more frequently than people without MS and are associated with greater physical and cognitive impairment,lower health-related quality of life,and increased mortality [117].In long-term management,one goal is to potentially avoid comorbidities.Due to the predisposition to polyautoimmunity,thyroid diseases (Hahimoto’s thyroiditis,Graves’disease) are not uncommon as comorbidities [118]. An infection of the endocrine system with SARS-CoV-2 (e.g. thyroid, adrenal gland, pituitary gland, etc.) is possible and the virus has been detected in post-mortem samples [119]. SARS -CoV-2 also mainly penetrates here the main receptor ACE2 and its co-receptor TMPRSS2 into the host cells. ACE2 protein expression was detected in about 87% of deceased COVID-19 patients. Pathological thyroid function tests correlated with the severity of the disease [119,120]. People with one already existing autoimmune disease and Covid-19 were 23% more likely to be diagnosed with another autoimmune disease [112]. In patients with comorbidities, advanced age and SARS-Cov-2, overactivation of T cells, overproduction of proinflammatory cytokines (IL-1 beta, IL-2R, IL-6, IL-8, IL-17, TNF alpha, IFN beta) and a reduction in Treg cells are confirmed [121]. Infections with SARS-CoV-2 and mRNA vaccines can trigger the clinical onset of an autoimmune disease [122]. So it must be during and after the SARS-CoV-2 infection, subacute thyroiditis, Graves disease and Hashimoto’s thyroiditis are expected [122] and the PwMS should be monitored accordingly in the event of clinical symptoms. The immunomodulatory function of vitamin D could be used as part of an early treatment strategy , as vitamin D deficiency increases the risk of autoimmune thyroid diseases [123]. There is a negative relationship between anti-thyroid antibodies (TPO-Ab, TgAb, TSHR Ab) and a sufficient serum 25(OH)D level. A Vit D suppl. led to a decrease in thyroid antibodies and in hypothyroidism, TSH levels decreased. Vit D positively influenced Hashimoto’s thyroiditis and graves disease [124-137].
A Covid-19 cohort showed a significantly higher risk of IBD and celiac disease [138]. Patients with ulcerative colitis were more likely to develop a severe form of Covid-19 than the general population [139]. Despite partly contradictory results of studies on the relationship between vitamin D, Covid-19 and IBD, it can be recognised that 25(OH)D levels above 30ng/mL can exert a protective function [140].
As Covid-19 is not a thing of the past and appears to be here to stay, an easy-to-use and inexpensive vitamin D supplement is needed and should be offered to at-risk groups. The active form of Vit D not only shows a dual effect on SARS-CoV-2 and MS, but also has a versatile spectrum of action on MS.
Summary
People with multiple sclerosis could proactively influence the course of their disease and reduce the risk of infections with possible complications through long-term prophylaxis with daily vitamin D supplementation. The immunomodulatory influence of vitamin D is undisputed and cytokine storms (COVID-19) as well as a severe course of the disease could be prevented. 25(OH)D serum values of over 50 ng/mL should be aimed for through individual daily vitamin D supplementation. The 25(OH)D serum values obtained in studies in the general population with daily doses of 5000-10,000 IU/day cannot be adequately transferred to people with multiple sclerosis and must be titrated individually. Due to the known immunopathological mechanisms of vitamin D and its benefits, it would be desirable to integrate this add-on therapy into standard clinical care.
