Abstract
TH17 cells displayed multiple immune functions in viral human infections including SARS-COV-2. The objective of the present opinion paper was to deduce the actual contribution of these cells in various infection phases of SARS-COV-2 in man. The deduction tempts to: (i) map the immune-typing of TH17 cells in: severe, critical, deceased and Vaccinee via show case analysis and (ii) suggest the pathogenic mechanism of TH17 cells in this disease. The show case analysis of five research papers published between 2020 and 2022 indicated that: TH17 cells are of two main subsets, the nonpathogenic and the pathogenic was with, marked plasticity, pleomorphism and instability. On the onset of the clinical infection through hospital admission the patient peripheral blood has shown twice TH17 counts than in normal controls. In uncontrolled progressed COVID-19, the TH17 cell count drop in peripheral blood and enriched in lungs with marked elevation in counts and clonal expansion therein in severe critical and deceased cases. Critical cases on recovery showed TH17 cell counts restoration to normal. Peripheral blood Th17 cells inhibit Teg while in lung both of TH17 and T reg counts were elevated. TH17 cell recruit neutrophils during the infection progress and interacts with various subsets of macrophages and DCs with an outcome of hypercytokinemia and tissue pathology. Based on these facts, the opinion suggested: (i) TH17 as predictor of severity, critical and deceased as well as (ii) the possibleTH17 cells pathogenic mechanisms operable in cases of SARS-COV-2 human disease.
Keywords
Cell, Clonal, COVID-19, Expansion, Pathogenic, TH17
Introduction
TH17 cells are heterogeneous distinct lineage of CD4+ T cells. They are differentiated from naïve T cells through the action of cytokine micro-environmental stimuli. TH17 are basically of two subsets the nonpathogenic and the pathogenic [1]. These helper cells take part in extra-cellular bacterial infections, yeast infections, viral infections including SARS-COV-2 and auto-immune diseases. TH17 performed dual immune functions: the immune-pathogenic and to lesser extent the immune-protective [1-6]. The objective of the present opinion paper was to: (i) map the role of TH17 through the show case analysis of five immune-typing studies of TH17 cells and (ii) suggest the pathogenic mechanisms of these cells in COVID-19, during the period of 2020 till 2022.
Show Case Analysis Approaches
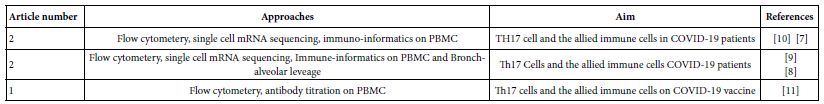
To assess the current holdings of the scientific workers in immunology of SARS-COV-2 infections in man, a sum of 150 current published works through the period of 2020 till 2022 were allocated. These efforts were analyzed so far concerning the CD4+ T cell subsets in COVID-19. Among which ten were concern the role of TH17 in this disease. Of the ten, one was proving TH17 role indirectly from cytokine profiles, five adopted flow cytometery, single cell mRNA sequencing and immunoinformatic approaches to the immune cells recovered from peripheral blood and broncho-alveolar leavage. The rest four review articles were already depending on flow cytometery proving that TH17 cells in association with severe COVID-19 disease and considered as supplementary to this work. The adopted five research works (Table 1) were the raw materials for the show case analysis to deduce the role of TH17 in various phases of human COVID-19 disease [1-12].
