Abstract
Severe acute respiratory syndrome (SARI) is a compulsory notification disease. The new molecular biology-based diagnostic methodologies have increased sensitivity for respiratory viruses (RVs) identification, evidencing their representativeness in SARI. Usually, they account for 22% of community-acquired pneumonias in adults. However, the epidemiology and burden of these infections in adults with SARI remain unclear. This study aimed to determine the clinical, epidemiological, risk factors and mortality rate of hospitalized adults with SARI, based on virus positivity. This descriptive, cross-sectional study included patients with SARI aged over than 14 years who were hospitalized from 2010 to 2016. A total of 592 cases were identified, of which 295 (49.8%) had samples collected. A total of 113 (38.4%) patients tested positive for RVs, and most of them were infected with influenza viruses (30.1%). Death occurred in 27.5% vs. 17.7% (p=0.09) of patients in the negative and positive virus group, respectively. SARI is an important condition in hospitalized adults, and is caused predominantly by influenza. It is associated with high mortality, independent of its etiology, without significant difference among those infected by positive or negative viruses.
Keywords
respiratory infections, respiratory viruses, influenza, and epidemiologic surveillance
1. Introduction
Lower respiratory tract infections are the leading cause of global morbidity and mortality, accounting for 292 million cases and 2.7 million deaths in 2015 [1]. The incidence reaches to 24.8 cases/100, 000 adults and 164.3 cases/100, 000 elderly people over 80 years old [2]. In Brazil, respiratory diseases are the fourth cause of death, concentrating on the extremes of age and in population with low socioeconomic status [3]. Community-acquired pneumonia (CAP) are caused by respiratory viruses (RVs) in 22.4% of cases according to a meta-analysis [4], which is the main etiologic agent in adults hospitalized, as reported in a US population study [2]. Influenza viruses (IFV) are among the major etiologies of CAP in adults, and influenza A H1N1pdm has been associated with more severe cases [5].
RVs can occur through monoinfection or coinfection with other viruses or bacteria [6] and may lead to severe forms [7, 8]. Some clinical factors in CAP are mostly associated with viral infections such as rhinorrhea and ground-glass opacity on computed tomography [9], but clinical and radiological signs should not be used alone to determine the etiology, due to the overlap of findings with other etiological agents [10].
Multiplex PCR-based diagnostic methods have been used to investigate cases of severe acute respiratory infection (SARI) identified by influenza virus surveillance [11, 12]. This methodology allowed the identification of multiple viral pathogens. In addition, to the benefit of epidemiological knowledge, the identification of virus in a clinical sample makes it possible to diagnose the etiology, support control measures to prevent transmission, and, together with clinical and laboratory data, guide antibiotics stewardship [13].
The high potential for pathogenicity of FLUs, human parainfluenza viruses (HPIVs), human adenovirus (HAdV), human respiratory syncytial viruses (HRSVs), and human metapneumovirus (HMPV) is well recognized [14]. Although there are difficulties in interpreting the detection of other RVs such as human rhinovirus (HRV), human bocavirus (HBoV), human enterovirus (HEV), and human coronaviruses (HCoVs), although evidence showed that they are associated with the development of lower respiratory tract infections [15–17].
In this study, we evaluated the clinical and epidemiological aspects of SARI in hospitalized adults identified during influenza active surveillance, and compared the clinical findings and outcomes based on virus positivity.
2. Materials and Methods
A cross-sectional study was performed to provide information on the active surveillance of influenza in hospitalized patients. In Brazil, notification of SARI is mandatory, and in the Hospital de Clínicas Complex, a tertiary academic hospital in southern Brazil, an active surveillance is conducted to detect cases, notify and collect samples to investigate RVs. Clinical, laboratory, Charlson comorbidity index, and outcome data are updated in a specific form after discharge or death. The Ethics Committee for Institutional Research approved this study (#18714013.4.0000.0096).
To be included, eligible patients were identified in the influenza active surveillance and older than 14 years. The following data were evaluated: age, sex, race, comorbidities, Charlson comorbidity index score, number of intensive care unit admissions, use of mechanical ventilations, death, collection or non-collection of specimen to detect the presence of a RV, antiviral use, time of symptom onset, period of hospitalization, and initiation of antiviral therapy (when performed), number of virus isolated, and identification of the virus isolated in case of positivity. The distribution of positive cases was evaluated monthly, as well severe cases and deaths. Severe disease was considered in case of ICU hospitalization, mechanical ventilation, or death.