References
- Wiendl H, Gold R, Berger T, Derfuss T, et al. (2021) ‘Multiple Sclerosis Therapy Consensus Group’ (MSTCG) Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper) Ther Adv Neurol Disord 14: 17562864211039648. [crossref]
- Giovannoni G, Popescu V, Wuerfel J, et al. (2022) Smouldering multiple sclerosis: the ‘real MS’. Ther Adv Neurol Disord 15: 17562864211066751. [crossref]
- Heming M, Wiendl H (2023) Learning multiple sclerosis immunopathogenesis from anti-CD20 therapy. Proc Natl Acad Sci 120: e2221544120. [crossref]
- Lublin FD, Häring DA, Ganjgahi H, et al. (2022) How patients with multiple sclerosis acquire disability. Brain 145: 3147-3161. [crossref]
- Rolf L, Damoiseaux J, Huitinga I, et al. (2018) Stress-Axis Regulation by Vitamin D3 in Multiple Sclerosis. Front Neurol 9: 263. [crossref]
- Anwar MJ, Alenezi SK, Alhowail AH (2023) Molecular insights into the pathogenic impact of vitamin D deficiency in neurological disorders. Biomed Pharmacother 162: 114718. [crossref]
- Miclea A, Bagnoud M, Chan A, Hoepner R (2020) A Brief Review of the Effects of Vitamin D on Multiple sclerosis. Front Immunol 11: 781. [crossref]
- Hupperts R, Smolders J, Vieth R,et al. (2019) Randomized trial of daily high dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β-1a. Neurology 93: e 1906-e1916. [crossref]
- Correale J, Ysrraelit MC, Gaitán MI (2009) Immunomodulatory effects of vitamin D in multiple sclerosis. Brain 132: 1146-1160. [crossref]
- Wang C, Zeng Z, Wang B, Guo S (2018) Lower 25-Hydroxyvitamin D Is Associated with Higher Relapse risk in patients with Relapsing-Remitting Multiple Sclerosis. J Nutr Health Aging 22: 38-43. [crossref]
- Souza PFN, Mesquita FP, Amaral JL, et al. (2021) The human pandemic coronaviruses on the show: The spike glycoprotein as the Main actor in the coronaviruses play. Int J Biol Macromol 179: 1-19. [crossref]
- Zhang L, Kempf A, Nehlmeier I, et al. (2024) SARS-CoV-2 BA.2.86 enters lung cells and evades neutralizing antibodies with high efficiency. Cell. [crossref]
- Qu P, Xu K, Faraone JN, et al. (2024) Immune evasion, infectivity, and fusogenicity of SARS-CoV-2 BA.2.86 and FLip variants. Cell. [crossref]
- MacDougall M, El-Hajj Sleiman J, et al. (2022) SARSCoV-2 and Multiple Sclerosis: Potential for Disease Exacerbation. Front Immunol 13: 871276. [crossref]
- Liao S, Huang Y, Zhang J, Xiong Q, Chi M, Yang L, et al. (2023) Vitamin D promotes epithelial tissue repair and host defense responses against influenza H1N1 virus and Staphylococcus aureus infections. Respir Res 24: 175. [crossref]
- Mansur JL, Tajer C, Mariani J, et al. (2020) Vitamin D high doses supplementation could represent a promising alternative to prevent or treat COVID-19 infection. Clin Investigate Arterioscler 32: 267-277. [crossref]
- Kaufman HW, Niles JK, et al. (2020) SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One 15: e 0239252. [crossref]
- Argano C, Mallaci Bocchio R, et al. (2023) Protective Effect of Vitamin D Supplementation on COVID-19-Related Intensive Care Hospitalization and Mortality: Definitive evidence from Meta-Analysis and Trial Sequential Analysis. Pharmaceuticals (Basel) 16: 130. [crossref]
- Grant WB, Lahore H, McDonnell SL, et al. (2020) Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 12: 988. [crossref]
- Mercola J, Grant WB, Wagner CL (2020) Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 12: 3361. [crossref]
- Gibbons JB, Norton EC, McCullough JS, et al. (2022) Association between vitamin D supplementation and COVID-19 infection and mortality. Sci Rep. 2022; 12: 19397. [crossref]
- Shah K, Varna VP, Sharma U, Mavalankar D (2022) Does vitamin D supplementation reduce COVID 19 severity?: a systematic review. QJM 115: 665-672. [crossref]
- Varikasuvu SR, Thangappazham B, et al. (2022) COVID-19 and vitamin D (Co-VIVID study): a systematic review and meta-analysis of randomized controlled trials. Expert Rev An Infect Ther 20: 907-913. [crossref]