Table 1: The show case analysis test research articles
TH17 Cells
Basic TH17 Cell Biology
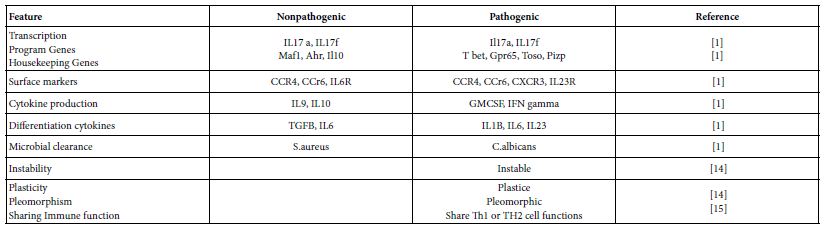
TH17 cells are distinct lineage of CD4+ T cells that differentiated from naïve T cells, secret the cytokines IL17 A and IL17f and express the lineage specific transcription factor RORC. Both of TH17 and TH17/Th1 clones showed selective expression of IL23R and CCR6 in addition to RORC. Th17 help B cells, express low cytotoxicity and low susceptibility to action of autologous T reg. and critical in clearance of extracellular microbial pathogens [14]. They are of two subsets pathogenic and nonpathogenic [1] (Table 2). These helper cells are pleomorphic, instable and exhibit a sort of plasticity. The TH17/TH1 subset can revert to Th1 cells. Hence some workers denote them as heterogenic helper cells [1,14].
Table 2: Th17 cell subset characteristics
TH17 Cell Differentiation
T helper lymphocytes featured by the expression of CD4+ T cells surface markers are the central cell subset of adaptive immunity. CD4 T cells can recognize proteins of microbial pathogens by their unique surface TCR. TCR can shape antigens and organize against them. Both of the TCR-antigen recognition and the signal of TCR engagement integrated stimuli initiate sort of transcriptional changes that guide naïve T cells towards a specialized function. These stimuli include cytokine, soluble mediators or bacterial products in the microenvironment. This differentiation process needs the regulatory interplay of specific intracellular signal transducer and activator STAT protein in the process. STAT eventually induces the dominant transcription factor TF. TF represent the master lineage specific factor. TF controls the transcriptional program of the cell covering: cytokine production and chemokine receptor expression that mediate trafficking to various organs: this network helps each T cell subsets to exert their specific functions in response to antigens available in the assigned tissue. The T cell TF is a T-Box protein in TH1, GATA b binding protein in TH2, and retinoic acid related orphan receptor gamma-t RORCg-t in TH17 and fork head box3 in T regs [16-19]. Any insult of what so ever nature to this differentiation mechanisms lead to dys-regulation mechanism in various steps of the T cell growth, maturation and response to challenge. Such dysregulation can contribute to pathological responses just as in case of immune mediated diseases. For TH1 and TH17 cells and allergenic responses for TH2. The fate decision of the naïve T cells is largely affected by the cytokine surrounding environment. The Th17 differentiation process encoded by the expression of two effector genes: IL17a and IL17f together with multiple player processes are involved in the different stages of differentiation. The STAT, RORC-g-t axis, RORA, Ahr IPF4 and BATF markers set the initial chromatin accessibility that allows the transcriptional programs. Among which the RO RCg-t is determinative for the expression of IL17a and IL17f genes.
Two different cytokine cocktails lead to two different TH17 subsets. TGFB and IL6 induce nonpathogenic TH17 cells characterized by the co-expression of IL10. While IL6 and IL23 but not TGFB lead to differentiation of the pathogenic TH17 cells. Both of the subsets would express RORCg-t but the pathogenic subset of TH17 cells are more plastic, polymorphic and have tendency for transition towards TH1 cells. For any naïve T cell differentiation, the concentration and the gradient of TGFB is crucial, high concentration induces T regs associated genes. While restraining T bet and TH1 genes possibly inhibit TH17 pathogenic responses. There is a developmental overlap between TH17, Th1 and T regs. Such overlap might be caused by the complex cytokine dynamics [1,2,20-22].
TH17 Cell and Cellular Interactions
TH17 cells are known to inhibit T reg. responses in peripheral blood of COVID-19 patients [7]. They can induce the neutrophil and epithelial responses provided by the presence of environmental microbial insults [2]. Within the continuum of COVID-19 pneumonic lungs, TH17 cells interact with pro-inflammatory, pro-fibrotic macrophages, DCs and pDCs [9].
