Abstract
The Summer of 2022 caused China’s heat wave to shatter records, bringing the country’s hydropower electricity into question, while in the U.S. it delivered five 1,000-year rain events in five weeks to several Midwest states.1 It is proposed that the linear, inextricably tight correlation between global CO2 values and global temperature, delineated and publicized by James Hansen and others, will finally create an urgency in the minds and hearts of all people, so that international direct air carbon capture by the gigaton can begin in earnest and in parallel with carbon-free fuels, zero carbon emissions, renewable energy, and even negative carbon emissions, implemented worldwide. This dual track proposal can immediately begin the reversal of global temperature in direct proportion to the decrease in the excess, heat-trapping atmospheric CO2. In that way, more time will be allocated for the world to convert to 100% renewable energy and thus begin to drastically reduce its carbon emissions, which add about 40 gigatons of CO2 to the air blanket above us every year. The second phase of the X-Prize Carbon Removal for the 2022 million dollar candidates, among other industrial processes already in motion, will demonstrate novel and inexpensive methods for capturing billions of tons of atmospheric carbon dioxide for permanent removal in 2025. This contest adds to other hopeful signs for global reversal of temperature and sea level rise.
Introduction
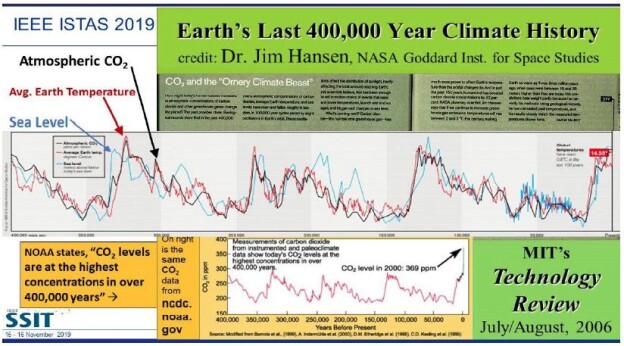
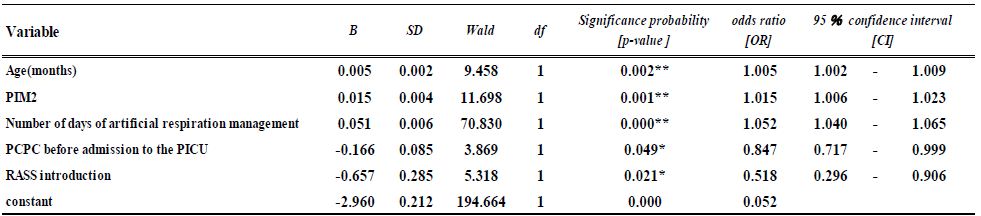
As global warming becomes more pronounced, obvious, and severe, the term “climate emergency” is re- emerging as a clarion call. Bill McGuire, author of Hothouse Earth: An Inhabitant’s Guide, says we need to be “hard-hitting enough to galvanize action and trigger behavioral change” with the aftermath of wildfires and floods [1,2]. Others, such as Professor Naomi Oreskes from Harvard University, have a more pessimistic view of “Wishful Thinking in Climate Science” since many programs around the world have small throughput, high cost per ton, or have simply been canceled after millions spent on the attempt [3]. Adam Vaughan agrees, noting that most major carbon capture and storage projects have not met their targets [4]. Meanwhile, the Amazon rainforest may have reached a crucial tipping point which will turn forest into a savannah according to a recent report from nine countries and territories that encompass the Amazon region [5]. Therefore, we need to have a “longtermism” attitude instead of a dystopian view, according to Professor William MacAskill, author of What We Owe the Future, as he insists that “improving the world for future generations is one of humanity’s most pressing tasks that urgently demand our attention [6]. My work in this area [7-9] has focused on the stellar work by James Hansen, who in 1988 was the first to predict a global “greenhouse effect” from a 3-D model developed at the NASA Goddard Institute for Space Studies but using the onerous phrase “climate forcing [10].” Since such a concept was not yet accepted by the public, the paper was shunned by academic critics and Hansen was declared to be “wrong” by most of the media at the time, threatened to lose his job, and generally criticized by everyone. In Figure 1, a slide from one of my presentations (ref. 7), offers a reproduction of Hansen’s now famous climate graph of the earth’s 400,000 year history, including the surprisingly overlapping plots of global temperature, CO2 and sea levels, along with a corroborating NOAA graph for the same period of time.
