DOI: 10.31038/CST.2020531
Abstract
Cancer patients are more vulnerable to acquiring COVID 19 infection and may also experience higher morbidity and mortality. In the context of COVID-19, cancer patients may be affected through delayed diagnosis and have significant impact on management in resource strained settings. Cancer treatment typically involves a possible combination of surgical resection, chemotherapy and radiation therapy (RT). RT delivery requires often daily attendance to a cancer center, is complex and poses potentially additional risks for infection as well as treatment related complications. Optimization of infection control measures and RT treatment schedules is paramount to minimize the impact of the pandemic on patients and optimize outcomes. This review aims to summarize the existing limited literature surrounding RT administration and optimization in the context of COVID-19.
Introduction
Since the recognition of the COVID-19 pandemic, evidence has become available that a cancer diagnosis is considered one of the comorbid conditions that increase the risk of COVID 19 infection [1- 3]. Meanwhile COVID-19 infection in cancer patients is associated with higher morbidity and mortality [4]. Data is still preliminary, but it is likely that both the increased risk of acquiring COVID-19 and more severe consequences thereof in cancer patients are multifactorial in nature likely involving complex relationships between the type of cancer site and extent or location of the disease as well as more nuanced patient and treatment related factors. While a number of publications have delved into the presentation and management of COVID-19, and its relationship to comorbid conditions including malignancy, and yet others into the implications of systemic management (chemotherapy, targeted agents), fewer publications specifically discuss the implications for patients who undergo radiation therapy (RT) and the operational considerations of radiation therapy (RT) departments. RT is administered mostly on a daily basis in cancer centers or facilities with complex logistics, unique organizational demands, high possibility of interaction between vulnerable patients and risk of exposure to multiple patients and staff. The lack of evidence surrounding best practices has left radiation oncology providers and patients with many questions still unanswered.
These questions include the level of vulnerability of cancer patients, who for example may undergo radiation but may not be undergoing systemic management and may therefore not necessarily be immune- compromised, as well as the implication for daily attendance to a RT facility and the associated infection risk and how to best mitigate it. Other questions involve the ability to and safety of delaying or altering RT treatment schedules to minimize risk of infection. Important additional considerations abound in particular settings such a RT emergencies and treatment of the COVID-19 positive patient as well as the administration of radioactive isotopes such as radioactive iodine in thyroid patients who then need to self-isolate at home or as inpatients for several days. Since the COVID-19 pandemic has raised unprecedented challenges and questions, with ongoing lack of robust data, periodic review of the available evidence is important to help guide providers and patients in order to enable evidence-based care hence prompting this scoping review.
Materials and Methods
To carry out the scoping review, publications that specifically addressed the impact and management of cancer patients undergoing radiation therapy in the context of the COVID 19 pandemic were identified in PubMed using the following mesh terms: ((“risk”[MeSH Terms] OR “risk”[All Fields]) OR “risk of”[All Fields]) AND (((((((“covid 19”[All Fields] OR “covid 2019”[All Fields]) OR “severe acute respiratory syndrome coronavirus 2”[Supplementary Concept]) OR “severe acute respiratory syndrome coronavirus 2”[All Fields]) OR “2019 ncov”[All Fields]) OR “sars cov 2”[All Fields]) OR “2019ncov”[All Fields]) OR ((“wuhan”[All Fields] AND (“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields])) AND (2019/12/1:2019/12/31[Date – Publication] OR 2020/1/1:2020/12/31[Date – Publication]))) AND ((((“patient s”[All Fields] OR “patients”[MeSH Terms]) OR “patients”[All Fields]) OR “patient”[All Fields]) OR “patients s”[All Fields]) AND (((((((((“cancer s”[All Fields] OR “cancerated”[All Fields]) OR “canceration”[All Fields]) OR “cancerization”[All Fields]) OR “cancerized”[All Fields]) OR “cancerous”[All Fields]) OR “neoplasms”[MeSH Terms]) OR “neoplasms”[All Fields]) OR “cancer”[All Fields]) OR “cancers”[All Fields]) AND ((((((((((((“radiate”[All Fields] OR “radiated”[All Fields]) OR “radiates”[All Fields]) OR “radiating”[All Fields]) OR “radiation”[MeSH Terms]) OR “radiation”[All Fields]) OR “electromagnetic radiation”[MeSH Terms]) OR (“electromagnetic”[All Fields] AND “radiation”[All Fields])) OR “electromagnetic radiation”[All Fields]) OR “radiations”[All Fields]) OR “radiation s”[All Fields]) OR “radiator”[All Fields]) OR “radiators”[All Fields]). 87 abstracts were identified.
Results
As of July 10, 2020: 1706 papers were published on the topic of COVID 19 and oncology in 2020 to date of which 457 (27%) of these discussed cancer as a risk factor for the development of the COVID 19 infection. The MeSH term was generated to capture papers specific to the context of the cancer patient undergoing radiation therapy during the COVID 19 pandemic. 87 papers were identified using the above MeSH term. Of these 5 (6%) were literature reviews or broad guideline recommendation papers, 12 (14%) dealt related to oncology but were not RT specific (eg. systemic management of hematological malignancies), 63 (72%) were oncology and RT specific and 7 (8%) were not directly relevant to either oncology or radiation but were relevant to COVID 19 more broadly. All of the abstracts were published between March and July, with 75% of the abstracts between the end of April and beginning of July 2020.