TH17 Cell Immune Functions
TH17 cells performed dual immune function in immune- pathogenesis of viral infections and/or immune protection [6]. They are involved in neutrophil and epithelial cell immune response to extracellular microbes and the initiation of autoimmune diseases [2]. Th17 cell may interplay with the pathogenicity of allergy, asthma and human inflammatory diseases [14].
TH17 Cells in Viral Infections
TH17/IL17 hinders and limits viral infections via several mechanisms as: Enhancing TH1 responses, promoting cytotoxic T cell activity, modulating antiviral B cell responses and inducing protective inflammatory responses. They may limit the viral induced organ pathology, inhibiting inflammation and mediating protective immune responses. Th17 cells/IL17 cytokine may promote viral infection through different mechanisms as: Antagonizing antiviral TH1, T regs and CTL responses, enhancing survival of infected cells, promoting viral intracellular replication, take part in evolution of tissue pathology and fibrosis [6].
TH17 Cells in Various Phases of SARS-COV-2 Human Infection
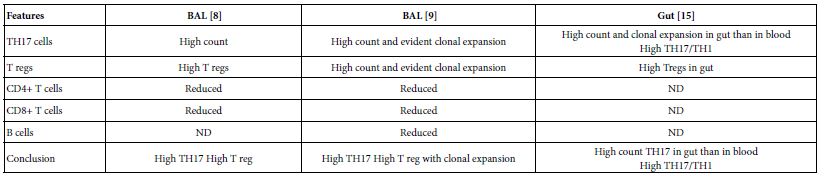
The TH17 cells were confirmed both in peripheral blood and lung compartments of various phases of COVID-19, Tables 3-6. Early acute infection and on admission to hospital, TH17 cells were of twice count than that of asymptomatic and controls. T regs were reduced in count and function [1]. On progress under un-controlled conditions, severe state, TH17 counts were reduced in circulation. Both TH17 cells and T regs were increased with clonal expansion in lung compartment in severe COVID-19. In critical or deceased cases both TH17 and T regs were amplified in counts and expansion. But on recovery from severe or critical states TH17 and T reg counts were restored to normal. In line with Th17 count elevation these cases there were reduction in CD4+ T cells, Cd8+ T cells both in circulation and lung compartment. TH17 suppress the T regs and triggers neutrophils causing recruitment to the affected tissue compartment and interacts with each of pro-inflammatory, and pro-fibrotic macrophages, DCs, pDCs, and monocytes. Such intercellular interactions may terminated by an overt inflammatory cytokine production leading to a state of hyper-cytolinemia, the cytokine storm [9]. Th17 cell clone expansions in lung compartment were higher than that in circulation. Lung resident TH17 cell clones can be either merely resident or of mixed resident and migratory forms from circulation. Other T cell subsets were showing various degrees of clonal expansion [9].
Table 3: Circulatory TH17 cells in severe and vaccine of COVID-19 as evident in the three show case analyzed groups
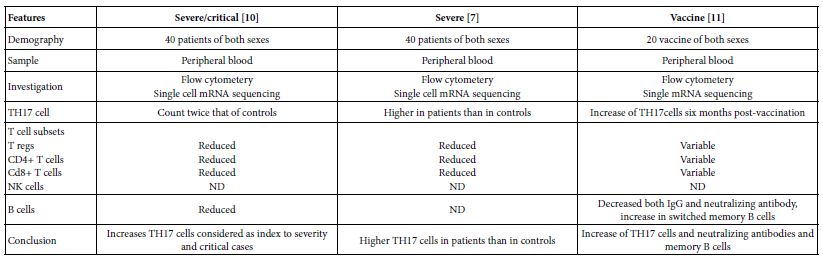
Table 4: Circulatory and pulmonary Th17 cells severe, critical, deceased and vaccine of COVID-19 as evident in the two show case analyzed groups
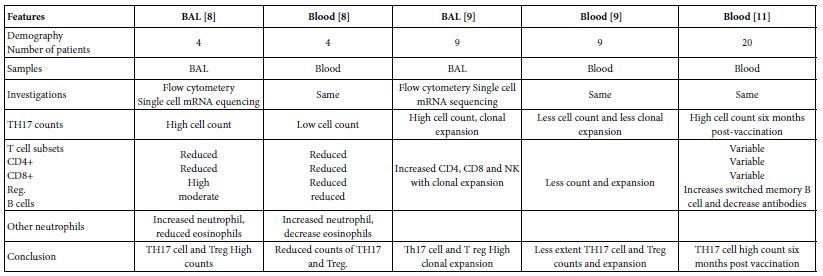