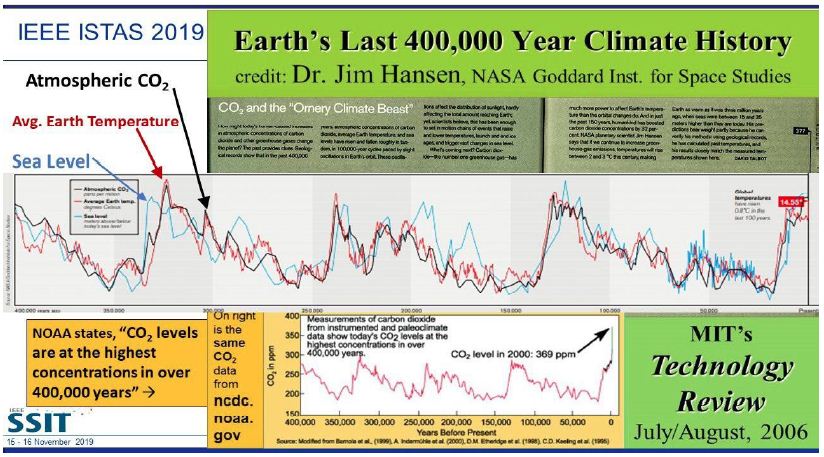
Figure 1: Earth’s 400,000 year history of temperature, carbon dioxide and sea levels
In 1999, the Vostok ice core 420,000-year record of carbon dioxide was published by Petit et al. [11] Exhibiting great stability, the CO2 levels clearly have never exceeded 290 ppm worldwide even through four ice ages. However, in the isolated monitoring station cited above for our modern, with our wanton fossil fuel carnage, the latest global carbon dioxide levels have now exceeded 410 ppm, with apparent disregard for the consequences. Notice in Figure 1 the important clearly tight correlation of temperature (blue graph) and carbon dioxide (red graph) for the past 420,000 years, which leads us to the obvious problem of the red line at the far-right side (present time), which extends dramatically above the maximum seen in the graph for the past 400,000 years. We are forced to admit the historic red and blue data lines don’t lie, so the axis label of CO2 concentration on the left necessarily correlate to the axis label of global temperature on the right. This is the most important realization that arises from Hansen’s Table of vertical axis variables that are found in this 2006 MIT Technology Review article [12]. While climatologists know this relationship exists, the United Nations Environmental Program (UNEP) and the International Panel on Climate Change (IPCC) among others are slow to realize the consequences of the present CO2 excess, which now surpasses 40% of the maximum 290 ppm the earth has ever experienced in over 420,000 years. The Hansen Equation that emerges from his Table of data becomes the key with:
(+/-) 20 ppm of CO2 = 1°C = 20 m change in sea level. Eq. (1).
Thus, it is vital to understand from Hansen’s careful measurement and plotting of the Vostok data, into the Hansen Equation summarizing it in a condensed form that we are indebted for at least 8 degrees C increase in temperature, which will manifest approximately by 2100 with the business-as-usual scenario [13]. Even a 6°C increase worldwide by 2100 will be devastating and is considered a mass extinction event [14]. Mark Lynas, author of Our Final Warning: Six Degrees of Climate Emergency, predicts that a five-degree world is where the tropics and sub-tropical regions are subjected to year-round deadly heat with large areas of uninhabitable zones, due to the high temperatures. Global food production will be decimated with ground-based agriculture only possible in diminishing zones of habitability in the highest latitudes. He also suggests that a five-degree world will have surviving humans crammed into refugee areas in Greenland and the Antarctic Peninsula, similar to the Eocene Period 50 million years ago with similar temperatures and CO2 levels. “Six-degrees sees the greatest mass extinction ever on Earth, greater than the end-Permian cataclysm that destroyed 90% of species alive at the time.” He further notes that our present rate of carbon emissions is about ten times the rate that of the end-Permian period and actually unprecedented in all of geological history [15]. The uninhabitable earth scenario is presently upon us, with each decade becoming hotter by about one degree C, unless something drastic is done to the atmosphere globally, such as iron fertilization of the oceans to periodically create algae blooms, geoengineering the atmosphere with albedo reflectivity particles which have been proven to settle on the earth’s surface soon after, or the simplest and perhaps the safest: gigaton direct air capture to reduce the amount of carbon dioxide in the air [16].
