DOI: 10.31038/IDT.2024511
Introduction
Febrile neutropenia (FN) frequently complicates cancer treatment, contributing to overall morbidity and the burden of hospitalization in children with cancer [1-6]. Myelosuppression is a side-effect of cytotoxic chemotherapy, resulting in recurring episodes of neutropenia; fever complicates about 27-34% of neutropenic episodes among children receiving chemotherapy or undergoing hematopoietic stem cell transplant (HSCT) [1-3]. Due to the risk of serious bacterial or fungal infections in children with FN, the difficulty of localizing infections in neutropenic children, and the mortality rate associated with inadequate treatment [4,5] the historical standard in pediatric hematology-oncology was hospitalization with empiric broad-spectrum antibiotics until the fever resolves and the neutropenia improves [6-8]. More recently, however, there is evidence of greater practice variation [9,10] based on practice guidelines emphasizing risk-stratification of children with chemotherapy-induced FN and the benefits of decreasing inpatient hospitalization [9,11-27]. Most FN episodes resolve without diagnosis of a serious infection; bacteremia, the most common infection complicating FN episodes, has a prevalence of 20%-29 [11,28-32]. Invasive fungal infection (IFI) is less common, occurring in less than 5% of FN episodes [32] and data on bacterial infection of sites other than the bloodstream are more limited. There is no single approach to risk-stratification in pediatric FN [26] which necessitates ongoing analysis of risk factors for serious infection, which facilitate risk-stratification and step-down management of children at lower risk [25,27,32,33].
Materials and Methods
Study Design
To evaluate the prevalence and potential predictors of bacterial and fungal infection among pediatric oncology patients with FN at our institution, we conducted a retrospective cohort study containing a nested case-control study. Using hospital billing codes and electronic medical records, we obtained a consecutive 3-year sample of children admitted with FN to the pediatric hematology-oncology teams at UCSF Benioff Children’s Hospital Oakland (Oakland, CA, US), with the end of the sample period preceding the Covid-19 pandemic. Children receiving treatment for cancer were included in the cohort if they had an absolutely neutrophil count (ANC) <500 x109/L or (if no ANC was reported) a total white blood cell count (WBC) <500 x109/L, as well as a single temperature >38.3°C or a sustained temperature >38°C [34]. Participants were excluded if they were receiving or had previously received allogeneic or autologous HSCT or had an underlying syndrome (such as Fanconi anemia) associated with chronic neutropenia. This study was approved by our hospital’s institutional review board and conducted in accordance with the Declaration of Helsinki.
Statistical Methods
Continuous variables, including participants’ ages and days to infection diagnosis, were not normally distributed and are described using median and interquartile range (IQR). Children with multiple episodes of FN during the sample period reentered the cohort for each episode. For the case-control analysis, we randomly sampled one episode per participant. Cases were defined by culture-proven bacteremia, urinary tract infection (UTI), meningitis, cellulitis, osteomyelitis, neutropenic colitis (typhlitis), Clostridium difficile enterocolitis, or invasive fungal infection (IFI). Clinical and radiographic findings were accepted for diagnosis of typhlitis, osteomyelitis, and IFI if cultures were not available [35]. Per institutional standards of care, any positive blood culture from a central venous catheter (CVC) was considered infectious, including coagulase-negative staphylococci. Controls were sampled at a two-to-one ratio with cases. To compare clinical and laboratory findings between the case and control groups, we used rank-sum tests for continuous variables and standard two-by-two tables with Fisher exact tests for categorical variables. Associations were considered significant with an uncorrected p-value <0.05. We also report each association’s relative risk ratio (RR) with a 95% confidence interval. Data analysis was performed using Stata 13 (Statacorp, College Station, TX).
Results
Study Cohort
The cohort (Table 1) consisted of 199 FN episodes among 140 participants, 43% female, with a median age at cohort entry of 6.1 years (3.1-12.3). Most participants (71.4%) were hospitalized once for FN during the study period; among the rest, the number of hospitalizations ranged from 2 to 8. The most common diagnoses were acute leukemia and lymphoma. There were 5 participants (3.6%) with trisomy 21, all of whom had acute leukemia. Nearly all of the participants had a CVC, and 31 (22.1%) had a history of at least one prior infection, including bacteremia (N=21), another bacterial infection (N=7), or IFI (N=4). All participants received empiric intravenous antibiotics with antipseudomonal activity upon the onset of fever. Most of the FN episodes (81.9%) developed in outpatients who were then admitted; 36 FN episodes (18.1%) occurred in children who were already hospitalized, especially those receiving high-intensity chemotherapy for acute myeloid leukemia (AML) or brain tumors. Among participants with acute lymphoblastic leukemia (ALL), 26 FN episodes (13.1% of the total) occurred during the lower-intensity maintenance phase of therapy.