- Longineti E, Bower H, McKay KA, et al. (2022) COVID-19 clinical outcomes and DMT of MS patients and populationbased controls . Ann Clin Transl Neurol 9: 1449-1458. [crossref]
- Subramanian S, Griffin G, Hewison M, et al. (2022) Vitamin D and COVID-19 Revisited. J Intern Med 292: 604-626. (crossref]
- Steenblock C, Toepfner N, Beuschlein F, et al. (2023) SARSCoV-2 infection and its effects on the endocrine system . Best Practice Res Clin Endocrinol Metab 37: 101761. [crossref]
- Cauchi M, Willis M, Andrews A, et al. (2022) Multiple sclerosis and the risk of infection: Association of British Neurologists consensus guidelines . Pract Neurol 003370. [crossref]
- Ballering AV, van Zon SKR (2022) Lifelines Corona Research Initiative. Persistence of somatic symptoms atier COVID-19 in the Netherlands: an observational cohort studies. Lancet 400: 452-461. [crossref]
- Barrea L, Verde L, Grant WB, et al. (2022) Vitamin D: A Role So in Long COVID-19? Nutrients 14: 1625. [crossref]
- Annweiler C, Beaudenon M, Gau er J, et al. (2022) COVIT TRIAL study group. High dose versus standard dose vitamin D supplementation in older people adults with COVID 19 (COVIT-TRIAL): A multicenter, open-label, randomized controlledsuperiority trial. PLoS Med 19: e 1003999. [crossref]
- Annweiler C, Cao Z, Sabatier JM (2020) Point of view: Should COVID-19 patients be supplemented with vitamin D? Maturitas 140: 24-26. [crossref]
- Shenoy S (2022) Gut microbiome, vitamin D, ACE2 interactions are critical factors in immune senescence and inflammatory: key for vaccine response and severity of COVID-19 infection. Inflamm Res 71: 13-26. [crossref]
- Bassatne A, Basbous M, Chakhtoura M, et al. (2021) The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metabolism 119: 154753. [crossref]
- Peng MY, Liu WC, Zheng JQ, Lu CL, Hou YC, Zheng CM, et al. (2021) Immunological aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of vitamin D. Int J Mol Sci 22: 5251. [crossref]
- Chiu SK, Tsai KW, Wu CC, et al. (2021) Putative role of Vitamin D for COVID-19 Vaccination. Int J Mol Sci 22: 8988. [crossref]
- Goncalves-Mendes N, Talvas J, et al. (2019) Impact of Vitamin D Supplementation on Influenza Vaccine Response and Immune Functions in Deficient Elderly Persons: A Randomized Placebo-Controlled Trial. Front Immunol 10: 65. [crossref]
- Chillon TS, Demircan K, Heller RA, et al. (2021) Relationship between Vitamin D Status and Antibody Response to COVID-19 mRNA Vaccination in Healthy Adults. Biomedicines 9: 1714. [crossref]
- Bae JH, Choe HJ, Holick MF, Lim S (2022) Association of vitamin D status with COVID-19 and its severity: Vitamin D and COVID-19: a narrativereview. Rev Endocrin Metab Disord 23: 579-599. [crossref]
- Gerhard A, Prüß H, Franke C (2022) [Manifestations of the central nervous system after COVID-19]. Nervenarzt 93: 769-778.[crossref]
- Orrù B, Szekeres-Bartho J, et al. [2020]. Inhibitory effects of Vitamin D on inflammation and IL-6 release. A further support for COVID-19 management?. Eur Rev Med Pharmacol Sci 24: 8187-8193. [crossref]
- Silberstein M (2020) Correlation between premorbid IL-6 levelsand COVID-19 mortality: Potential role for Vitamin D. Int Immunopharmacol 88: 106995. [crossref]
- White JH (2022) Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity. Nutrients 14: 284. [crossref]
- Campi I, Gennari L, Merlo D, et al. (2021) Vitamin D and COVID 19 severity and related mortality: a prospective studying in Italy. BMC Infect Dis 21: 566. [crossref]
- Chen T, Song J, Liu H, Zheng H, Chen C (2021) Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci Rep 11: 10902. [crossref]
- Gold JE, Okyay RA, Light WE, Hurley DJ (2021) Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 10: 763. [crossref]
- Klein J, Wood J, Jaycox J, et al. (2023) Distinguishing features of Long COVID identified through immune profiling. Nature 623;139-148 [crossref]