Viral laboratory investigation was carried out using a multiplex PCR technology (Seeplex® RV15 ACE Detection Kit, Seegene Inc., Korea), which enables the simultaneous detection of 15 RVs: HAdVs; HMPV; HPIVs types 1, 2, 3, and 4 (HPIV-1, HPIV-2, HPIV-3, and HPIV-4); IFVA subtypes H1N1pdm and H3N2; IFV type B; HRSVs types A and B (HRSVA and HRSVB); HRV types A, B, and C; HEV; HBoV; and HCoV types 229E/NL63 (alpha coronaviruses) and OC43/HKU1 (beta coronaviruses). Clinical findings and outcome between groups with positive and negative viruses were evaluated.
2.1 Statistical analysis
A statistical analysis was carried out using the program XLSTAT version 2018.2.50494. Baseline demographic and clinical characteristics with normal and non-normal distributions were presented as means ± standard deviation and medians with interquartile ranges, respectively. Fisher’s exact test, independent t-test, Wilcoxon-Mann‐Whitney test, and U tests were used where appropriate. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for the variables included. For the results in which the observed frequencies were less than 5, no statistical analysis was performed.. The level of significance was set at p<0.05. Time-to-event analyses (discharge or death) were performed using the Kaplan-Meier method.
3. Results
In the study period, a total of 1, 840 SARI cases were notified, of which 592 (32%) were from patients over 14 years old. Of these, only 295 (49.8%) patients were evaluated to detect RVs, and 113 (38.3%) of these cases were positive (Figure 1).
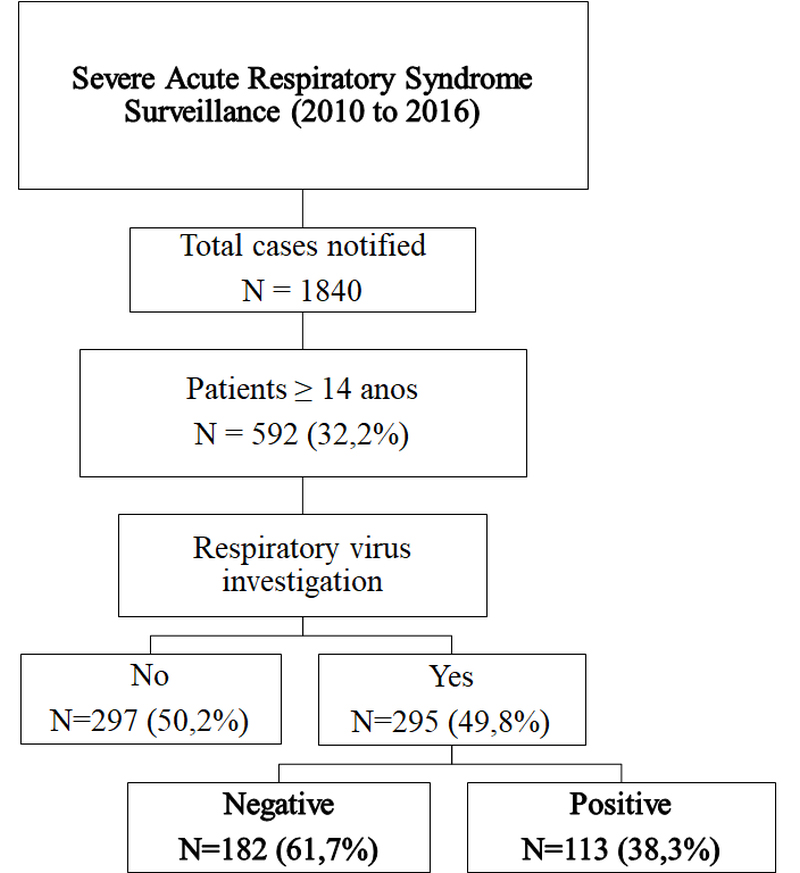
Figure 1. Flow chart of study design
Of the positive cases, influenza viruses were the most frequent pathogen (34 cases, 30.1%), and IFVA H1N1pdm represented more than half of IFVs samples (Fig 2B). HRV were present in 23.3%, HRSV in 13.8%, HPIV in 13%, HCoV in 7.2%, HAdV in 7.2%, HEV in 6.5% and HMPV in 4.3% of positive cases (Fig 2A). Monoinfection was detected in 89 cases (78.8%), and viral coinfection with 2 and 3 viruses were observed in 23 (20.4%) cases and 1 (0.9%) case, respectively.