Types of Publications Identified
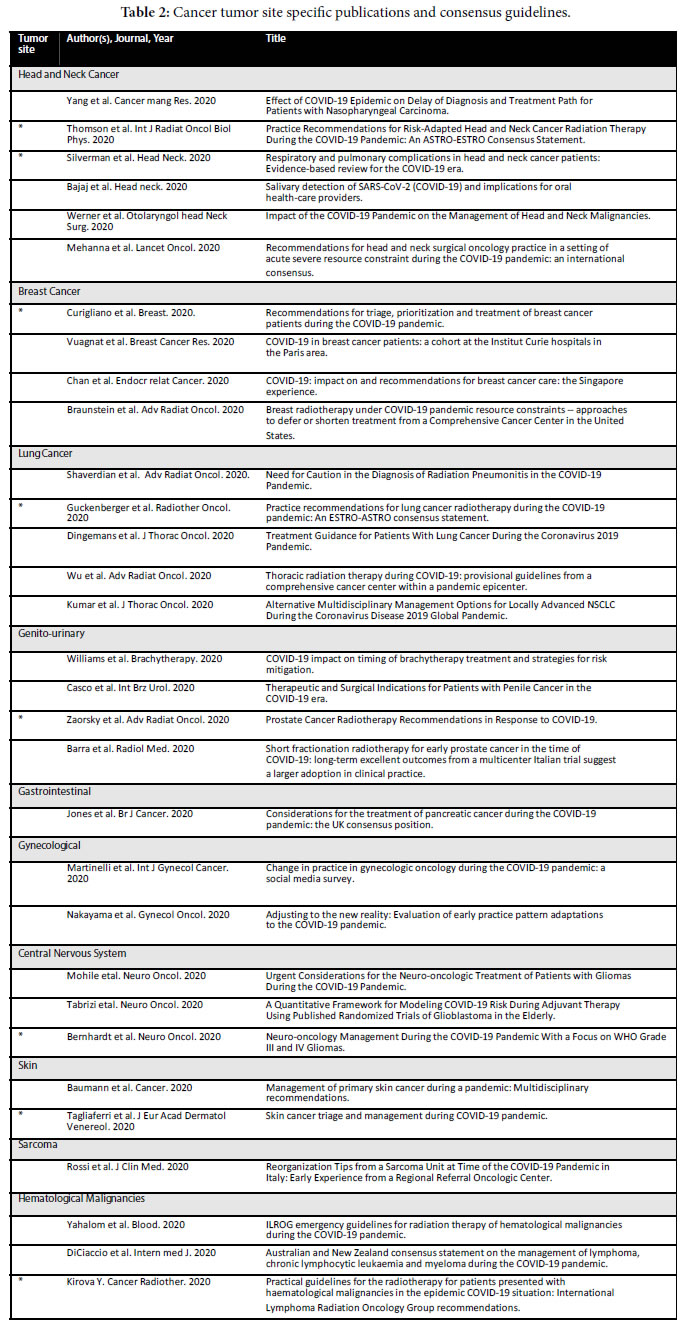
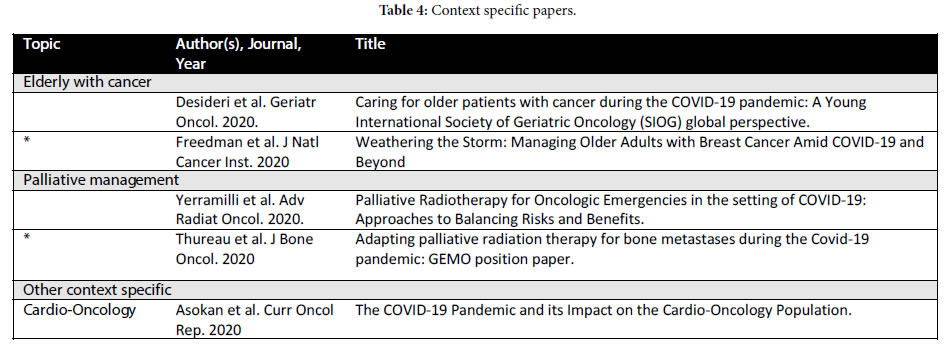
Four types of publications were broadly identified: 1) Literature reviews and operational/planning guidelines which did not contain patient data but rather represent institutional or expert opinion (Table 1); 2) consensus guidelines generated by governing bodies and site- specific groups for specific cancer histologies (Table 2); 3) retrospective single or multiple institution papers (Table 3) and 4) more context focused papers eg. management of the elderly with cancer during COVID-19 and palliative management (Table 4).
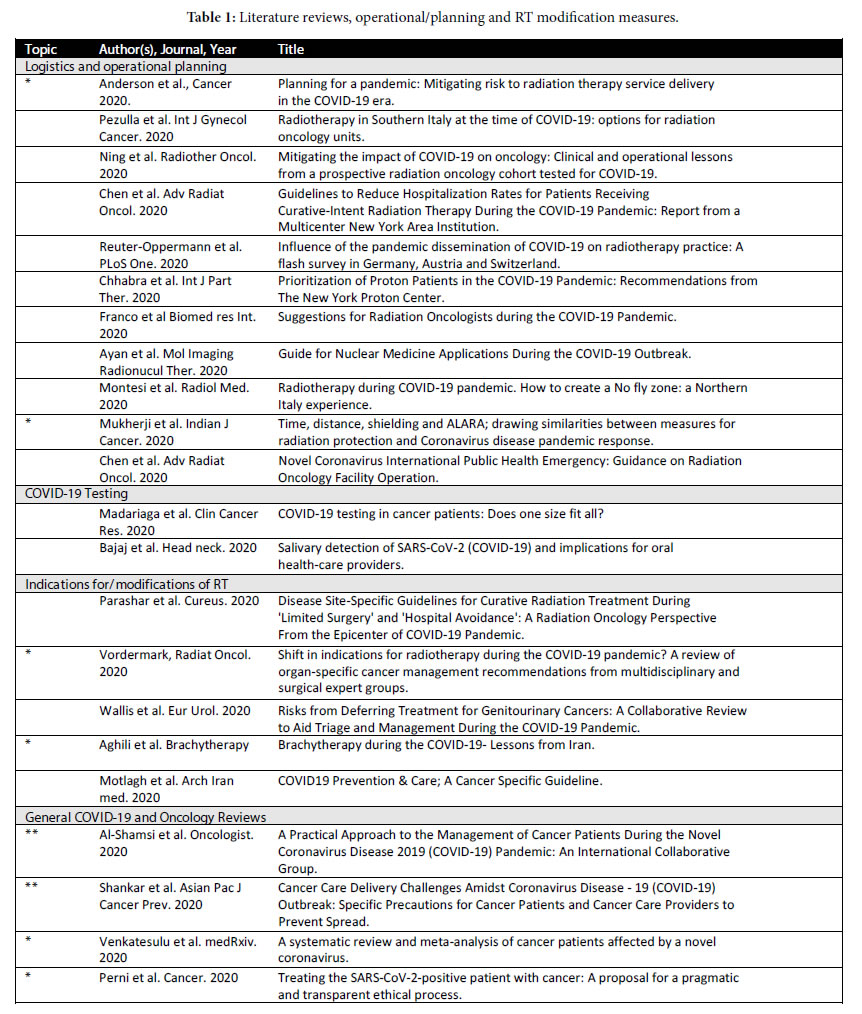

Literature reviews involving COVID-19 and oncology identified with ** were made available by Al-Shamsi et al., Shankar et al. and Anderson et al. (Table 1 ** denotes encompassing reviews), * smaller important reviews) [1-3]. These broader reviews (**) were not necessarily RT specific but contained some information regarding RT. Discussion of RT departmental planning, logistics and operational considerations were available in other reviews (Table 1). Site specific consensus guideline papers with recommendations (organ or histology specific) were also identified specifically for head and neck, lung, genitourinary and hematological malignancies and to a lesser extent in other sites (Table 2). Some papers dealt more generally with single center experiences which also provided some guidelines while others reported on testing for COVID 19 in cancer patients undergoing or about to undergo radiation therapy (Table 1). Finally, there were also papers that were more specifically targeting the elderly patient with cancer, palliative management and other smaller topics (Table 4).