Table 1: Demographic and clinical characteristics
|
Parameter |
N (%) |
|
Sex |
|
| Female |
60 (42.9) |
| Male |
80 (57.1) |
| Age at onset (years), median (IQR) |
6.1 (3.1-12.3) |
| Trisomy 21 |
5 (3.6) |
| Diagnosis | |
| ALL |
61 (43.6) |
| Brain tumor |
19 (13.6) |
| Sarcoma |
18 (12.9) |
| Lymphoma |
13 (9.3) |
| AML |
7 (5.0) |
| Neuroblastoma |
7 (5.0) |
| Wilms tumor |
6 (4.3) |
| Hepatoblastoma |
5 (3.6) |
| Other diagnosis* |
4 (2.9) |
| History of cancer relapse |
21 (15.0) |
| Central venous catheter |
127 (90.7) |
IQR: Interquartile Range; ALL: Acute Lympoblastic Leukemia; AML: Acute Myelogenous Leukemia; UTI: Urinary Tract Infection.
*Desmoplastic small round cell tumor (N=1), renal carcinoma (N=2), and rhabdoid liver tumor (N=1).
Infectious complications
Of the 199 FN episodes studied, 43 (21.6%) led to a diagnosis of bacterial or fungal infection (Figure 1), with 6 episodes (3%) involving multiple infections. The most common was bacteremia, of which there were 29 cases (14.6%); cultures were positive for Gram-positive organisms in 18 (including 8 with coagulase-negative staphylococci), Gram-negative organisms in 8, and mixed flora in 3. Bacteremia was diagnosed a median of 1 day (1-3) after fever onset. There were 16 cases (8%) of other bacterial infections, which were diagnosed a median of 4 days (2-6) after fever onset and included typhlitis (N=5), Clostridium difficile enterocolitis (N=4), UTI (N=3), and cellulitis (N=3); 5 of these infections occurred along with bacteremia. There were 5 cases of IFI, most commonly pulmonary aspergillosis, diagnosed a median of 5 days (0-9) after fever onset. Overall, the median time from fever onset to diagnosis was 2 days (1-4). Distributive shock requiring intensive care occurred in 4 FN episodes (2%) due to bacteremia or meningitis, and one of these children died.
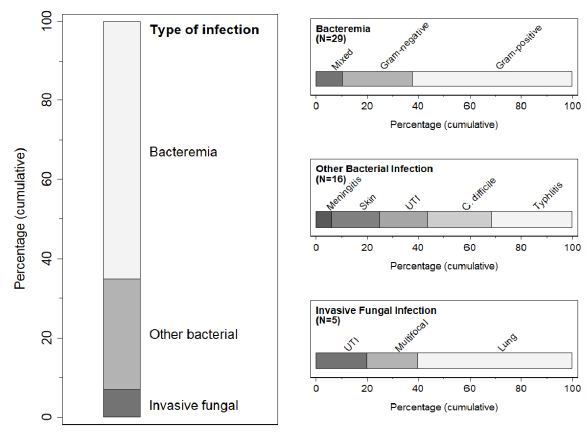
Figure 1: Overview of bacterial and fungal infections in the cohort. For episodes with multiple infections, the left panel categorizes the first diagnosed. UTI, urinary tract infection; C. difficile, Clostridium difficile enterocolitis.