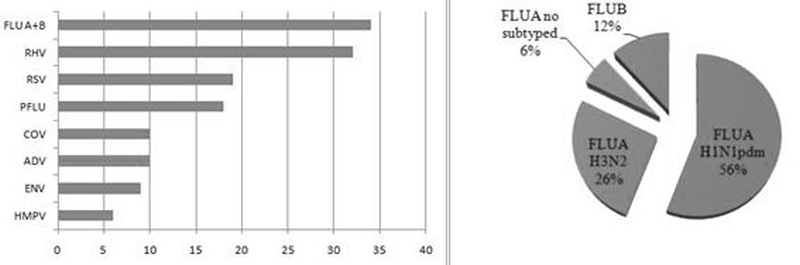
Figure 2. Respiratory viruses detected and influenza virus distribution
Patients whose samples were positive or negative for RVs had their demographic, clinical, and laboratory findings compared. No statistical difference was observed between both groups, except for the duration of symptoms until hospital admission that was higher in patients with negative results (p = 0.019). Mortality was also higher in patients with negative test results (27.5% vs. 17.7%, p = 0.09), but with no significant difference (Table 1).
Table 1. Demographic, clinical, and laboratory findings among positive and negative cases for respiratory viruses
|
Characteristics |
Total N = 295 (%) |
Positive virus N = 113 (%) |
Negative virus |
p-value |
|
Age – median (IQR) |
45.1 (30.8/60.3) |
43.7 (29.2/61.3) |
45.7 (33.1/59.6) |
0.521 |
|
>14–<=18 |
19 (6.5) |
7 (6.2) |
12 (6.6) |
NS |
|
> 18–<=50 |
152 (51.6) |
60 (53.1) |
92 (50.6) |
|
|
>50 |
124 (42.1) |
46 (40.8) |
78 (42.9) |
|
|
Gender |
||||
|
female |
135 (45.8) |
57 (50.5) |
78 (42.9) |
0.229 |
|
IFV vaccine last season |
60 (20.4) |
27 (23.9) |
33 (18.2) |
0.237 |
|
Signs/symptoms |
||||
|
Dyspnea |
287 (97.3) |
109 (96.5) |
178 (97.9) |
0.487 |
|
Desaturation (O2Sat <95%) |
192 (80.7) |
72 (78.3) |
120 (82.2) |
0.709 |
|
respiratory discomfort |
192 (85.4) |
75 (86.3) |
117 (84.8) |
0.801 |
|
Comorbidities (yes) |
228 (77.3) |
93 (82.4) |
135 (74.2) |
0.117 |
|
Pneumopathy |
93 (31.6) |
40 (35.4) |
53 (29.2) |
NS |
|
Cardiopathy |
53 (18.1) |
19 (16.9) |
34 (18.8) |
|
|
Immunodeficiency |
106 (36.1) |
47 (41.6) |
59 (32.6) |
|
|
Other comorbidities |
130 (44, 1) |
47 (41.6) |
83 (45.6) |
|
|
Pregnant/puerperium |
15 (5.1) |
9 (8) |
6 (3.3) |
NS |
|
Charlson comorbidity index score |
||||
|
0 |
89 (30.6) |
27 (24.4) |
62 (34.5) |
NS |
|
1–2 |
105 (36.1) |
49 (44.2) |
56 (31.2) |
|
|
3–4 |
68 (23.4) |
21 (19) |
47 (26.2) |
|
|
>=5 |
29 (10) |
14 (12.7) |
15 (8.4) |
|
|
Duration of symptoms until hospital admission – days (IQR) |
4 (1.5/7) |
3 (3/6) |
4 (2/7) |
0.019 |
|
Pulmonary X-ray |
||||
|
Normal |
30 (11.6) |
14 (15.3) |
16 (9.6) |
NS |
|
Interstitial pattern |
119 (46) |
42 (45.7) |
77 (46.2) |
|
|
Consolidation |
80 (30.9) |
27 (29.4) |
53 (31.8) |
|
|
Mixed pattern |
24 (9.3) |
12 (13.1) |
12 (7.2) |
|
|
Other |
22 (8.5) |
7 (7.7) |
15 (9) |
|
|
Severe disease |
196 (75, 7) |
69 (75) |
127 (76.1) |
0, 129 |
|
Mechanical ventilation |
130 (44.3) |
42 (37.5) |
88 (48.4) |
NS |
|
ICU |
189 (64.3) |
64 (57.2) |
125 (68.7) |
|
|
Death |
70 (23.8) |
20 (17.7) |
50 (27.5) |
IQR = interquartile range. NS = not significant. In bold = significant value
No significant difference was observed between patients infected with IFVs and those infected with other RVs (ORVs). Severe disease was found in 70.9% and 77% (p = 0.93) of cases and the mortality rate was 22.3% and 21% (p = 0.91), respectively. A Kaplan-Meier curve comparing monoinfection by IFV and ORV cases did not show a statistical difference (p = 0.91) (Fig 3).