Logistical and Operational Focused Publications
Logistics and Operational Considerations – Limit the Risk of Infection
From a practical day to day perspective the ability to effectively manage new logistical and operational challenges in order to mitigate risk of infection to patients and staff with need of frequent adaptation, poses one of the greatest challenges to RT in the context of COVID-19 [5-7]. In the absence of both data and homogenous higher-level guidelines, cancer therapy centers and individual radiation therapy departments have created their own guidelines and this is reflected in the publications uncovered in this review. Important insights originate in virus epicenters like Italy [8], New York in the US [9,10]. According to a survey by Opperman et al., who conducted an online survey among medical physicists in Germany, Austria and Switzerland from March 23rd to 26th 2020, 72.4% of the respondents stated that their processes were affected due to COVID-19, with longer processing times (54.2%), patient no-shows (42.5%) and staff reduction (36.7%). 75.8% expected further unavailability of their personnel in the upcoming weeks [11].
COVID-19 Testing in Cancer Patients Undergoing RT – Address Testing Practices
Another challenge is that of the selection, timing and actions taken with respect to patient and staff testing for COVID-19. As noted with respect to other aspects of the management of the pandemic, guidelines are lacking and complex standard of care practices had to be rapidly adapted to include patient (and staff) testing when appropriate [12]. This is particularly complex when patients may need to undergo brachytherapy or radioactive iodine and carry significant risk on infecting other staff if they themselves are infected and/or pose significant logistical challenges eg. Isolation as in or outpatient in the case of radioactive iodine administration. We now understand that testing negative for COVID carries a not in-insignificant possibility that the patient may have a false negative test and that the timing of the test is also impacting on its usefulness and accuracy. Testing itself is not readily available in many jurisdictions and may take some time to obtain results. Patients have to be pre-screened and directed to undergo testing, which may be difficult or impossible. However, data does support the notion that a positive result may render cancer patients more vulnerable to COVID-19 [4,13]. Bajaj et al. report on the salivary detection of SARS-CoV-2 (COVID-19) and implications for oral health-care providers summarizing guidelines for oral care specialists [14]. This literature review notes that salivary specimens have a higher than 90% concordance rate with nasopharyngeal specimens which while enabling PCR testing of salivary samples also reveals that saliva poses additional risk to health care providers. Since there is currently no data available to assess the risk of transmission of COVID-19 in dental practices, and since cancer patients in particular with head and neck primaries require dental interventions, this is an area where further data is required to optimize management and outcomes. Madriaga et al. reviewed the literature for COVID-19 testing in cancer patients and describe the approach to COVID-19 testing adopted in a large cancer center in Toronto [15].
Indications for/Modification of RT – Modify RT When Possible to Mitigate Risk
Parashar et al. from a tertiary cancer center in New York provides a general set of robust guidelines for curative RT in the context of the pandemic echoing other site-specific papers and setting some “ground rules” for this approach while summarizing alternative dose and fractionation options for each tumor site that may be employed in the context of COVID-19 to minimize risk to patients and staff [16]. They discuss the scenarios of RT as an alternative to surgery when immediate surgery is not possible, RT as a ‘bridge’ to surgery and radiation options as an alternative to chemotherapy given the risk of hospitalization with high-dose chemotherapy. It should be noted that while enrollment of patients on clinical trials is considered in this paper and would be preferable when fractionation schemes with lesser evidence are employed or patients are documented COVID-19 positive, in practical terms this is often curtailed as clinical trials are on hold in many centers due to the pandemic. Vordermark also provides a review of organ-specific cancer management [17]. In this publication the author searched for multidisciplinary and expert recommendations to guide potential shift in RT indications and found limited data as of April 2020 when it was published however provides a good summary of the available data at that point. Chen et al. and Franco et al. provide broader frameworks for prioritization of patients [6,18]. Franco et al. also provides guidelines for the management of patients with COVID-19 [18].
Brachytherapy
Williams et al. provide a thorough review of the impact of delaying or prolonging brachytherapy treatment courses in multiple disease sites including gynecological sites, prostate and breast [19]. While the timing and duration of brachytherapy is highly sensitive in sites like cervix and vaginal cancer, breast and in particular prostate may allow for some postponement of brachytherapy. Additional alternative fractionation schemes are also discussed for each cancer site. Aghili et al. review brachytherapy guidelines in the context of COVID-19 highlighting the need to consider the best regimens as opposed to discontinuation or postponement of brachytherapy [20].