Risk Factors for Bacterial or Fungal Infection
The case-control sample consisted of 40 cases and 80 controls (Table 2). There was not a statistically significant difference in age or sex between the cases and controls, although the case group contained a larger proportion of children <1 year of age and a larger proportion of children who were already hospitalized at fever onset. at the onset of FN. The relative risk of infection was markedly higher in children with trisomy 21 (RR 3.11 [2.39-4.03]) and those with AML (RR 2.11 [1.13-3.95]), although these p-values were >0.05. While cases were slightly more likely to have a temperature ≥39°C, presenting temperature and laboratory values were not significantly different between cases and controls, nor were clinical findings like mucositis and gastrointestinal upset. Cases were more likely to have fever recurrence after >24 hours afebrile and also to have fevers lasting ≥7 days, although these associations were not statistically significant. There was a significantly increased risk of infection (p<0.004) for participants with a prior history of prior bacterial or fungal infection (RR 2.16 [1.34 to 3.48]).
Table 2: Univariate analysis of a nested case-control sample of pediatric patients with febrile neutropenia (FN)
|
Risk factor, N (%) |
Cases (N=40) | Controls (N=80) | p |
Relative risk (95% CI) |
| Demographic and historical features | ||||
| Sex |
27 (67.5) |
41 (51.2) | 0.118 | 1.59 (0.91 to 2.77) |
|
Relapsed |
6 (15.0) | 12 (15.0) | 1.000 |
1.00 (0.49 to 2.03) |
| Age at onset (years)* |
6.6 (4.2-15.9) |
7.2 (3.1-12.3) | 0.432 | — |
|
Age <1 year |
3 (7.5) | 2 (2.5) | 0.332 |
1.86 (0.87 to 4.00) |
| Trisomy 21 |
2 (5.0) |
0 (0.0) | 0.109 | 3.11 (2.39 to 4.03) |
|
Diagnosis of AML |
4 (10.0) | 2 (2.5) | 0.095 |
2.11 (1.13 to 3.95) |
| Prior infection |
18 (45.0) |
15 (18.8) | 0.004 |
2.16 (1.34 to 3.48) |
| Findings at FN onset | ||||
| Temperature (oC)* |
39 (38.6-39.7) |
38.8 (38.4-39.3) | 0.052 | — |
|
Presenting WBC (x109/L)* |
0.3 (0-0.8) | 0.5 (0.2-0.9) | 0.106 |
— |
| Presenting ANC (x109/L)* |
115.5 (0-250) |
86.0 (11-348) | 0.538 | — |
|
Already admitted |
10 (25.0) | 10 (12.5) | 0.118 |
1.67 (0.98 to 2.83) |
| Rhinitis or rhinorrhea |
5 (12.5) |
16 (20.0) | 0.445 | 0.67 (0.30 to 1.51) |
|
Severe mucositis |
7 (17.5) | 12 (15.0) | 0.793 |
1.13 (0.59 to 2.16) |
| Abdominal pain |
8 (20.0) |
11 (13.8) | 0.430 | 1.33 (0.73 to 2.42) |
|
Vomiting |
13 (32.5) | 15 (18.8) |
0.111 |
1.58 (0.95 to 2.63) |
| Findings at reevaluation | ||||
| Fever duration (days)* |
2 (1.5-5) |
2 (1-4) | 0.346 | — |
|
Fever recurrence |
12 (30.0) | 13 (16.3) | 0.097 |
1.63 (0.97 to 2.72) |
| Fever for ≥7 days |
7 (17.5) |
8 (10.0) | 0.255 |
1.48 (0.81 to 2.73) |
*Reported as median and interquartile range.
P-values are from Fisher exact tests for proportions and rank-sum tests for continuous variables. CI: Confidence Interval; AML: Acute Myeloid Leukemia; WBC: White Blood Cells; ANC: Absolute Neutrophil Count.
Discussion
In this consecutive sample of 199 FN episodes in a typical pediatric oncology population at a United States tertiary-care hospital, 21.6% were complicated by a bacterial or fungal infection, most frequently Gram-positive bacteremia. UTI was more common than expected, likely reflecting our emergency department’s practice of obtaining non-catheterized urine samples from most febrile children. Although undiagnosed UTI would likely be treated by empiric antibiotics, this source of pathology in children with FN warrants further investigation. In a nested case-control analysis, the relative risk of bacterial or fungal infection was higher in children with trisomy 21 and those with AML and considerably higher in those with a prior history of infection. Infections diagnosed during the study period were generally not relapses of prior infection; instead, infection risk may reflect cumulative person-level factors, including duration of chemotherapy, cumulative antibiotic exposure, and differences in the microbiome.