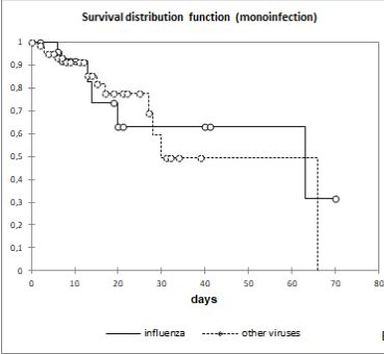
Figure 3. Kaplan-Meier curve comparing influenza vs. other respiratory virus infection
Comparing the clinical and laboratory findings of fatal and non-fatal cases, no significant differences were observed regarding presence of comorbidities, Charlson score and X-ray findings, only the identification of more than 1 virus were more common in non-fatal cases (10.3 vs. 1.5%, p = 0.02), although no signification association to a specific virus was observed (Table 2).
Table 2. Demographic, clinical, and laboratory findings of fatal and non-fatal cases
|
Characteristics |
Total cases with collected samples (N = 295) |
Fatal cases (N = 70) |
Non-fatal cases (N = 225) |
p-value |
|
Age – median (IQR) |
45.1 (30.8/60.3) |
47.9 (32.8/57.9) |
44.5 (29.9/60.9) |
0.477 |
|
Gender – Female |
135 (45.8) |
25 (35.8) |
110 (48.9) |
0.056 |
|
Influenza vaccine last season |
60 (20.4) |
8 (11.5) |
52 (23.2) |
0.046 |
|
Signs/symptoms |
||||
|
Dyspnea |
287 (97.3) |
68 (97.2) |
219 (97.4) |
1.000 |
|
Desaturation |
192 (80.7) |
47 (87.1) |
145 (78.9) |
0.774 |
|
Comorbidities (yes) |
228 (77.3) |
58 (82.9) |
170 (75.6) |
0.253 |
|
Virus result |
||||
|
Positive |
113 (38.4) |
20 (28.6) |
93 (41.4) |
0.067 |
|
IFV |
34 (11.6) |
6 (8.6) |
28 (12.5) |
0.520 |
|
IFV A H1N1pdm |
19 (6.5) |
3 (4.3) |
16 (7.2) |
NA*
|
|
IFV A H3N2 |
9 (3.1) |
1 (1.5) |
8 (3.6) |
|
|
IFV not subtyped |
2 (0.7) |
0 (0) |
2 (0.9) |
|
|
IFV B |
4 (1.4) |
2 (2.9) |
2 (0.9) |
|
|
HRV |
32 (10.9) |
5 (7.2) |
27 (12) |
NA* |
|
HRSV |
19 (6.5) |
2 (2.9) |
17 (7.6) |
|
|
HPIV |
18 (6.2) |
3 (4.3) |
15 (6.7) |
|
|
HAdV |
10 (3.4) |
1 (1.5) |
9 (4) |
|
|
HCoV |
10 (3.4) |
2 (2.9) |
8 (3.6) |
|
|
HEV |
9 (3.1) |
1 (1.5) |
8 (3.6) |
|
|
HMPV |
6 (2.1) |
1 (1.5) |
5 (2.3) |
|
|
Number of virus identified |
||||
|
1 |
89 (30.2) |
19 (27.2) |
70 (31.2) |
0.555 |
|
>1 |
24 (8.2) |
1 (1.5) |
23 (10.3) |
NA* |
In bold: significant values; IQR = interquartile range; NS = Not significant; NA = Not applicable; *Frequency < 5
4. Discussion
Due to the importance of RVs in SARI, high morbidity and mortality, and the capacity to cause outbreaks and pandemics, clinical and epidemiological studies must be conducted to improve RVs knowledge, mainly in developing countries [18]. As a result of the high representativeness of RVs in pediatrics, few data are specific for the adult population, especially in Brazil and Latin American countries. In this study we showed a high prevalence of respiratory viral infection among adult SARI patients.
