Abstract
Background: More than 8 in 10 neonatal deaths result from illnesses that are curable and preventable. In countries like Ethiopia, more frequently, male partners or spouses make the health care decision. Due to the majority of women entering the workforce, fathers now have the burden of raising their children, which has altered the traditional role of the father. Therefore, it is crucial to find out the paternal knowledge of neonatal danger signs.
Methods: A community-based cross-sectional study was conducted in Bishoftu city among 621 fathers from April 1 to 27, 2022. Participants were recruited using simple random sampling techniques and 610 completed the questionnaire. Data were collected through face-to-face interviews and entered into Epi-data version 3.1 and analyzed using SPSS version 26. Binary logistic regression was used for the analysis.
Results: The results of this study showed that fathers with good knowledge were found to be 44.3% (95% CI 40-48). The chances of having good knowledge were positively associated with urban residents (AOR=2.99, 95% CI 1.86-4.8), respondents whose wives had a history of operative delivery (AOR=2.95, 95 % CI 1.81-4.81), who accompanied their wives during the ANC visit (AOR=2.29, 95% CI 1.56 to 3.37), who had 3 or more children (AOR=3.86, 95% CI 2.19-6.8) and who obtained information from healthcare providers (AOR=2.34, 95% CI 1.58-3.45).
Conclusions: In this study, fathers’ knowledge of neonatal danger signs was found to be low. Therefore, all concerned bodies should strengthen the provision of health education on neonatal danger signs for mothers’ husbands.
Keywords
Father, Neonatal danger signs, Knowledge, Bishoftu town, Ethiopia
Introduction
The 28-day time following birth is known as the neonatal period, and it can be further divided into three categories: very early (birth to less than 24 hours), early (birth to <7 days), and late neonatal periods (7 days to <28 days). This period is the most dangerous time for a child’s survival with the highest risk of death during the first hour of the period. Neonatal danger signs are common manifestations related to a potentially serious problem that can be recognized by nonclinical individuals, such as the mother and other family members [1-3] and are used in the integrated management of neonatal and child illness (IMNCI) by professionals for recognizing infants who need medical treatment [1-4].
Approximately, 5.0 million children died before turning five, with 2.4 million of those deaths occurring in newborns. With one million newborn deaths in 2020, sub-Saharan Africa was responsible for 43% of all newborn deaths worldwide. After a gradual decline from 39 to 29 between 2005 and 2016, the neonatal death rate in Ethiopia increased to 30 per 1,000 live births in 2019. If current trends continue, more than 60 countries, 70% of which are in sub-Saharan Africa, will miss the SDG target for neonatal mortality, which is set at 12 deaths per 1,000 live births [5,6].
This alarmingly high death toll, most of which occurred at home where few mothers and families recognize the early warning signs of a newborn’s illness, serves as a stark reminder of the urgent need to end preventable deaths of children and young people. A study carried out in sub-Saharan African countries found that half of children under five years of age died at home and that this was associated with poor recognition of illness and care-seeking. Further research also revealed that one of the common reasons for delays in receiving appropriate care for serious newborn complications is a lack of understanding of the problem or danger signs by mothers, families, and other newborn caregiver [5-10].
Early identification of neonatal danger indicators by caregivers and prompt and appropriate referral have been the main strategies used to reduce neonatal mortality [4,11]. Young infants are more likely to show subtle disease symptoms, which are only noticeable to closest caregivers who are aware of the signs to look for. Therefore, the risk of neonatal death is reduced when caregivers are aware of the warning signs of newborn disease and act in their infant’s best interests by seeking medical attention [12].
Studies conducted in many different countries revealed that fathers or husbands’ knowledge of neonatal risk indicators was deficient. A study carried out in Kathmandu, Nepal, revealed that in 28.2% of cases, fathers’ awareness of newborn risk indicators was low, in 63.1% of cases, it was moderate, and in 8.7% of cases, it was high. According to a study conducted in Bungoma County, Kenya, 50% of fathers were aware of at least one neonatal danger sign. Ethiopian reports indicate that 40.7% of fathers knew three or more neonatal danger signs [13- 15]. Furthermore, only 34% of women delivered in Ethiopia seek PNC checks, and more than half of births take place outside of medical facilities [6]. Given the high proportion of home births and the lower utilization of PNC service, it is critical to investigate and improve parents’ awareness of the warning signs of newborn disease.
Paternal knowledge about neonatal danger signs has been influenced by several factors such as urban residence, source of information, the wife of respondent having a history of instrumental birth, accompanying the wife during ANC visits, income and educational level [14,15].