Oncology Site Specific Publications and Consensus Guidelines
Head and Neck Cancers
Head and neck cancer patients are vulnerable to COVID-19 because in addition to the cancer diagnosis they may also share other risk factors (smoking, nutritional depletion, swallowing and/ or breathing dysfunction) [21,22]. In addition, many head and neck primaries are rapidly progressive causing significant clinical deterioration and often requiring hospital admission for nutritional support and refeeding syndrome. Delay in diagnosis is particularly detrimental [23] and in the case of nasopharyngeal cancer additional chemotherapy could be employed to counteract the delay in diagnosis [23]. Head and neck cancers also require significant PPE due to the diagnoses requiring examination under anesthesia or direct laryngoscopy with biopsy [24]. Thomson et al. provide the ASTRO-ESTRO consensus to risk adapted head and neck cancer RT [25]. Two pandemic scenarios: early (risk mitigation) and late (severely reduced radiation therapy resources), were evaluated and a panel of experts developed treatment recommendations for 5 HNC cases. They evaluated potential symptomatic benefit with the risk of active COVID-19 infection balancing potential for cure and risk of progression as well as patient fitness to recommended rational patient triage. With respect to interventions in the upper aerodigestive tract region (eg. rhinoscopy or flexible laryngoscopy in the outpatient setting and tracheostomy or rigid endoscopy under anesthesia), it is recommended that all health care personnel wear personal protective equipment such as N95, gown, cap, eye protection, and gloves [26]. Additional guidelines are provided by Werner et al. and Mehanna et al. [24,27].
Breast Cancer
Breast cancer patients make up a large proportion of the patients on treatment and follow-up in most cancer treatment centers and therefore a robust approach to risk stratification is crucial to ensure adequate access to care and diminish the risk of adverse outcomes while minimizing risk of COVID-19 infection. Curigliano et al. creates a framework on how to approach these using scenarios often encountered in clinical practice [28]. The use of primary systemic therapy is also discussed as an alternative to upfront surgery in the context of COVID-19. RT is prioritized according to the risk categorization of the cancer and whether the patient is already on treatment. Palliative treatments and acute spinal cord compression are considered urgent, followed by high risk patients while postoperative RT for low risk patients and post treatment visits are of lower priority. Additional recommendations include the omission of boost RT and accelerated partial breast RT for low risk patients. It is recognized that patients receiving chemotherapy regimens with intermediate/ high risk of immunosuppression, such as anthracyclines, 3-weekly docetaxel or 3 weekly platinum are at intermediate or high risk of immunosuppression. Vuagnat et al. set up a prospective registry for 15600 patients actively treated for early or metastatic breast cancer in the last 4 months [29]. They found that the COVID-19 mortality rate depended more on the comorbidities prior to RT that the treatment itself. Other recommendations are also provided by Chan et al. and Braunstein et al. but patient outcome data is still lacking [30,31].
Lung Cancer
Lung cancer patients pose a uniquely challenging scenario in the context of COVID-19 in part because they are already vulnerable to lung infections and in the context of RT because they are at risk for radiation pneumonitis which can be challenging to distinguish clinically and radiographically [32] but is also treated with high dose steroids which may worsen COVID-19 related lung injury. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic are provided in an ESTRO-ASTRO consensus statement by Guckenberger et al. Singh et al. and Dingemans et al. also make recommendations on standardizing the care of lung cancer patients during COVID-19 while recommending that general standard principles of practice be followed [33-35]. Wu et al. provide guidelines for thoracic radiation therapy specifically while Kumar et al. discusses alternative management options [36,37].
Genito-Urinary
Delaying treatment in genito-urinary cancers is potentially of significant detriment [38]. In penile cancer, surgery should proceed when possible due to the aggressive nature of the disease, with RT as an organ preserving approach [39]. Zaorsky et al. provide RT recommendations for prostate cancer [40]. However, since prostate cancer is a more indolent malignancy in early stages, treatment can be avoided or delayed for very low, low, and favorable intermediate- risk disease For unfavorable intermediate-risk, high-risk, clinical node positive, recurrence post-surgery, oligometastatic, and low- volume M1 disease neoadjuvant hormone therapy for 4-6 months was recommended [40]. Ultrahypofractionation may be preferred for localized, oligometastatic, and low volume M1, and moderate hypofractionation may be preferred for post-prostatectomy and clinical node positive disease. Postoperatively, salvage RT is preferred to adjuvant radiation [40]. Short fractionation RT for early prostate cancer is at the forefront of the field and is discussed in the context of COVID-19 by Barra et al. [41].
Central Nervous System Cancers
In the context of central nervous system cancers, the unique aspects include the often older age of the patients and co-existing neurological symptoms requiring ongoing steroid use to decrease increased intracranial pressure. In addition to these, the prognosis is often guarded, and advanced care planning will be more important than ever to address upon diagnosis and initiation of management. Guidelines are provided by Mohile et al. [42]. These include mitigating risk through social distancing and discussions of goals of care (considering that ventilator use may unfortunately be denied to some of these patients considering that high grade glioma such as glioblastoma will be considered a terminal diagnosis particularly in the elderly). Nonetheless maximal safe resection is recommended both to decrease intracranial pressure but also to improve longevity and diminish steroid use. In lower grade glioma prognosis may be far superior and thus some interventions may be deferred. Tabrizi et al. extracted patient level data from 1321 elderly glioblastoma patients to provide a quantitative framework for modelling COVID-19 risk using published randomised trials in the elderly with glioblastoma [43]. They support hypofractionated RT and increased utilization of temozolomide alone in patients with MGMT methylation when the risk of COVID-19 infection is high. With respect to WHO grade III and IV gliomas Bernhardt et al. combined the opinion of 6 international experts in a consensus-based practice recommendation including neuro-oncologists, neurosurgeons, radiation -oncologists and a medical physicist [44]. Overall agreement was had that treatment cannot be significantly delayed and initiating therapy should not be outweighed by COVID-19.
Hematological Cancers
Patients with hematological cancers are vulnerable to COVID-19 as they may suffer for cytopenias and may be immunosuppressed. Yahalom et al. offer guidelines to potentially omit RT in order to decrease the risk of exposure as a result of daily attendance to the cancer center [45]. They recommend possible omission of RT in: palliative settings where alternatives can be offered, for completely excised localized low-grade lymphomas and localized nodular lymphocyte- predominant Hodgkin lymphoma and for consolidation RT for diffuse large B-cell lymphoma/aggressive non-Hodgkin lymphoma (NHL) in patients who have completed a full chemotherapy course and achieved a complete remission [45]. Some lymphomas can safely delay RT (eg. asymptomatic localized low-grade lymphomas, localized nodular lymphocyte-predominant Hodgkin lymphoma, patients who develop COVID-19 infection prior to commencing RT), while others can benefit from shortened RT courses (eg. 20 Gy in 10 fractions or 30.6 Gy in 17 fractions). A consensus statement is available from Di Ciaccio et al. and Kirova et al. [46,47].