In recent years, there has been an important improvement in the knowledge on the epidemiological aspects of the etiology of CAP. This probably occurred after the introduction of vaccines for Streptococcus pneumoniae and Haemophilus influenzae and, especially, access to new molecular diagnostic methods (PCR) [19, 20], which considerably increased the sensitivity for the identification of RVs. The notification of SARI in adults accounted for practically one-third of the cases. The active surveillance guarantees the identification of almost all cases, but only half of them had virus sampling performed due to the low adherence to epidemiological surveillance procedures. In this study, among the samples collected, 38.3% were positive for some RVs, similar to the incidence previously reported [21], and confirming the importance of viruses as the main cause of SARI in this population.
Comparing virus positive and negative cases, no clinical and demographic difference was found, nor in the proportion of severe cases and deaths, despite the increasing mortality among negative cases. Unfortunately, no systematic investigation has been performed for other etiologic agents. Bacterial infections are present in 14%–23% of patients with community-acquired pneumonia, but no etiologic agent was identified in 46%–62% of the cases [2, 22]. Therefore, in our study, some of the negative cases are probably bacterial infections, or even viral infections, which were not identified by the method used to evaluate the case. Furthermore, patients with negative results had a significant higher duration of symptoms until hospital admission, which could contribute for viral negative results.
Regarding the viruses identified, the importance of the influenza virus in the adult population studied was confirmed, being present in 11.6% of the SARI cases with virus investigation and in 30.1% of the positive cases for RVs. Similar this finding, the IFVs were the major pathogen associated with the development of community-acquired pneumonia in adults, according to a previous meta-analysis (4). However, the differences between the results of epidemiological studies on RVs may be related to geographic location and seasonal factors. In the present study, we decided to collect the data from 2010, following the 2009 pandemic, which was considered as an atypical year in relation to the clinical and epidemiological aspects of RVs infections.
Regardless of RVs detection (positive or negative), high lethality was found among cases of SARI (23.8%), reaching 34.9% between cases requiring ICU and 45.4% among those who required mechanical ventilation. Therefore, all hospitalized patients who met the SARI criteria, notwithstanding of etiology and presence or absence of comorbidities, should be considered as high risk for poor outcome.
Also, viral coinfected patients had higher mortality rates than monoinfected individuals (p<0.02), but the low frequency of viral monoinfection in the studied group hinders any conclusion. We have previously reported a similar finding after comparing patients with viral monoinfection and coinfection with bacteria, in which the detection of mixed respiratory pathogens is frequent in hospitalized patients with acute respiratory infections, but its impact on the clinical outcome does not appear substantial [23].
This study presents some limitations: (i) the low rate of RVs sampling performed among adult patients with SARI, (ii) and the failure to investigate other etiological agents that are possibly associated with SARI such as bacteria, fungi, and mycobacteria. (iii) In addition, the high proportion of patients with immunosuppression limits the extrapolation of these data to other populations. However, the frequency of respiratory viruses found in adults with SARI with percentage values above those previously reported should serve as an alert to the need for viral investigation in all cases of SARI in adults, seeking to know the impact of these infections on this age group
5. Conclusion
As a conclusion, for the population evaluated in the present study, respiratory viruses accounted for more than a third of cases of SARI in adults, being IFVs the main etiological agent. SARI is associated with high lethality, regardless of virus positivity, and presence of comorbidities. Surveillance measures should therefore be maintained and strengthened in adults, seeking to establish risk factors possibly associated with fatality rates and the etiologies of negative cases for RVs to identify emerging pathogens.
Conflict of Interests: Nothing to declare.
Acknowledgements: We would like to thank all epidemiological division staff who supported the routine collection and registration of data: Adeli Ribeiro P. Medeiros, Celia Targa, Cristina Garcia Beckert Batista, Fabiana Costa de Senna Ávila Farias, Juçara Maleoni de Oliveira, Lili Gonçalves, Monica Klimczuk Fernandes, Rosa Helena Silva Souza, and Suzana Dal-Ri Moreira.
We would also like to thank the following virology laboratory staff: Meri B. Nogueira, Luine R. Vidal, and Luciane A. Pereira
Authors Contributions: SMR participated to the conception/design of the study, supervised the analysis, interpreted the data, and wrote the manuscript. BMA participated to the conception of the study, analyzed the data and performed the statistical analysis. NS and MLP collected and analyzed the data. All authors provided contributions to the manuscript, and approved the final version.