As with any observational retrospective study, these findings are not definitive. Our broadly inclusive definition of infection was designed to reflect clinical decision-making, with emphasis on clinical data that would indicate a change in management or a longer course of inpatient observation. The overall similarity between groups in the case-control analysis, as well as the fact that infections occurred during relatively low-intensity chemotherapy (like maintenance ALL therapy) emphasizes the challenge of risk-stratifying children with FN. Most infections in this cohort, however, were diagnosed within the first 4 days after the onset of fever. For children without trisomy 21, AML, or a prior infection history, who do not have overt signs of infection, there may be less benefit of hospitalization longer than through neutrophil recovery, as long as careful outpatient follow-up can be assured.
Conflict of Interest
The authors have no conflicts of interest or external funding sources to disclose.
References
- Bagnasco F, Haupt R, Fontana V, et al. (2012) Risk of repeated febrile episodes during chemotherapy-induced granulocytopenia in children with cancer: a prospective single center study. J Chemother 24(3): 155-160. doi: 10.1179/1973947812Y.0000000002
- Castagnola E, Fontana V, Caviglia I, et al. (2007) A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clin Infect Dis 45(10): 1296-1304. [crossref]
- Castagnola E, Garrè ML, Bertoluzzo L, et al. (2011) Epidemiology of febrile neutropenia in children with central nervous system tumor: results from a single center prospective study. J Pediatr Hematol Oncol 33(7): e310-315. [crossref]
- Davis K, Wilson S (2020) Febrile neutropenia in paediatric oncology. Paediatr Child Health (Oxford) 30(3): 93-97. [crossref]
- Boccia R, Glaspy J, Crawford J, Aapro M (2022) Chemotherapy-Induced Neutropenia and Febrile Neutropenia in the US: A Beast of Burden That Needs to Be Tamed? Oncologist 27(8): 625-636. [crossref]
- Pizzo PA (1993) Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med 328(18): 1323-1332. [crossref]
- Freifeld A, Marchigiani D, Walsh T, et al. (1999) A Double-Blind Comparison of Empirical Oral and Intravenous Antibiotic Therapy for Low-Risk Febrile Patients with Neutropenia during Cancer Chemotherapy. New England Journal of Medicine 341(5): 305-311. [crossref]
- Basu SK, Fernandez ID, et al. (2005) Length of Stay and Mortality Associated With Febrile Neutropenia Among Children With Cancer. JCO 23(31): 7958-7966. [crossref]
- Boragina M, Patel H, Reiter S, et al. (2007) Management of febrile neutropenia in pediatric oncology patients: a Canadian survey. Pediatr Blood Cancer 48(5): 521-526. [crossref]
- Maxwell RR, Egan-Sherry D, Gill JB, et al. (2017) Management of chemotherapy-induced febrile neutropenia in pediatric oncology patients: A North American survey of pediatric hematology/oncology and pediatric infectious disease physicians. Pediatr Blood Cancer 64(12). [crossref]
- Agyeman P, Kontny U, Nadal D, et al. (2014) A Prospective Multicenter SPOG 2003 FN Study of Microbiologically Defined Infections in Pediatric Cancer Patients with Fever and Neutropenia. Pediatr Infect Dis J. [crossref]
- Brack E, Bodmer N, Simon A, et al. (2012) First-day step-down to oral outpatient treatment versus continued standard treatment in children with cancer and low-risk fever in neutropenia. A randomized controlled trial within the multicenter SPOG 2003 FN study. Pediatr Blood Cancer 59(3): 423-430. [crossref]
- Dommett R, Geary J, Freeman S, et al. (2009) Successful introduction and audit of a step-down oral antibiotic strategy for low risk paediatric febrile neutropaenia in a UK, multicentre, shared care setting. Eur J Cancer 45(16): 2843-2849. doi: 10.1016/j.ejca.2009.06.003 [crossref]
- Gupta A, Swaroop C, Agarwala S, et al. (2009) Randomized controlled trial comparing oral amoxicillin-clavulanate and ofloxacin with intravenous ceftriaxone and amikacin as outpatient therapy in pediatric low-risk febrile neutropenia. J Pediatr Hematol Oncol 31(9): 635-641. [crossref]