Other Cancer Subtypes
Guidelines have been published for pancreatic cancer [48], representing the UK consensus position. Endoscopy is recommended to continue for malignant biliary obstruction, however as it is an aerosol-generating procedure it is recommended that all elective and non-essential endoscopic procedures not be performed. Chemotherapy is suggested when surgery is not possible in the context of COVID-19 as are hypofractionated RT approaches (eg. 5-15 fraction regimens). Skin cancer management and triage was discussed by Baumann et al. and Tagliaferri et al. [49,50]. For patients with Merkel cell carcinoma, the authors recommend prioritizing treatment, unless favorable T1 disease. For patients with melanoma, the authors recommend delaying the treatment of patients with T0 to T1 disease for 3 months if there is no macroscopic residual disease at the time of biopsy. Treatment of tumors ≥T2 can be delayed for 3 months if the biopsy margins are negative. For squamous cell carcinoma, early disease can have treatment delayed for 2 to 3 months unless there is rapid growth, symptomatic lesions, or the patient is immunocompromised. The treatment of tumors ≥T2b should be prioritized, but a 1-month to 2-month delay is considered acceptable. For squamous cell carcinoma in situ and basal cell carcinoma, treatment can be deferred for 3 months unless symptomatic [49]. With respect to gynecological cancers Martinelli et al. carried out a survey showing that responders prioritized treatment of early stage high-risk uterine cancers (45%), newly diagnosed epithelial ovarian cancer (41%), and locally advanced cervical cancer (41%) [51]. 77% of respondents reported no changes in surgical treatment for early stage cervical cancer in COVID-19- negative patients, but treatment was postponed by 54% if the patient tested COVID-19-positive. Responders also considered neoadjuvant chemotherapy for advanced ovarian cancers and hypofractionation of RT for locally advanced cervical cancers. A similar survey was carried out by Nakayama et al. Rossi et al. report on their early experience in the management of sarcoma with priority given to bone and soft tissue sarcomas, metastases and aggressive benign tumors at risk of impending or pathologic fracture. For these and other sites further publications are as yet lacking [52,53].
Single Center Experiences
Single center experiences provide an “on the ground” perspective of the transformative experience COVID-19 has had on radiation oncology practice and health care resources and can provide an avenue for practical guidelines. Several papers exemplify this in particular in areas that were/are epicenters for the disease. Tey et al. provides a workflow for the COVID-19 positive patient on RT [54]. Chen et al. reports from a multicenter New York area institution with experience- based guidelines for disease sites that require concurrent chemo- irradiation and thus were more likely to result in patient presentation to the emergency room or in hospital admission [6]. The priority framework in this publication implemented as of April 13, 2020 is extremely practical in that it addresses efficiency from a systemic standpoint to optimize care for all patients within a RT department. Three priority levels are described; the first for cases where delay may result in loss of life, progression of disease or permanent loss of neurologic or other function (oncologic emergencies, advanced head and neck, gastrointestinal, gynecologic and lung cancers); priority 2 for cases that may be delayed for up to 4 weeks (early stage head and neck, lung and lymphoma, benign central nervous system cases) and priority 3 for cases that may be delayed for 30 days or more (early prostate, breast or prostate already on androgen deprivation therapy). This publication also presents patient data and addresses approaches to toxicity management in the context of COVID-19. Press et al. also described a single institution experience from a proton center in Manhattan quantifying the impact of treatment delays and interruptions [9]. Chhabra et al. also provide recommendations for prioritization of proton patient in the New York Proton Center [55]. Additional radiation oncology center experiences are published by Tan et al. (Singapore), Montesi et al. (Italy), Wu et al. (Wuhan), Handoko et al. (Indonesia), Mishra et al. (New York) [8,56-59].
Specific Considerations
The Elderly: Desideri et al. provides a very good summary of the data surrounding COVID-19 and the elderly [60]. Freedman et al. report specifically on managing older adults with breast cancer noting appropriately that considerations for management are highly relevant “within the new normal” considering that 30% of breast cancer patients are 70 years old or older [61]. They provide options for the most commonly encountered scenarios within the framework of existing evidence. Interestingly they also recommend deferral of routine follow-up and routine breast imaging and anticipate that this postponement will prompt discussion of the limited utility of these measures beyond the pandemic, as will no doubt be the case for other low value interventions. Asokan et al. provide a review of the impact of COVID-19 on the cardio-oncology population [62]. This is a population that also includes elderly patients with pre- existing cardiac comorbidities and possibly additional cardiotoxic insults such as chemotherapy and/or radiation or systemic treatment such as androgen deprivation therapy. Data surrounding the risk of COVID-19 infection and outcomes in this population is currently lacking.
Palliative RT: Yerramilli et al. provide a review of palliative RT for oncologic emergencies with emphasis on balancing risk and benefit [63]. Palliative treatments make up a large proportion of the workload of the RT department and the patients who require palliative RT often require it within days if not hours. This patient population is particularly vulnerable to the impact of the pandemic in a resource strained environment. Yerramilli et al. provides a framework for the triaging a patient with an oncologic emergency which is not dissimilar from frameworks already employed in resources strained environments [63]. Patients who are symptomatic and/or have an oncologic emergency and a more prolonged life expectancy are recommended to receive short course palliative RT, delay of RT or best supportive in the case of limited life expectancy is otherwise recommended. Thureau et al. in their GEMO (the European Study Group of Bone Metastases) position paper, astutely note that the indications and treatment modalities for palliative bone metastases must be re-discussed in the context of COVID-19 [64-67]. Palliative treatments often require the most clinical judgement and can benefit the most from available evidence surrounding reduction in the number of treatments and the complexity thereof in a resource strained setting. Their recommendations to carry out simulation planning and treatment at the same time as the consult, carry out tele- consults when appropriate, and use existing criteria to assess bone instability to allow for optimization of decision making enabling the least invasive technique. They also provide guidelines for retreatment of bone metastasis, spinal cord compression and SBRT (Stereotactic Body Radiation Therapy) for which they consider the level of evidence too low to be considered in the current situation.