- Su Y, Yuan D, Chen DG, et al. (2022) Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185: 881-895. [crossref]
- Cui J, Yan W, Xu S, et al. (2018) Anti-Epstein-Barr virus at VC bodies in Beijing during 2013-2017: What we have found in the different patients. PLoS ONE 13: e0193171. [crossref]
- Lanz TV, Brewer RC, Ho PP, et al. (2022) Clonally expanded B cells in multiple sclerosis binds EBV EBNA1 and GlialCAM. Nature 603: 321-327. [crossref]
- Bernal KDE, Whitehurst CB (2023) Incident of Epstein-Barr virus reactivation is elevated in COVID 19 patients. Virus Res 334: 199157. [crossref]
- Peluso MJ, Sans HM, Forman CA, et al. (2022) Plasma Markers of Neurologic Injury and Inflammation in People With Self-Reported Neurologic Postacute Sequelae of SARSCoV-2 infection. Neurol Neuroimmunol Neuroinflammation 9: e200003. [crossref]
- Bruno A, Dolceti E, Azzolini F, et al. (2022) Interleukin 6 SNP rs1818879 Regulates Radiological and Inflammatory Activity in Multiple Sclerosis. Genes (Basel) 13: 897. [crossref]
- Musella A, Fresegna D, et al. (2020) Prototypical ‘proinflammatory cytokine (IL-1) in multiple sclerosis: role in pathogenesis and therapeutic targeting. Expert opinion Ther Targets 24: 37-46. [crossref]
- Fresegna D, Bullitia S, Musella A, et al. (2020) Re-examination the Role of TNF in MS Pathogenesis and Therapy.Cells 9: 2290. [crossref]
- Hedström AK, Huang J, Brenner N, et al. (2021) Low sun exposure acts synergistically with high Epstein-Barr nuclear antigen 1 (EBNA-1) antibody levels in multiple sclerosis etiology. Eur J Neurol 28: 4146-4152. [crossref]
- Najafipoor A, Roghanian R, et al. (2015) The beneficial effects of vitamin D3 on reducing antibody titers against Epstein-Barr virus in multiple sclerosispatients. Cell Immunol 294: 9-12. [crossref]
- Ascherio A, Munger KL, Lünemann JD (2012) The initiation and preventionof multiple. Nat Rev Neurol 8: 602-612. [crossref]
- Rolf L, Muris AH, Mathias A, et al. (2018) Exploring the effect of vitamin D3 supplementa on the anti-EBV antibody response in relapsing-remitting multiple sclerosis. Mult Scler 24: 1280-1287. [crossref]
- Virhammar J, Nääs A, Fällmar D, et al. (2021) Biomarkers for central nervous system Injury in cerebrospinal fluid are elevated in COVID-19 and associated with neurologicalsymptoms and diseases severity. Eur J Neurol 28: 3324-3331. [crossref]
- Ameres M, Brandstetier S, Toncheva AA, et al. (2020) Association of neuronal injury blood marker neurofilament light chain with mild-to-moderate COVID-19. J Neurol 267: 3476-3478.
- Aamodt AH, Høgestøl EA, et al. (2021) Blood neurofilament light concentration at admitiance: a potential prognostic markers in COVID-19. J Neurol 268: 3574-3583. [crossref]
- Hashimoto K (2023) Detrimental effects of COVID-19 in the brain and therapeutic options for long COVID: The role of Epstein-Barr virus and the gut-brain axis. Mol Psychiatry. [crossref]
- Røsjø E, Lossius A, Abdelmagid N, et al. (2017) Effect of high-dose vitamin D3 supplementation on antibody responses against Epstein-Barr virus in relapsing remitting multiple sclerosis. Mult Scler 23: 395-402. [crossref]
- Ricigliano VA, Handel AE, et al. (2015) EBNA2 binds to genomic intervals associated with multiple sclerosis and overlaps with vitamin D receptor occupancy. PLoS One 10: e0119605. [crossref]
- Brüting C, Stangl GI, Staege MS (2021) Vitamin D, Epstein-Barr virus, and endogenous retroviruses in multiple sclerosis-facts and hypotheses.J Integr Neurosci 20: 233-238. [crossref]
- Marcucci SB, Obeidat AZ (2020) EBNA1, EBNA2, and EBNA3 link Epstein-Barr virus and hypovitaminosis D in multiple sclerosis pathogenesis.J Neuroimmunol 339: 577116. [crossref]
- Schwalfenberg GK (2021) Treatment of Infectious Mononucleosis with High Dose Vitamin D3 in Three Cases. Ann Nutr Disord & Ther 8: 1068.