- Kern WV (2006) Risk Assessment and Treatment of Low-Risk Patients with Febrile Neutropenia. Clin Infect Dis 42(4): 533-540. [crossref]
- Lucas KG, Brown AE, Armstrong D, et al. (1996) The identification of febrile, neutropenic children with neoplastic disease at low risk for bacteremia and complications of sepsis. Cancer 77(4): 791-798. [crossref]
- Manji A, Beyene J, Dupuis LL, et al. (2012) Outpatient and oral antibiotic management of low-risk febrile neutropenia are effective in children—a systematic review of prospective trials. Supportive Care in Cancer 20(6): 1135-1145. [crossref]
- Mullen CA, Petropoulos D, Roberts WM, et al. (1999) Outpatient treatment of fever and neutropenia for low risk pediatric cancer patients. Cancer 86(1): 126-134. [crossref]
- Phillips B, Wade R, et al. (2010) Systematic review and meta-analysis of the discriminatory performance of risk prediction rules in febrile neutropaenic episodes in children and young people. European Journal of Cancer 46(16): 2950-2964. [crossref]
- Rackoff WR, Gonin R, Robinson C, et al. (1996) Predicting the risk of bacteremia in childen with fever and neutropenia. J Clin Oncol 14(3): 919-924. [crossref]
- Rondinelli PIP, Ribeiro K de CB, de Camargo B (2006) A proposed score for predicting severe infection complications in children with chemotherapy-induced febrile neutropenia. J Pediatr Hematol Oncol 28(10): 665-670. [crossref]
- Santolaya ME, Alvarez AM, Avilés CL, et al. (2002) Prospective evaluation of a model of prediction of invasive bacterial infection risk among children with cancer, fever, and neutropenia. Clin Infect Dis 35(6): 678-683. [crossref]
- Teuffel O, Ethier MC, Alibhai SMH, et al. (2011) Outpatient management of cancer patients with febrile neutropenia: a systematic review and meta-analysis. Ann Oncol 22(11): 2358-2365. [crossref]
- Cennamo F, Masetti R, Largo P, et al. (2021) Update on Febrile Neutropenia in Pediatric Oncological Patients Undergoing Chemotherapy. Children (Basel) 8(12): 1086. [crossref]
- Lehrnbecher T, Phillips R, Alexander S, et al. (2012) Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol 30(35): 4427-4438. [crossref]
- Lehrnbecher T, Robinson P, Fisher B, et al. (2017) Guideline for the Management of Fever and Neutropenia in Children With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. JCO 35(18): 2082-2094. [crossref]
- Lehrnbecher T, Robinson PD, Ammann RA, et al. (2023) Guideline for the Management of Fever and Neutropenia in Pediatric Patients With Cancer and Hematopoietic Cell Transplantation Recipients: 2023 Update. J Clin Oncol 41(9): 1774-1785. [crossref]
- Alexander SW, Wade KC, Hibberd PLMD, Parsons SKMD (2002) Evaluation of Risk Prediction Criteria for Episodes of Febrile Neutropenia in Children with Cancer. Journal of Pediatric Hematology 24(1): 38-42. [crossref]
- Ammann RA, Hirt A, Lüthy AR, Aebi C (2003) Identification of children presenting with fever in chemotherapy-induced neutropenia at low risk for severe bacterial infection. Med Pediatr Oncol 41(5): 436-443. [crossref]
- Hakim H, Flynn PM, Knapp KM, et al. (2009) Etiology and clinical course of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol,(9): 623-629. [crossref]
- Hakim H, Flynn PM, Srivastava DK, et al. (2010) Risk Prediction in Pediatric Cancer Patients With Fever and Neutropenia: The Pediatric Infectious Disease Journal 29(1): 53-59. [crossref]
- Boeriu E, Borda A, Vulcanescu DD, et al. (2022) Diagnosis and Management of Febrile Neutropenia in Pediatric Oncology Patients-A Systematic Review. Diagnostics (Basel) 12(8): 1800. [crossref]
- Teuffel O, Sung L (2012) Advances in management of low-risk febrile neutropenia. [Miscellaneous Article]. Current Opinion in Pediatrics 24(1): 40-45. [crossref]
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis 52(4): e56-93. [crossref]
- Ascioglu S, Rex JH, de Pauw B, et al. (2002) Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 34(1): 7-14. [crossref]