Conclusions
Although multiple attempts at guideline and consensus generation have been made with respect to RT administration in the context of the COVID-19 pandemic in several tumor sites, evidence for the effectiveness or adequacy of these is lacking and some cancer sites have as yet very little or no guidelines. This is equally so the case with respect to the utilization of altered fractionation schemes being potentially proposed to diminish patient visits to cancer centers which should likely be approached with some caution and emphasis on following standard of care practice whenever possible. Several frameworks have been published for the optimization of logistics and operational planning that may be employed in radiation therapy centers and departments. Over time it is likely that more data will become available with respect to patient management and outcome, however as of July 2020, very few small retrospective data sets are available with respect to the outcomes of COVID-19 positive cancer patients undergoing RT. It is as yet unclear to what extent adverse outcomes in cancer patients may be related to preexisting comorbidities, the cancer diagnosis and its implications or the treatment of the cancer itself. Additional areas where evidence is lacking include:
1) The impact on of COVID-19 on older patients with cancer
2) The impact of treatment delay in patients currently considered intermediate or low risk for tumor progression
3) The impact of altered fractionation schedules
4) The long and short-term psychological impact of COVID-19 and altered cancer management on patients and staff.
Declarations
Ethics approval and consent to participate: This study is a literature review and does not report on data collected from humans and is exempt from ethics approval.
Consent for publication: Not applicable.
Availability of data and material: The data supporting the conclusions of this article are included within the article as references.
Competing Interests: The author declares that they have no competing interests.
Authors’ contributions: AVK conceived the idea for the review, reviewed the literature, created the accompanying material, and wrote the manuscript.
References
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, Coomes EA, Chemaly RF, et al. (2020) A Practical Approach to the Management of Cancer PatientsDuring the Novel Coronavirus Disease 2019 (COVID-19) Pandemic: An InternationalCollaborative Group. Oncologist 25:e936-e945. [crossref]
- Shankar A, Saini D, Roy S, MosaviJarrahi A, Chakraborty A, et al. (2020) Cancer Care Delivery Challenges Amidst Coronavirus Disease- 19 (COVID-19) Outbreak: Specific Precautions for Cancer Patients and CancerCare Providers to Prevent Spread. Asian Pac J Cancer Prev 21:569-573. [crossref]
- Anderson N, Thompson K, Andrews J, Chesson B, Cray A, et al. (2020) Planning for a pandemic: Mitigating risk toradiation therapy service delivery in the COVID-19 era. J Med Radiat Sci. [crossref]
- Zhang H, Wang L, Chen Y, Wu Q, Chen G, et al. (2020) Outcomes of novel coronavirus disease 2019 (COVID-19)infection in 107 patients with cancer from Wuhan, China. Cancer. [crossref]
- Mukherji A, Gupta T, Agarwal JP (2020) Time, distance, shielding and ALARA; drawing similarities between measures for radiation protection and Coronavirusdisease pandemic response. Indian J Cancer 57:221-223. [crossref]
- Chen W, Su XY, Wang VJ, Wang EC, Xu R, et al. (2020) Novel Coronavirus International Public Health Emergency: Guidance on Radiation Oncology Facility Operation. AdvRadiat Oncol. [crossref]
- Krengli M, Ferrara E, Mastroleo F, Brambilla M, Ricardi U (2020) Running a Radiation Oncology Department at the time of coronavirus: an Italian experience. AdvRadiat Oncol. [crossref]
- Montesi G, Di Biase S, Chierchini S, Pavanato G, Virdis GE, et al. (2020) Radiotherapy during COVID-19 pandemic. How to create a No fly zone: a Northern Italy experience. Radiol Med 125:600-603. [crossref]
- Press RH, Hasan S, Chhabra AM, Choi JI, Simone CB (2020) Quantifying the Impact of COVID-19 on Cancer Patients: A Technical Report of Patient Experience During the COVID-19 Pandemic at a High-volume Radiation Oncology Proton Center in New York City. Cureus 12:e7873. [crossref]
- Chen WC, Teckie S, Somerstein G, Adair N, Potters L (2020) Guidelines to Reduce Hospitalization Rates for Patients Receiving Curative-Intent Radiation Therapy During the COVID-19 Pandemic: Report from a Multicenter New York Area Institution. AdvRadiat Oncol. [crossref]
- Reuter-Oppermann M, Müller-Polyzou R, Wirtz H, Georgiadis A (2020) Influence of the pandemic dissemination of COVID-19 on radiotherapy practice: A flash survey in Germany, Austria and Switzerland. PLoS One 15:e0233330. [crossref]
- Perni S, Milligan MG, Saraf A, Vivenzio T, Marques A, et al. (2020) Treating the SARS-CoV-2-positive patient with cancer: A proposal for a pragmatic and transparent ethical process. Cancer. [crossref]
- Ning MS, McAleer MF, Jeter MD, Minsky BD, Ghafar RA, et al. (2020) Mitigating the impact of COVID-19 on oncology: Clinical and operational lessons from a prospective radiation oncology cohort tested for COVID-19. Radiother Oncol148:252-257. [crossref]
- Bajaj N, Granwehr BP, Hanna EY, Chambers MS (2020) Salivary detection of SARS-CoV-2 (COVID-19) and implications for oral health-care providers. Head Neck 42:1543-1547. [crossref]
- Madariaga A, McMullen M, Sheikh S, Kumar R, Liu F, et al. (2020) COVID-19 testing in cancer patients: Does one size fit all?.Clin Cancer Res.
- Parashar B, Chen WC, Herman JM, Potters L (2020) Disease Site-Specific Guidelines for Curative Radiation Treatment During ‘Limited Surgery’ and ‘Hospital Avoidance’: A Radiation Oncology Perspective From the Epicenter of COVID-19 Pandemic. Cureus 12:e8190. [crossref]
- Vordermark D (2020) Shift in indications for radiotherapy during the COVID-19 pandemic? A review of organ-specific cancer management recommendations from multidisciplinary and surgical expert groups. Version 2. Radiat Oncol 15:140.