- Sangha A, Quon M, Pfeffer G, Orton SM (2023) The Role of Vitamin D in Neuroprotection in Multiple Sclerosis: An update. Nutrients 15: 2978. [crossref]
- Malaguarnera L (2020) Vitamin D3 as Potential Treatment Adjuncts for COVID-19. Nutrients 12: 3512.[crossref]
- Borsche L, Glauner B, von Mendel J (2021) COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 ng / mL 25(OH) D3: Results of a Systematic Review and Meta-Analysis. Nutrients 13: 3596. [crossref]
- Xu Y, Baylink DJ, Chen CS, et al. (2020) The importance of vitamin D metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID 19. J Transl Med 18: 322. [crossref]
- Fiorino S, Zippi M, Gallo C, Sifo D, Sabbatani S, Manfredi R, et al. (2021) The rationale for a multi-step therapeutic approach based on antivirals, drugs and nutrients with immunomodulatory activity in patients with coronavirus-SARS2-induced disease of different severities. Br J Nutr 125: 275-293. [crossref]
- van Groningen L, Opdenoordt S, et al. (2010) Cholecalciferol loading dose guideline for vitamin D deficient adults. Eur J Endocrinol 162: 805-811. [crossref]
- Kearns MD, Alvarez JA, Tangpricha V (2014) Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocrin Practice 20: 341-351. [crossref]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. (2011) Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7): 1911-30. [crossref]
- Fabbri A, Infante M, Ricordi C (2020) Editorial-Vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections. Rev Med Pharmacol Sci 24: 4048-4052. [crossref]
- Quesada-Gomez JM, Lopez-Miranda J, et al. (2022) Vitamin D Endocrine System and COVID-19: Treatment with Calcifediol . Nutrients 14: 2716.
- Charoenngam N, Jaroenlapnopparat A, Mettler SK, Grover A (2023) Genetic Variations of the Vitamin D Metabolic Pathway and COVID-19 Susceptibility and Severity: Current Understanding and Existing Evidence. Biomedicines. [crossref]
- Gomaa AA, Abdel-Wadood YA, Thabet RH, et al. (2023) Pharmacological evaluation of vitamin D in COVID-19 and long COVID-19: recent studies confirm clinical validation and highlight metformin to improve VDR sensitivity and efficacy. Inflammopharmacology. [crossref]
- Shirvani A, Kalajian TA, Song A, Holick MF (2019) Disassociation of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci Rep 9(1): 17685. [crossref]
- Stawinski P, Dziadkowiec K N, Marcus A (2021) COVID-19-Induced Colitis: A Novel Relationship During Troubling Times. Cureus 13(6): e15870. [crossref]
- Pan L, Mu M, Yang P, et al. (2020) Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol 115(5): 766-773. [crossref]
- Carvalho A, Alqusairi R, Adams A, et al. (2020) SARS-CoV-2 Gastrointestinal Infection Causing Hemorrhagic Colitis: Implications for Detection and Transmission of COVID-19 Disease. Am J Gastroenterol 115(6): 942-946. [crossref]
- Rutigliani M, Bozzo M, Barberis A, et al. (2022) Case Report: A Peculiar Case of Inflammatory Colitis After SARS-CoV-2 Infection. Front Immunol 13: 849140. [crossref]
- Hutchinson KA, Karatzios C, Sant’Anna A, et al. (2023) Novel association between SARS-COV-2 infection and acute haemorrhagic colitis in a paediatric patient. J Paediatr Child Health 59(3): 563-564. [crossref]
- Gupta A, Madhavan MV, Sehgal K, et al. (2020) Extrapulmonary manifestations of COVID-19. Nat Med 26(7): 1017-1032. [crossref]
- Brunet E, Casabella A, Calzado S, et al. (2021) Ileitis as the exclusive manifestation of COVID-19. The first reported case. Gastroenterol Hepatol 44(8): 561-563. [crossref]
- Lerner A (2020) “Covid-19 and the Human Gut: A New Runner on the Tract.” International Journal of Celiac Disease. 8(2): 64-67.