Direct Air Carbon Capture
To be considered as mankind’s only real recourse to practically reverse the inexorable temperature rise in the same time frame it took to go up, while grabbing the same amount of carbon that went up, Direct Air Capture (DAC) is the latest and growing trend among large movers and shakers, such as the X-Prize Carbon Removal https://www.xprize.org/prizes/elonmusk and Project Vesta https://www.vesta.earth/. Using a special type of carbon-removing sand made of a natural mineral olivine, Project Vesta accelerates the earth’s own long-term process of rock weathering by grinding olivine minerals into sand and letting the ocean react with it to bind CO2 permanently in seawater over decades [17]. Vesta.earth has received endorsement from MIT Technology Review, The Guardian, and the National Academies of Sciences, Engineering, and Medicine, besides Bill Gates. Along the same lines, Professor David Beerling at the University of Sheffield, UK, has found that ground up basalt can also absorb carbon from the air. His plan is to sprinkle it on farm lands in the UK to absorb 6 to 30 million tonnes of carbon dioxide per year, which would meet about half of the UK’s net-zero target [18]. One of the 15 X-Prize Milestone winners, who go onto the three-year development stage is Captura from Pasedena CA, which is doing just the opposite process. Captura is developing CO2 capture and sequestration technology for extracting CO2 from oceanwater that is scalable to Mton/year – Gton/year to meet the rapidly growing demand in the carbon credit market. Captura’s approach leads capture of high-purity CO2 and restores pH balance in oceanwater. Another Milestone Award Winner is the Pennsylvania State University which proposes to use millions of African farms to sequester up to 1 gigaton per year of CO2 from the air https://plantvillage.psu.edu/. (Note: The “gigaton” and the metric version “gigatonne” are very close within a few percent in value so they are used interchangeably in this article). Credit must be given to Elon Musk for envisioning this standard process repeatedly used throughout the history of the technological age to rapidly develop a new process that does not presently exist: offer a huge cash prize for the winner. Here, the grand X-Prize winner in 2025 will receive $100 million from the Musk Foundation for the best gigaton carbon dioxide removal scheme. As the xprize.org website states, “The climate math is becoming clear that we will need gigaton-scale carbon removal in the coming decades to avoid the worst effects of climate change. The International Panel on Climate Change (IPCC) estimates the need at approximately 10 gigatonnes of net CO2 removal per year by the year 2050 in order to keep global temperature rise under 1.5 or 2C. As governments, companies, investors, and entrepreneurs make plans to meet this challenge, it is clear that we will need a range of carbon removal solutions to be proven through demonstration and deployment to complement work that is already underway. If humanity continues on a business-as-usual path, the global average temperature could increase 6°C by the year 2100.