Although mothers are more involved in taking care of their children, fathers contribute significantly to the parenting of their new borns. In today’s society, a lot of women are in the labor force, which has changed the role of father, making them more engaged in the responsibilities of child rearing [16,17]. In developing countries like Ethiopia, health care decisions for women and children are made more often by their husbands/partners without women’s involvement; also, fathers are considered the “final decision makers” even when their partners are included in discussions [18-22]. Improving fathers’ awareness of important issues such as danger signs, is crucial, if they are the final decision makers for neonatal health [14]. So this research aims to assess paternal knowledge about neonatal danger signs and associated factors in the town of Bishoftu.
Methods
Study Setting, Design and Period
A community-based cross-sectional study design was employed to conduct this study in Bishoftu town between April 1 and 27, 2022. Bishoftu is one of the town administrations in the Oromia region of Ethiopia, located47 kilometers east of Addis Abeba. The town administration is made up of fourteen kebeles, five of which are rural and nine of which are urban. There were two public hospitals, five health centers, two private hospitals, and ten private clinics in the town that offered the people living in the catchment area a variety of medical services, from basic curative and preventive to advanced services. According to the Bishoftu town health office report of 2022, the town had a total population of 234,970 and 48,972 total numbers of households [23].
Source and Study Population
Source Population
All husbands with children less than 6 months of age in Bishoftu town.
Study Population
Husbands with children less than 6 months of age in randomly selected kebeles of Bishoftu town.
Eligibility Criteria
This study included all husbands who had children under six months of age and had lived in the Bishoftu town for at least six months.
Sample Size Determination
The sample size was determined using the estimated prevalence of good knowledge of neonatal danger signs among husbands of mothers who gave birth in the past six months from an earlier study done in Gurage zone using a single population proportion formula. Where, Zα/2 = 1.96, the margin of error = 0.05, p = proportion of husbands who have good knowledge of the neonatal danger signs, which was 40.7%, and q = 1-p.
![]()
After accounting for the design effects of 1.5 and 10% non- response rates, the minimum sample size needed for this study was determined to be 621 [15].
Sampling Technique and Procedure
A multistage sampling method was used to draw the final sample size. The town of Bishoftu has 9 urban and 5 rural kebeles. Of these rural and urban kebeles (taking 35% of the total urban and rural kebeles), 3 urban and 2 rural kebeles was selected using simple random sampling technique (lottery method). From the rural kebeles, Kurkura and Kajima were selected, and from the urban kebeles, kebele 1, 7, and 9 were selected.
Households with fathers with children less than 6 months of age within the selected kebeles were listed from the family folder of the health extension workers. The total sample size was proportionally allocated to the selected kebeles based on the number of households with father who has children under 6 months of age in their respective kebeles. Then the final sample which was 621 was selected by a simple random sampling technique (using a computer generated list of random numbers) from a list of households with fathers with babies less than 6 months of age within selected kebeles which was extracted from the family folder of the health extension workers.
Study Variables
Dependent Variable
Paternal knowledge about neonatal danger signs.
Independent Variables
The independent variables include Socio-demographic factors (age of the father, age of the recent child, father level of education, wife level of education, father occupation, wife occupation, residence, religion, type of family and monthly household income), Obstetrical factors of the wives of the respondents (number of babies, place of birth of the last baby born, mode of delivery, wife ANC visit, accompanied by the husband during the antenatal care (ANC) visit and postnatal care (PNC), history of the last born baby sick, decision maker to seek care, history of child death ) and Information-related factors (source of information about neonatal danger signs).
Operational Deflnition and Measurements
Neonatal Danger Signs. signs that indicate abnormal health conditions and that occur during the first 28 days of life and includes difficulty breathing, fast breathing, lethargy or weakness, unconsciousness, convulsion, fever, coldness of the baby or hypothermia, umbilical Redness or discharge from the umbilicus, poor feeding or unable to suckle, Yellow palms/soles/eyes, Eye draining/pus/redness, and diarrhea [24-26].
Knowledge. Husbands’ level of awareness or mindfulness about neonatal danger signs. Consequently, fathers who were able to mention at least three neonatal danger signs among the 12 neonatal danger signs without prompt were regarded to have good knowledge. Fathers who mention two or less neonatal danger signs among the 12 neonatal danger signs without prompt were considered to have poor knowledge about neonatal danger signs [25,26].
Data Collection Tools and Techniques
A validated semi-structured questionnaire was used to gather the data. The questionnaire was prepared in English after reviewing pertinent literature. The questionnaire was translated into Afan Oromo and Amharic, then back-translated into English by professional translators to ensure consistency. To promote understanding, the questionnaire was given in both Amharic and Afan Oromo depending on the participants’ preferred language [14,15,24-30].