- Dvornikova KA, Bystrova EY, Churilov LP, et al. (2021) Pathogenesis of the inflammatory bowel disease in context of SARS-COV-2 infection. Mol Biol Rep, (7): 5745-5758. [crossref]
- Vanella G, Capurso G, Burti C, et al. (2021) Gastrointestinal mucosal damage in patients with COVID-19 undergoing endoscopy: an international multicentre study. BMJ Open Gastroenterol 8(1): e000578. [crossref]
- Lerner A, Benzvi C, Vojdani A (2023) SARS-CoV-2 Gut-Targeted Epitopes: Sequence Similarity and Cross-Reactivity Join Together for Molecular Mimicry. Biomedicines 11(7): 1937. [crossref]
- Ailioaie LM, Ailioaie C, Litscher G (2023) Gut Microbiota and Mitochondria: Health and Pathophysiological Aspects of Long COVID. Int J Mol Sci 24(24): 17198. [crossref]
- Preziosi NA, Rizvi AH, Feerick JD, Mandelia C (2022) De Novo Pediatric Ulcerative Colitis Triggered by SARS-CoV-2 Infection: a Tale of 2 Sisters. Inflamm Bowel Dis 28(10): 1623-1625. [crossref]
- Dotan A, Muller S, Kanduc D, et al. (2021) The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev 20(4): 102792. [crossref]
- Abdelhamid L, Luo XM (2022) Diet and Hygiene in Modulating Autoimmunity During the Pandemic Era. Front Immunol 12: 749774. [crossref]
- Deretzi G, Kountouras J, Polyzos SA, et al. (2015) Polyautoimmunity in a Greek cohort of multiple sclerosis. Acta Neurol Scand 131(4): 225-30. [crossref]
- Perga S, Martire S, Montarolo F, et al. (2018) The Footprints of Poly-Autoimmunity: Evidence for Common Biological Factors Involved in Multiple Sclerosis and Hashimoto’s Thyroiditis. Front Immunol 9: 311. [crossref]
- Marrie RA, Reider N, Cohen J, Stuve O, et al. (2015) A systematic review of the incidence and prevalence of autoimmune disease in multiple sclerosis. Mult Scler 21(3): 282-93. [crossref]
- Brummer T, Ruck T, Meuth SG, et al. (2021) Treatment approaches to patients with multiple sclerosis and coexisting autoimmune disorders. Ther Adv Neurol Disord 14: 17562864211035542. [crossref]
- Gutierrez-Arcelus M, Rich SS, Raychaudhuri S (2016) Autoimmune diseases-connecting risk alleles with molecular traits of the immune system. Nat Rev Genet 17(3): 160-74. [crossref]
- Wang X, Wan J, Wang M, et al. (2022) Multiple sclerosis and inflammatory bowel disease: A systematic review and meta-analysis. Ann Clin Transl Neurol 9(2): 132-140. [crossref]
- Konen FF, Möhn N, Witte T, et al. (2023) Treatment of autoimmunity: The impact of disease-modifying therapies in multiple sclerosis and comorbid autoimmune disorders. Autoimmun Rev 22(5): 103312. [crossref]
- de Sèze J, Maillart E, Gueguen A, et al. (2023) Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic. Front Immunol 14: 1004795. [crossref]
- Varley CD, Winthrop KL (2021) Long-Term Safety of Rituximab (Risks of Viral and Opportunistic Infections) Curr Rheumatol Rep 23(9): 74. [crossref]
- Smith JB, Gonzales EG, Li BH, Langer-Gould A (2022) Analysis of Rituximab Use, Time Between Rituximab and SARS-CoV-2 Vaccination, and COVID-19 Hospitalization or Death in Patients With Multiple Sclerosis. JAMA Netw Open 1;5(12): e2248664. [crossref]
- Abbadessa G, Maida E, Miele G, et al. (2022) Lymphopenia in Multiple Sclerosis patients treated with Ocrelizumab is associated with an effect on CD8 T cells. Mult Scler Relat Disord 60: 103740. [crossref]