Fundings: None
References
- Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Swartz S, et al. (2017) Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the global burden of disease study 2015. Lancet Infect Dis 17; 909–48.
- Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. (2015) Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 373, 415–27.
- Brasil, Ministério da Saúde. Protocolo de tratamento de influenza 2017. Available from http://bvsms.saude.gov.br/bvs/publicacoes/protocolo_tratamento_influenza_2017.pdf (accessed 02 June, 2018).
- Wu X, Wang Q, Wang M, Su X, Xing Z, Zhang W, Shi Y. (2015) Incidence of respiratory viral infections detected by PCR and real-time PCR in adult patients with community-acquired pneumonia: A meta-analysis. Respiration 2015; 89; 343–52. [Crossref]
- Qu JX, Gu L, Pu ZH, Yu XM, Liu YM, Li R, et al. (2015) Viral etiology of community-acquired pneumonia among adolescents and adults with mild or moderate severity and its relation to age and severity. BMC Infect Dis 15; 89. [Crossref]
- Musher DM, Thorner AR. (2014) Community-acquired pneumonia. N Engl J Med 371; 1619–28.
- Wu RG. (2013) Viral pneumonia in adults. J Int Med Taiwan 24; 317–27.
- Nguyen C, Kaku S, Tutera D, Kuschner WG, Barr J. (2016) Viral respiratory infections of adults in the intensive care unit. J Intensive Care Med 31; 427–41. [Crossref]
- Kim JE, Kim UJ, Kim HK, Cho SK, An JH, Kang SJ, et al. (2014) Predictors of viral pneumonia in patients with community-acquired pneumonia. PLoS One 9; 2014; 1–13. [Crossref]
- Jartti A, Rauvala E, Kauma H, Renko M, Kunnari M, Syrjälä H. (2011) Chest imaging findings in hospitalized patients with H1N1 influenza. Acta radiol; 52, 297–04. [Crossref]
- World Health Organization. Global epidemiological surveillance standards for Influenza 2014. Available from http://www.who.int/influenza/resources/documents/WHO_Epidemiological_Influenza_Surveillance_Standards_2014.pdf (accessed 02 June, 2018).
- World Health Organization. Operational guidelines for sentinel severe acute respiratory infection (SARI) surveillance 2015. Available from https://www.paho.org/revelac-i/wp-content/uploads/2015/10/2015-cha-operational-guidelines-sentinel-sari.pdf (accessed 02 June, 2018).
- Gadsby NJ, Russel CD, McHugh MP, Mark H, Conway Morris A, Laurenson IF et al. (2016) Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis 201; 62, 817–23. [Crossref]
- Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. (2011) Viral pneumonia. Lancet 377; 1264–75.
- Tan D, Zhu H, Fu Y, Tong F, Yao D, Walline J, et al. (2016) Severe community-acquired pneumonia caused by human adenovirus in immunocompetent adults: a multicenter case series. PLoS One 11; 1–12. [Crossref]
- Branche AR, Falsey AR. (2015) Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs & Aging 32, 261–69. [Crossref]
- Panda S, Mohakud NK, Pena L, Kumar S. (2014) Human metapneumovirus: review of an important respiratory pathogen. Int J Infect Dis 25; 45–52. [Crossref]
- Al-Tawfiq JA, Zumia A, Gautret P, Gray GC, Hui DS, Al-Rabeeah AA et al. (2014) Surveillance for emerging respiratory viruses. Lancet Infect Dis 2014; 14, 992–1000. [Crossref]
- Fang GD, Fine M, Orloff J, Arisumi D, Yu VL, Kapoor W, et al.(1990) New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore) 69, 307–16. [Crossref]
- Pavia AT. (2011) Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis 52, 0–5. [Crossref]
- Pavia AT. (2013) What is the role of respiratory viruses in community-acquired pneumonia? what is the best therapy for influenza and other viral causes of community-acquired pneumonia? Infect Dis Clin North Am 27, 157–75. [Crossref]
- Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H. (2013) Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: Results of a one-year study. J Infect 2013; 67, 11–18. [Crossref]
- Damasio GAC, Pereira LA, Moreira SD, Duarte dos Santos CN, Dalla-Costa LM, Raboni SM. (2015) Does virus–bacteria coinfection increase the clinical severity of acute respiratory infection? J Med Virol 2015; 87; 1456–61. [Crossref]