Data were collected by seven bilingual, trained data collectors, who were holders of bachelor’s degree in nursing under the guidance of health extension workers, through a face-to-face interview using a semi-structured questionnaire during home visits. Three experienced supervisors supervised the data collection process. The data was collected whenever the fathers were available, including on weekends and lunch time. Repeat visits were made when study households were found to be closed or when respondents were unavailable.
Data Quality Control
To ensure the quality of the data, a well-designed data collection instrument was constructed and pretested before the actual survey in a comparable setting in the town of Adama, involving 5% of the estimated sample size, following which the appropriate revisions and modifications were made accordingly. Data collectors and supervisors received two days of training on the instrument. The principal investigator reviewed the collected data daily to verify its completeness and consistency. The supervisors and the principal investigator were informed of the problems encountered during the data collection period for immediate action. Discussions were made with the interviewers to reduce errors made during the interview and to take timely corrective actions.
Data Processing and Analysis
The gathered data were manually checked and reviewed for completeness and consistency and then entered into Epi-Data V.3.1 and exported to SPSS V.26 for analysis. Cross tabulation was performed for the exploration of the data, to clean the missing values and to determine the expected count per cell. Bivariate and multivariable logistic regression models were used to investigate relationships between dependent and independent variables. Variables with a p-value < 0.2 in the bivariate analysis were considered candidates for inclusion in the multivariable logistic regression analysis.
Multicollinearity was checked to see the linear correlation among the independent variables by using variance inflation factor (VIF) and tolerance which was 4.33 and 0.25 respectively. The data was also assessed for potential confounding and interaction factor. The fitness of the model was tested using the Hosmer-Lemeshow goodness-of-fit test model, the value being 0.955. In the multivariable logistic regression analysis, variables with a p-value below 0.05 were considered to have a statistically significant relationship with the outcome variable. The adjusted odds ratio (AOR) at a 95% confidence interval (CI) was used to indicate the degree to which the independent variables explained the outcome variable. Descriptive statistics were employed to determine the frequency of different variables. The data were then presented using simple frequencies, tables, and figures.
Results
Socio-demographic Characteristics of the Respondents
A total of 610 respondents were included in the study, with a response rate of 98.2%. The mean age of the respondents was 34.35 years (SD ±6.509) and ranged from 22 to 49 years. Nearly two-thirds of the respondents (64.8%) had completed secondary school and 247(40.5%) of the study participants were government employees. More than half of the respondents (57.4%) were urban residents and 326(53.6%) were followers of the orthodox religion. More than half of the respondents (53.3%) had a female child (Table 1).
Table 1: Socio-demographic characteristics of husbands of mothers who gave birth in the last 6 months in Bishoftu town, Ethiopia, 2022 (n=610)
|
Variables |
Category | Frequency |
Percentage (%) |
|
Age of father |
20-24 |
44 |
7.2 |
| 25-29 |
132 |
21.6 |
|
| 30-34 |
124 |
20.3 |
|
| ≥35 |
310 |
50.8 |
|
| Age of the child in days | 0-28 |
81 |
13.3 |
| >28 |
529 |
86.7 |
|
|
Religion |
Protestant |
148 |
24.3 |
| Orthodox |
326 |
53.4 |
|
| Catholic |
34 |
5.6 |
|
| Muslim |
69 |
11.3 |
|
| Wakefeta |
33 |
5.4 |
|
| Father’s level of education | No formal education |
43 |
7.0 |
| Primary education |
172 |
28.2 |
|
| Secondary and above |
395 |
64.8 |
|
|
Father’s occupation |
Government employee |
247 |
40.5 |
| Private employee |
149 |
24.4 |
|
| Self-employed & daily labourer |
139 |
22.8 |
|
| Farmer |
75 |
12.3 |
|
| Mother’s level of education | No formal education |
52 |
8.5 |
| Primary education |
167 |
27.4 |
|
| Secondary and above |
391 |
64.1 |
|
|
Mother’s occupation |
Government employee |
83 |
13.6 |
| Private employee |
109 |
17.9 |
|
| Self-employed & daily labourer |
158 |
25.9 |
|
| Farmer |
15 |
2.5 |
|
| Housewife |
245 |
40.2 |
|
| Family type | Nuclear |
480 |
78.7 |
| Joint |
130 |
21.3 |
|
| Monthly household income | <=4950 |
298 |
48.9 |
| >4950 |
312 |
51.1 |
Obstetrics-related Characteristics of the Wives of the Respondents
Almost three-fourths of the wives of the respondents (72.1%) had a parity of greater than or equal to two, while the rest were primiparous. More than half of the wives of the respondents (56.9%) had delivered in the hospital. Almost all of the wives (97.4%) had attended ANC follow-up, and 292(47.9%) respondents had accompanied their wives during the visit.