“This four-year global competition invites innovators and teams from anywhere on the planet to create and demonstrate solutions that can pull carbon dioxide directly from the atmosphere or oceans, and sequester it durably and sustainably. To win the grand prize, teams must demonstrate a working solution at a scale of at least 1000 tonnes removed per year; model their costs at a scale of 1 million tonnes per year; and show a pathway to achieving a scale of gigatonnes per year in future. “Any carbon negative solution is eligible: nature-based, direct air capture, oceans, mineralization, or anything else that achieves net negative emissions, sequesters CO2 durably, and show a sustainable path to achieving low cost at gigatonne scale.” Mission Zero Technologies and Project Hajar became another $1M Milestone Award Winner while developing direct air capture (DAC) technology that will recover high-purity CO2 from the air while incurring only a fraction of the costs and energy it takes to do so today. It has received seed funding from a number of sources https://www.missionzero.tech/news to produce a 100+ ton/year DAC pilot plant in the UK and elsewhere, which stores atmospheric carbon in the peridotite rock of the upper mantle of the earth. However, very few of these competitors in the X-Prize seem to be capable of providing a “pathway” to even a “net 10 gigatons/year” of carbon removal from the air (which technically should be a gross amount of 50 gigatons/year to offset the 40 Gt/yr we send up there each year). One ray of hope, among many workable climate solutions, is the recent discovery of an alloy of gallium, indium, and tin that is liquid at room temperature and conducts electricity. By spiking the silvery mixture with a sprinkling of catalytically active cerium and placing it inside a glass tube, along with a splash of water, scientists have now proven a room temperature method to convert CO2 to carbon, instead of the usual high temperature procedure. Chemists Dorna Esrafilzadeh and Torben Daeneke at RMIT University in Melbourne, Australia, turned to a new class of catalysts made from metal alloys that are liquid at room temperature [19]. This is a process that can be scaled up, since it is catalytically driven, and may someday offer a novel and inexpensive method for capturing billions of tons of atmospheric carbon. Such inventions and ongoing projects are the main focus of this short update on carbon capture, removal and storage. A noteworthy comment summarizing large-scale gigaton carbon capture is Professor Chris Jones from Georgia Institute of Technology. “These approaches are low cost at under $100 per ton of captured CO2, ‘but there’s only so much land change you could make to capture a significant amount,’ he says. ‘We need to capture 10 gigatons per year for negative emissions by 2060. Land and biomass approaches only scale to a few gigatons.’ Of known carbon-removal techniques, two hold the most promise, he says, citing a recent National Academies report. One is DAC and the other is carbon mineralization. “Nothing prevents us from scaling these up to the 10 gigatons per year scale needed aside from a commitment, coordination and cooperation [20]. In the same article, Raghubir Gupta from Sustaera https://www.sustaera.com/ boasts the largest carbon capture record so far at the lowest cost (also a Milestone winner). “One big thing we have that not many others have is practical experience of scaling up the technology to 1,000 tonnes/day carbon dioxide capture,” he says. “With that background when we looked at CO2 removal from air, we thought the two things that are most important to really make a difference are cost and scale. It’s not the efficiency of the process [21].
Sustaera uses cheap sodium carbonate to adsorb CO2. It coats the material on a high-surface area ceramic scaffold used in catalytic converters. The high surface area increases access to the sorbent and increases CO2 adsorption rate significantly. The energy advantage comes from using electricity instead of heat to separate the CO2 and regenerate the sorbent, at a cost of under $100/ton. To finish this section, my favorite DAC company, working since 2015 on this technology, is Carbon Engineering from Canada (Figure 2) https://carbonengineering.com/our-story/ producing synthetic fuel from the captured CO2 with AIR TO FUELSTM plants in several markets around the world. Their latest facility in the Permian Basin, US, is expected to capture one million tons of CO2 from the air annually when complete, so it can be permanently and safely stored deep underground in geological formations, using a potassium hydroxide sorbent coupled with a calcium caustic recovery loop in the process. They have also partnered with Storegga in the UK https://www.storegga.earth/ to accomplish one million tonnes of DAC annually.

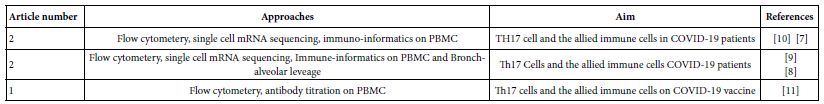
Figure 2: Hansen Challenge: All of the facts to convert any chosen number of excess CO2 in ppm to gigatons, where A2 is the business as usual scenario for carbon emissions worldwide.
The challenging calculation reflected in Figure 2 of the numbers results from an attempt to sequester 50 gigatons (with 40 Gt subtracted for many more decades to come) every year if the world is fortunate enough to discover a very inexpensive means to capture CO2 in that quantity reliably [22].