- Chen H, Lu R, Zhang YG, Sun J (2018) Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs. Tissue Barriers 6(4): 1-13. [crossref]
- Holick MF, Mazzei L, García Menéndez S, et al. (2023) Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases. Nutrients 15(3): 767. [crossref]
- Wimalawansa SJ (2023) Physiological Basis for Using Vitamin D to Improve Health. Biomedicines 11(6): 1542. [crossref]
- Gotelli E, Soldano S, Hysa E, et al. (2023) Understanding the Immune-Endocrine Effects of Vitamin D in SARS-CoV-2 Infection: A Role in Protecting against Neurodamage. Neuroimmunomodulation 30(1): 185-195. [crossref]
- Zierfuss B, Larochelle, Prat A (2024) Blodd-brain barrier dysfunction in multiple sclerosis: causes, consequences, and potential effects of therapies. Lancet Neurol 23: 95-109. [crossref]
- Tesch F, Ehm F, Vivirito A, et al. (2023) Incident autoimmune diseases in association with SARS-CoV-2 infection: a matched cohort study. Clin Rheumatol 42 2905-2914. [crossref]
- Peng K, Li X, Yang D, Chan SCW, et al. (2023) Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study. EclinicalMedicine 63: 102154.
- Lim SH, Ju HJ, Han JH, et al. (2023) Autoimmune and Autoinflammatory Connective Tissue Disorders Following COVID-19. JAMA Netw Open 6(10): e2336120. [crossref]
- Gracia-Ramos AE, Martin-Nares E, et al. (2021) New Onset of Autoimmune Diseases Following COVID-19 Diagnosis. Cells 10(12): 3592. [crossref]
- Sharma C, Bayry J (2023) High risk of autoimmune diseases after COVID-19. Nat Rev Rheuma 19(7): 399-400. [crossref]
- Marrie RA,Fisk JD,Fitzgerald K,Kowalec K,Maxwell C,Rotstein D, et al. (2023).Etiology,effects and management of comorbidities in multiple sclerosis: recent advances.Front Immunol14: 1197195. [crossref]
- Edwards NC,Munsell M,Menzin J,Phillips AL (2018).Comorbidity in US patients with multiple sclerosis.Patient Relat Outcome Meas 9: 97-102. [crossref]
- Steenblock C, Toepfner N, Beuschlein F, Perakakis N, Mohan Anjana R, Mohan V, et al.(2023) SARS-CoV-2 infection and its effects on the endocrine system. Best Pract Res Clin Endocrinol Metab. 37(4): 101761. [crossref]
- Köhler VF, Knösel T, Hasmann SE, Scherer C, Hellmuth JC, Muenchhoff M, et al. (2023) Thyroidal Angiotensin-Converting Enzyme 2 Protein Expression and Thyroid Function Tests in Patients with COVID-19: Results from a Retrospective Case Series and a Prospective Cohort Study. Thyroid. 33(2): 177-185. [crossref]
- Mobasheri L, Nasirpour MH, Masoumi E, Azarnaminy AF, Jafari M, Esmaeili SA. (2022) SARS-CoV-2 triggering autoimmune diseases. Cytokine. ;154: 155873. [crossref]
- Staruszkiewicz M, Pituch-Noworolska A, Skoczen S. (2023) SARS-CoV-2 and thyroid diseases. J Transl Autoimmun 100214. [crossref]
- Zhao R, Zhang W, Ma C, Zhao Y, Xiong R, Wang H, et al. (2021) Immunomodulatory Function of Vitamin D and Its Role in Autoimmune Thyroid Disease. Front Immunol. 12: 574967. [crossref]
- Babić Leko M, Jureško I, Rozić I, Pleić N, Gunjača I, Zemunik T. (2023) Vitamin D and the Thyroid: A Critical Review of the Current Evidence. Int J Mol Sci. 24(4): 3586. [crossref]
- Mackawy AM, Al-Ayed BM, Al-Rashidi BM. (2013) Vitamin d deficiency and its association with thyroid disease. Int J Health Sci (Qassim) 7(3): 267-75. [crossref]