Table 5: Circulatory Th17 cells severe, critical, critical deceased and vaccine COVID-19 as evident in the five show case analyzed groups
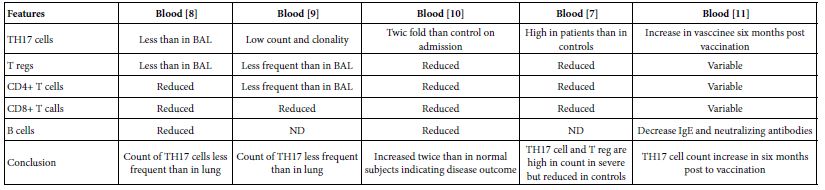
Table 6: Pulmonary existed TH17 cells in various forms of COVID-19 as evident in two show case analyzed groups in comparison to gut Th17 in Chron’s disease
TH17 Cell Suggested Pathogenic Mechanisms
Since TH17 cells expressed low cytotoxicity, though to be a pathogenic helper cell it should express other supportive means to make it able to perform its pathogenic influences. Hence, this opinion paper tempted to hypothesize theoretical suggested mechanisms operable in induction of immune tissue injuries in the lung compartments. They can be coined as follows:
- TH17 cells when interacts with pro-inflammatory macrophages and inflammatory macrophages, they will induce excessive inflammatory cytokines forming cytokine storm mediating tissue pathology consequences of COVID-19 [9].
- Th17 cells recruits neutrophils to lung compartment whereby the affected tissue niche, therein neutrophils produce excessive inflammatory cytokines and reactive O2 intermediates mediating immune tissue injury [9].
- On inhibition of T reg by TH17 cells, they lend the cellular microenvironment allowance of up regulation of auto-reactive T cells to initiate autoimmune pathologic tissue injury [7,10].
- The TH17 cell interaction with pro-fibrotic macrophages may initiate lung tissue fibrotic lesions, the known consequences of COVID-19 pneumonia [9].
- TH17/Th1 cells are known to be: plastic, pleomorphic and instable they my undergoes transition to TH1 cells producing IFNG cytokines and other inflammatory cytokines leading to hyper inflammation in lung paranechyma the sign of COVID-19 pneumonic lungs [15].
Conclusions
TH17 cells associated with the pathogenesis of COVID-19. Circulatory TH17 subset is distinct from lung tissue resident TH17 cell subset. The tissue resident TH17 cells are expanded as tissue specific subset, as mixed clones of migratory and tissue resident TH17 cell clones. On clinical onset of the disease they were of twice count level than controls and inhibit T regs. But in progression during uncontrolled affection, Th17 cell and T reg cells gaining higher counts and marked clonal expansion therein lung compartments as compared to peripheral blood both in severe and critical cases. Though they both reduced to variable degrees in circulation, on recovery of severe and critical cases TH17/T reg ratios restored to normal.
References
- Simone D, Stingo A, Ciccia F (2021) Genetic and environmental determinants of T helper 17 pathogenicity in Front Genetics 12: 703242. [crossref]
- kurebayashi Y, Nagai S, Ikejiri A, Koyasa S (2013) Recent advances in understanding the molecular mechanisms of development and function of TH17 Gene to Cell 18: 247-265. [crossref]
- Velikova TV, Rotsev SV, Georgiev DS, Batselova HM (2021) The role of TH17 cells in sars-cov-2 infection: implementation for the therapy of severe coid-19 Cell 4: e3058.