- Franco P, Kochbati L, Siano M, De Bari B (2020) Suggestions for Radiation Oncologists during the COVID-19 Pandemic. Biomed Res Int2020:4892382. [crossref]
- Williams VM, Kahn JM, Harkenrider MM, Chino J, Chen J, et al. (2020) COVID-19 impact on timing of brachytherapy treatment and strategies for risk mitigation. Brachytherapy 19:401-411. [crossref]
- Aghili M, Jafari F, VandRajabpoor M (2020) Brachytherapy during the COVID-19- Lessons from Iran. Brachytherapy 19:412-414. [crossref]
- Silverman DA, Lin C, Tamaki A, Puram SV, Carrau RL, et al. (2020) Respiratory and pulmonary complications in head and neck cancer patients: Evidence-based review for the COVID-19 era. Head Neck 42:1218-1226. [crossref]
- Civantos AM, Carey RM, Lichtenstein GR, Lukens JN, Cohen RB, et al. (2020) Care of immunocompromised patients with head and neck cancer during the COVID-19 pandemic: Two challenging and informative clinical cases. Head Neck 42:1131-1136. [crossref]
- Yang Y, Shen C, Hu C (2020) Effect of COVID-19 Epidemic on Delay of Diagnosis and Treatment Path for Patients with Nasopharyngeal Carcinoma. Cancer Manag Res12:3859-3864. [crossref]
- Werner MT, Carey RM, Albergotti WG, Lukens JN, Brody RM (2020) Impact of the COVID-19 Pandemic on the Management of Head and Neck Malignancies. Otolaryngol Head Neck Surg 162:816-817. [crossref]
- Thomson DJ, Palma D, Guckenberger M, Balermpas P, Beitler JJ, et al. (2020) Practice Recommendations for Risk-Adapted Head and Neck Cancer Radiation Therapy During the COVID-19 Pandemic: An ASTRO-ESTRO Consensus Statement. Int J Radiat Oncol BiolPhys 107:618-627. [crossref]
- Kowalski LP, Sanabria A, Ridge JA, Ng WT, de Bree R, et al. (2020) COVID-19 pandemic: Effects and evidence-based recommendations for otolaryngology and head and neck surgery practice. Head Neck 42:1259-1267. [crossref]
- Mehanna H, Hardman JC, Shenson JA, Abou-Foul AK, Topf MC, et al. (2020) Recommendations for head and neck surgical oncology practice in a setting of acute severe resource constraint during the COVID-19 pandemic: an international consensus. Lancet Oncol 21:e350-e359. [crossref]
- Curigliano G, Cardoso MJ, Poortmans P, Gentilini O, Pravettoni G, et al. (2020) Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast52:8-16. [crossref]
- Vuagnat P, Frelaut M, Ramtohul T, Basse C, Diakite S, et al. (2020) COVID-19 in breast cancer patients: a cohort at the Institut Curie hospitals in the Paris area. Breast Cancer Res 22:55.
- Chan JJ, Sim Y, Ow SGW, Lim JSJ, Kusumawidjaja G(2020) COVID-19: impact on and recommendations for breast cancer care: the Singapore experience. EndocrRelat Cancer.
- Braunstein LZ, Gillespie EF, Hong L, Xu A, Bakhoum SF (2020) Breast radiotherapy under COVID-19 pandemic resource constraints — approaches to defer or shorten treatment from a Comprehensive Cancer Center in the United States. AdvRadiat Oncol. [crossref]
- Shaverdian N, Shepherd A, Rimner A, Wu AJ, Simone CB 2nd, et al. (2020) Need for Caution in the Diagnosis of Radiation Pneumonitis in the COVID-19 Pandemic. AdvRadiat Oncol. [crossref]
- Guckenberger M, Belka C, Bezjak A, Bradley J, Daly ME, et al. (2020) Practice Recommendations for Lung Cancer Radiotherapy During the COVID-19 Pandemic: An ESTRO-ASTRO Consensus Statement. Int J Radiat Oncol BiolPhys 107:631-640. [crossref]
- Singh AP, Berman AT, Marmarelis ME, Haas AR, Feigenberg SJ, et al. (2020) Management of Lung Cancer During the COVID-19 Pandemic. JCO Oncol Pract.
- Dingemans AC, Soo RA, Jazieh AR, Rice SJ, Kim YT, et al. (2020) Treatment Guidance for Patients With Lung Cancer During the Coronavirus 2019 Pandemic. J Thorac Oncol 15:1119-1136. [crossref]
- Wu AJ, Rimner A, Shepherd AF, Gelblum DY, Shaverdian N, et al. (2020) Thoracic radiation therapy during COVID-19: provisional guidelines from a comprehensive cancer center within a pandemic epicenter. AdvRadiat Oncol. [crossref]
- Kumar S, Chmura S, Robinson C, Lin SH, Gadgeel SM, et al. (2020) Alternative Multidisciplinary Management Options for Locally Advanced NSCLC During the Coronavirus Disease 2019 Global Pandemic. J Thorac Oncol 15:1137-1146. [crossref]
- Wallis CJD, Novara G, Marandino L, Bex A, Kamat AM, et al. (2020) Risks from Deferring Treatment for Genitourinary Cancers: A Collaborative Review to Aid Triage and Management During the COVID-19 Pandemic. EurUrol 78:29-42. [crossref]
- Casco NC, Carmona MJ, Soto ÁJ (2020) Therapeutic and Surgical Indications for Patients with Penile Cancer in the COVID-19 era. IntBraz J Urol 46:86-92. [crossref]
- Zaorsky NG, Yu JB, McBride SM, Dess RT, Jackson WC, et al. (2020) Prostate Cancer Radiotherapy Recommendations in Response to COVID-19. AdvRadiat Oncol. [crossref]
- Barra S, Guarnieri A, di Monale E Bastia MB, Marcenaro M, Tornari E, et al. (2020) Short fractionation radiotherapy for early prostate cancer in the time of COVID-19: long-term excellent outcomes from a multicenter Italian trial suggest a larger adoption in clinical practice. Radiol Med 1-5. [crossref]
- Mohile NA, Blakeley JO, Gatson NTN, Hottinger AF, Lassman AB, et al. (2020) Urgent Considerations for the Neuro-oncologic Treatment of Patients with Gliomas During the COVID-19 Pandemic. Neuro Oncol. 22:912-917. [crossref]
- Tabrizi S, Trippa L, Cagney D, Tanguturi S, Ventz S (2020) A Quantitative Framework for Modeling COVID-19 Risk During Adjuvant Therapy Using Published Randomized Trials of Glioblastoma in the Elderly. Neuro Oncol. [crossref]
- Bernhardt D, Wick W, Weiss SE, Sahgal A, Lo SS, et al. (2020) Neuro-oncology Management During the COVID-19 Pandemic With a Focus on WHOGrade III and IV Gliomas. Neuro Oncol. [crossref]
- Yahalom J, Dabaja BS, Ricardi U, Ng A, Mikhaeel NG, et al. (2020) ILROG emergency guidelines for radiation therapy of hematological malignancies during the COVID-19 pandemic. Blood 135:1829-1832. [crossref]
- Di Ciaccio P, McCaughan G, Trotman J, Ho PJ, Cheah CY, et al. (2020) Australian and New Zealand consensus statement on the management of lymphoma, chronic lymphocytic leukaemia and myeloma during the COVID-19 pandemic. Intern Med J 50:667-679. [crossref]
- Kirova Y (2020) Guide pratique pour la radiothérapie des hémopathiesmalignesdans la situation d’épidémie de COVID-19 :recommandations de l’InternationalLymphomaRadiationOncologyGroup [Practical guidelines for the radiotherapy for patients presented with haematological malignancies in the epidemic COVID-19 situation: International Lymphoma Radiation Oncology Group recommendations]. Cancer Radiother 24: 194-195. [crossref]
- Jones CM, Radhakrishna G, Aitken K, Bridgewater J, Corrie P, et al. (2020) Considerations for the treatment of pancreatic cancer during the COVID-19 pandemic: the UK consensus position. Br J Cancer. 8:1-5. [crossref]
- Baumann BC, MacArthur KM, Brewer JD, Mendenhall WM, Barker CA, et al. (2020) Management of primary skin cancer during a pandemic: Multidisciplinary recommendations. Cancer.