- Appunni S, Rubens M, Ramamoorthy V, et al. (2021). Association between vitamin D deficiency and hypothyroidism: results from the National Health and Nutrition Examination Survey (NHANES) 2007-2012. 21, 224. [crossref]
- Waterhouse M, Pham H, Rahman ST, Baxter C, Romero BD, Armstrong B, et al.(2023) The Effect of Vitamin D Supplementation on Hypothyroidism in the Randomized Controlled D-Health Trial. Thyroid. 33: 11, 1302-1310.[crossref]
- Galușca D, Popoviciu MS, Babeș EE, Vidican M, Zaha AA, Babeș VV, et al. (2022) Vitamin D Implications and Effect of Supplementation in Endocrine Disorders: Autoimmune Thyroid Disorders (Hashimoto’s Disease and Grave’s Disease), Diabetes Mellitus and Obesity. Medicina. 58(2): 194. [crossref]
- Villa A, Corsello A, Cintoni M, Papi G, Pontecorvi A, Corsello SM, et al. (2020) Effect of vitamin D supplementation on TSH levels in euthyroid subjects with autoimmune thyroiditis. Endocrine 70(1): 85-91. [crossref]
- Pankiv, V., Yuzvenko, T., Koval, S., Singh, K., Pankiv, I., Sehgal, T. et al. (2020) Correlation of vitamin D level with thyroid status and TSH antibody titers in patients with Graves’ disease. INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine). 16;305-309.
- Khozam SA, Sumaili AM, Alflan MA, Shawabkeh RAS. (2022) Association Between Vitamin D Deficiency and Autoimmune Thyroid Disorder: A Systematic Review. Cureus. 14(6): e25869. [crossref]
- Ma J, Wu D, Li C, et al. (2015) Lower serum 25-hydroxyvitamin D level is associated with 3 types of autoimmune thyroid diseases. Medicine (Baltimore). 94: e1639. [crossref]
- Kivity S, Agmon-Levin N, Zisappl M, et al. (2011) Vitamin D and autoimmune thyroid disease. Cell Mol Immunol 8: 243-247. [crossref]
- Tamer G, Arik S, Tamer I, Coksert D.(2011) Relative vitamin D insufficiency in Hashimotoʼs thyroiditis. Thyroid. 21: 891-896. [crossref]
- Wang X, Zynat J, Guo Y, et al.(2015) Low serum vitamin D is associated with anti-thyroid-globulin antibody in female individuals. Int J Endocrinol 2015: 1-6.
- Goswami R, Marwaha RK, Gupta N, et al. (2009) Prevalence of vitamin D deficiency and its relationships with thyroid autoimmunity in Asian Indians: a community-based survey. Br J Nutr. 102: 382-386. [crossref]
- Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. (2013) High vitamin D status in younger individuals is associated with low circulation thyrotropin. Thyroid 23: 25-30.
- Chang R, Yen-Ting Chen T, Wang SI, Hung YM, Chen HY, Wei CJ. (2023) Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. EClinicalMedicine. 56: 101783. [crossref]
- Yang, J., Ke, J., Jiang, X. et al.(2024) The association between ulcerative colitis and COVID-19 severity: a systematic review and meta-analysis systematic review. Int J Colorectal Dis. 39, 5. [crossref]
- De Nicolò A, Cusato J, Bezzio C, Saibeni S, Vernero M, Disabato M, et al. (2022) Possible Impact of Vitamin D Status and Supplementation on SARS-CoV-2 Infection Risk and COVID-19 Symptoms in a Cohort of Patients with Inflammatory Bowel Disease. Nutrients. 15(1): 169. [crossref]
Part of this publication appeared in:
Goischke H.-K. (2023) What immunopathogenic similarities exist between SARS-CoV-2 infection and multiple sclerosis?: A plea for daily vitamin D supplementation to improve quality of life! J Neurol Transl Neurosci 8(1): 1093.