- Martonik D, Parfieniuk-Kowerda A, Rogalska M, Filisiak R (2021) The role of TH17 responses in covid-19. Cells 10: [crossref]
- Samiento-Monroy JC, Parra-Medina R, Garavito E, Rojas-Villarraga A (2021) TH17 response to severe acute respiratory syndrome coronavirus 2: A type of immune response with possible therapeutic Viral Immunol 34: 190-200. [crossref]
- Ma WT, Yao XT, Peng Q, Chen DK (2019) The protective and pathogenic roles of IL17 in viral infections; friend or Open Biol 9: 190109. [crossref]
- Sadeghi A , Tahaschi S ,Mahmood A, Kuznetsova M, Valizadeh H, et (2020) Th17 and T reg cell function in sars-cov-2 in patients compared to healthy controls. J Cell Physiol 236: 2829-2839. [crossref]
- Ronit A, Berg RMG, Bay JT, Haugaard AK, Ahlstorm MG, et al. (2020) Compartmental immunotyping in covid-19; A case series. J Allergy Clin Immunol 147: 81-91. [crossref]
- Zhao Y, Killan C, Turner JE, Bosurgi L, Roedl K, et (2021) Clonal expansion and activation of tissue resident memory-like TH17 cell expressing GM-CSF in the lung of patients with severe covid-19. Sci Immunol 6: 6692. [crossref]
- Samson M, Nicholas B, Ciudad M, Greigert H, Guilhem H, et al. (2022) T cell immune response predicts the risk of critical sars-cov-2 infection in hospitalized patients. Eur J Inetrnal Med 102:104-109. [crossref]
- Gandolfo C , Anichini G , Mugaini M, Bocchia M, Terrosi C, et (2022) Overview of anti-Sars-cov-2 immune responses six months after BNT162b2 mRNA vaccine. Vaccines 10: 171. [crossref]
- Biasi SD, Meschiari M, Gibellini L, Bellinazzi C, Borella R, et al. (2020) Marked T cell activation ,senescence ,exhaustion and skewing towards TH17 in patients with covid-19 pneumonia. Nat Comm 11: 3434. [crossref]
- Muyayalo KP, Huang DH, Zhao SJ, Xie T, Mor G, et (2020) Covid-19 and T reg./ TH17 imbalance:Potential relationship to pregnancy outcomes. AM J Rep Immunol 64: e13304. [crossref]
- Maddur MS, Miossec P, Karveri SV, Buyry J (2012) Biology, pathogenesis of auto- immune and inflammatory disease and Am J Pathol 181: 8-18. [crossref]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, et (2007) Phenotypes and functional features of human TH17. J Exp Med 204: 1849-1861. [crossref]
- Szabo S, Kim ST, Costa GL, Zhang X, Fathman CG, et (2000) A novel transcription factor, T bet, directs TH1 lineage commitment. Cell 100: 655-669. [crossref]
- Fontlnot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs, the development and function of CD4+Cd25+ regulatory T Nat Immunol 4: 330-336. [crossref]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, et al. (2005) Interleukine 17 producing CD4+ effector T cells develop via a lineage distinct from T helper type 1 and 2 Nat Immunol 6: 1123-1132. [crossref]
- Ciofani M, Madar A, Sellars M, Mace K, Pauli F, et (2012) A validated regulatory network for TH17 cell specification. Cell 151: 289-303. [crossref]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, et (2010) Generation of pathogenic TH17 cells in the absence of TGFB signaling. Nature 467: 967-971. [crossref]
- Kebir H, Kreymborh K, Ifergan I, Dodelet-Devillers A, Cayrol R, et (2007) Human Th17 lymphocytes promote blood-brain barrier distrubtion and central nervous system inflammation. Nat Med 13: 1173-1175. [crossref]
- Zheng W, Flavell RA (1997) The transcription factor is necessary and sufficient for TH2 cytokine gene expression in CD4 T cells. Cell 89: 587-586. [crossref]