Conclusion
A proposed world recovery plan is directed the world’s government leaders and approximately one thousand billionaires to form a DAC Gigaton Consortium that takes multi-gigaton DAC seriously, beginning as soon as possible, in order to actually LOWER the earth’s 412 ppm of CO2 steadily each year for decades until 350 ppm and furthermore, the long-sought-after 290 ppm global level is reached. It is a guarantee, even as IPCC is now in 2022 beginning to realize, that without any more excess levels above 290 ppm (global maximum seen in 400,000 years of Figure 1), the world’s temperature will literally, inevitably, and steadily lower itself back down to a comfortably temperate zone. To the mathematicians and scientists who read this, Equation 1 is both positive and negative with all three variables proportionally and linearly dependent on the leading change which occurs first in time (CO2, temperature, or sea level), whether plus or minus. We as a species have been spoiled with about 10,000 years of an interglacial period, giving us moderate temperatures worldwide and now we have to pay dearly for the self-created disruption in the former stability in order to regain it once again. Each year that we wait to perform 50 GT/yr of DAC adds about 40 Gt to the total excess 466 Gt that needs to be removed to restore our planet to livable conditions.
References
- Cappucci, Matthew, Five 1,000-year rain events hit the U.S. in five weeks, Washington Post, Aug. 26, 2022.
- McGuire Bill (2022) Advertising Crisis, New Scientist.
- Oreskes, Naomi (2022) Wishful Thinking in Climate Science. Scientific American.
- Vaughan, Adam (2022) Most major carbon capture and storage projects haven’t met targets. New Scientist.
- Taylor Luke (2022) The Amazon rainforest has already reached a crucial tipping point. New Scientist.
- MacAskill William (2022) Planning Ahead. New Scientist.
- Valone Thomas (2019) Jacqueline Panting. Quantitative Carbon Dioxide, Temperature, and Sea Level Relation for the Future of Terrestrial Fossil-Fueled Technology, An Accurate Predictive Model Based on Vostok 420 kY Historical Record. IEEE International Symposium on Technology and Society (ISTAS) Proceedings, 15-16 November, School of Engineering, Tufts University, Medford MA. https://wwslideshare.net/ThomasValonePhD/valone-ieee-istas2019ppt-ver4-with-extra-slide-withlinks-at-end, sponsored by the IEEE Society for Social Implications of Technology.
- Valone, Thomas, Predictive Connection for 2100 between Atmospheric Carbon, Global Warming and Ocean Height Based on Climate History, International Journal of Environment and Climate Change, 9(10): 562-594, 2019; Article no.IJECC.2019.048, ISSN: 2581-8627, DOI: 10.9734/IJECC/2019/v9i1030140
- Valone Thomas (2020) Global Environmental Forecast and Roadmap Based on 420 kY of Paleoclimatology. Journal of Atmospheric Science Research 3.
- Hansen J, Fung I, Lacis A, Rind D, Lebedeff S, et al. (1988) Global climate changes as forecast by Goddard Institute for Space Studies three-dimensional model. Geophys. Res. 93: 9341-9364.
- Petit JR, Jouzel J, Raynaud D, Barkov NI, Barnola JM, et al. (1999) Climate and Atmospheric History of the Past 420,000 years from the Vostok Ice Core, Antarctica. Nature 399: 429-436.
- Hansen James (2006) CO2 and the Ornery Climate Beast. Technology Review.
- Brown Patrick, Ken Caldeira (2017) Greater future global warming inferred from Earth’s recent energy budget. Nature 552: 45-50.
- Six Degrees Could Change the World Geographic Society.
- Lynas, Mark (2020) Our Final Warning: Six Degrees of Climate Emergency, HarperCollins Publishers.
- Goodell Jeff (2010) How to Cool the Planet, Mariner Books, Houghton Mifflin Harcourt.
- Temple James (2020) How green sand could capture billions of tons of carbon dioxide. MIT Technology Review.
- Kantzas EP, Maria VM, Mark RL, Rafael ME, Phil R et al. (2022) Substantial carbon drawdown potential from enhanced rock weathering in the United Kingdom. Nature Geoscience 15: 382-389.
- Service, Robert F (2019) New way to turn carbon dioxide into coal could ‘rewind the emissions clock’, Science.
- Patel P (2022) XPrize Competitors Capture Carbon > $100 million at stake in CO2-removal face-off, IEEE
- Ibid, p. 6.
- Zeman F (2014) Reducing the cost of Ca-based direct air capture of CO2. Sci. Technol 48: 11730-11735.