- Tagliaferri L, Di Stefani A, Schinzari G, Fionda B, Rossi E, et al. (2020) Gemelli Skin-Cancer Multidisciplianry Tumour Board (S-MDTB). Skin cancer triage and managementduring COVID-19 pandemic. J EurAcadDermatolVenereol 34:1136-1139. [crossref]
- Martinelli F, Garbi A (2020) Change in practice in gynecologic oncology during the COVID-19 pandemic: a social media survey. Int J Gynecol Cancerijgc-2020-001585. [crossref]
- Nakayama J, El-Nashar SA, Waggoner S, Traughber B, Kesterson J (2020) Adjusting to the new reality: Evaluation of early practice pattern adaptations to the COVID-19 pandemic. Gynecol Oncol. [crossref]
- Rossi B, Zoccali C, Baldi J, Scotto di Uccio A, et al. (2020) Reorganization Tips from a Sarcoma Unit at Time of the COVID-19 Pandemic in Italy: Early Experience from a Regional Referral Oncologic Center. J Clin Med 9:E1868. [crossref]
- Tey J, Ho S, Choo BA, Ho F, Yap SP, et al. (2020) Navigating the challenges of the COVID-19 outbreak: Perspectives from the radiation oncology service in Singapore. Radiother Oncol148:189-193. [crossref]
- Chhabra AM, Choi JI, Hasan S, Press RH, Simone CB 2nd (2020) Prioritization of Proton Patients in the COVID-19 Pandemic: Recommendations from The New York Proton Center. Int J Part Ther 6:38-44. [crossref]
- Tan BF, Tuan JKL, Yap SP, Ho SZ, Wang MLC (2020) Managing the COVID-19 Pandemic as a National Radiation Oncology Centre in Singapore. Clin Oncol(R CollRadiol) 32:e155-e159. [crossref]
- Wu Y, Wang J, Luo C, Hu S, Lin X (2020) A Comparison of Burnout Frequency Among Oncology Physicians and Nurses Working on the Frontline and Usual Wards During the COVID-19 Epidemic in Wuhan, China. J Pain Symptom Manage 60:e60-e65. [crossref]
- Handoko, Permata TBM, Giselvania A, Nuryadi E, Octavianus S, et al. (2020) Ensuring safety and sustainability of radiotherapy services during the COVID-19 pandemic in resources constrain country: An Indonesian experience. Radiother Oncol150:57-60. [crossref]
- Mishra KK, Afshar A, Thariat J, Shih HA, Scholey JE (2020) Practice considerations for proton beam radiotherapy of uveal melanoma during the COVID-19 pandemic: PTCOG Ocular experience. AdvRadiat Oncol. [crossref]
- Desideri I, Pilleron S, Battisti NML, Gomes F, de Glas N, et al. (2020) Caring for older patients with cancer during the COVID-19 pandemic: A Young International Society of Geriatric Oncology (SIOG) global perspective. J Geriatr Oncol. [crossref]
- Freedman RA, Sedrak MS, Bellon JR, Block CC, Lin NU, et al. (2020) Weathering the Storm: Managing Older Adults with Breast Cancer Amid COVID-19 and Beyond. J Natl Cancer Inst. [crossref]
- Asokan I, Rabadia SV, Yang EH (2020) The COVID-19 Pandemic and its Impact on the Cardio-Oncology Population. Curr Oncol Rep 22:60. [crossref]
- Yerramilli D, Xu AJ, Gillespie EF, Shepherd AF, Beal K, et al. (2020) Palliative Radiotherapy for Oncologic Emergencies in the setting of COVID-19: Approaches to Balancing Risks and Benefits. AdvRadiat Oncol. [crossref]
- Thureau S, Faivre JC, Assaker R, Biver E, Confavreux CB, et al. (2020) Adapting palliative radiation therapy for bone metastases during the Covid-19 pandemic: GEMO position paper. J Bone Oncol100291. [crossref]
- Venkatesulu BP, Chandrasekar VT, Girdhar P, Advani P, Sharma A, et al. (2020) A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. medRxiv.[crossref]
- Pezzulla D, Macchia G, Taccari F, Sticca G, Deodato F (2020) Radiotherapy in Southern Italy at the time of COVID-19: options for radiation oncology units. Int J Gynecol Cancer 30:917-919. [crossref]
- Wu F, Song Y, Zeng HY, Ye F, Chen B, et al. (2020) [Discussion on diagnosis and treatment of hepatobiliary malignancies during the outbreak of COVID-19]. ZhonghuaZhong Liu ZaZhi 42:187-191. [crossref]