Of those who accompanied their wife during ANC visits, 168(57.5%) have accompanied one time and only 3(1%) have accompanied 4 times. Four hundred fourty-nine (73.6%) respondents had visited their wives at the health facility postnatal care unit after they gave birth and 191(31.3%) of the respondents had accompanied their wives during discharge from the postnatal care unit and among them only 82(42.9%) of the respondents received postnatal discharge counseling on neonatal danger signs (Table 2).
Table 2: Obstetric characteristics of respondents’ wives who gave birth in the last 6 months in Bishoftu town, Ethiopia, 2022 (n=610).
|
Variables |
Category | Frequency |
Percentage (%) |
|
Number of babies |
1-2 |
348 |
57.05 |
|
>=3 |
262 |
42.95 |
|
| Place of birth of the last baby born |
Health center |
263 | 43.1 |
|
Hospital |
347 |
56.9 |
|
|
Mode of delivery |
SVD |
435 |
71.3 |
| Instrumental |
109 |
17.9 |
|
|
CS |
66 |
10.8 |
|
| Accompany wife during PNC discharge | Yes |
191 |
31.3 |
|
No |
419 |
68.7 |
|
|
History of last baby sick |
Yes |
189 |
31 |
|
No |
421 |
69 |
|
|
Place of seeking care (n=189) |
Health center |
83 |
43.9 |
|
Hospital |
104 |
55 |
|
| Traditional healers |
2 |
1.1 |
|
|
Decision-maker to seek care (n=189) |
Husband |
75 |
39.7 |
| Wife |
29 |
15.34 |
|
| Wife’s relative |
4 |
2.1 | |
|
Husband and wife |
81 |
42.86 |
|
|
Wife attends ANC |
Yes |
594 |
97.4 |
|
No |
16 |
2.6 |
|
|
Frequency of ANC (n=594) |
One times |
6 |
1 |
|
Two times |
15 |
2.52 |
|
| Three times |
152 |
25.6 | |
|
Four times and above |
421 |
70.88 |
|
| Accompanied by the husband during a visit | Yes |
292 |
47.9 |
|
No |
318 |
52.1 |
|
|
History of child death |
Yes |
5 |
0.8 |
|
No |
605 |
99.2 |
|
| Time to reach the nearest health facility | <30 minutes |
355 |
58.2 |
Notes: CS: Cesarean section; SVD: Spontaneous vaginal delivery; ANC: antenatal care
Paternal Knowledge about Neonatal Danger Signs
In general, 44.3% (95% CI 40-48) of the fathers had good knowledge about neonatal danger signs. Of the total of the respondents, 379 (62.1%) mentioned fever and 30(4.9%) mentioned jaundice or yellowish discoloration of palms / soles. Vomiting was also a commonly recognized danger sign by 358(58.9%) respondents in this study (Figure 1).
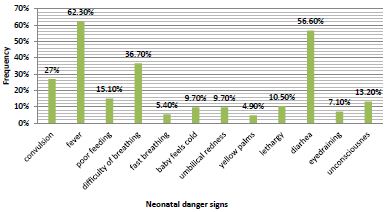
Figure 1: Percentages of fathers who mentioned each neonatal danger signs in Bishoftu town, Ethiopia, 2022 (n=610).
Source of Information on Neonatal Danger Signs
The source of information on neonatal danger signs for more than half 343(56.2%) of the respondents were health professionals, while others received the information from the media, friends, and relatives.
Factors Associated with Paternal Knowledge of Neonatal Danger Signs
In the bi-variable logistic regression analysis factors such as father’s age, father’s level of education, residence, accompanying the wife during the ANC visit, mode of delivery, having at least three children and health professionals as a source of information showed a significant association with paternal knowledge of neonatal danger signs and then included in the multivariable analysis. In the multivariable logistic regression analysis, place of residence, accompanying the wife during the ANC visit, modes of delivery, having three or more children, and health professionals as a source of information were independently associated with paternal knowledge about neonatal danger signs in multivariable logistic regression analysis.
Compared to respondents who live in rural areas, those living in urban areas were 2.9 times more likely to have a good knowledge about neonatal danger signs (AOR= 2.99, 95% CI 1.86 to 4.81). Participants whose wives had a history of operative delivery were 2.9 times more likely to have a good knowledge of neonatal danger signs than those whose wives had a spontaneous vaginal delivery (AOR=2.95, 95 % CI 1.81 to 4.81).
Husbands who accompanied their wives during the ANC visit were 2.3 times more likely to have a good knowledge of neonatal danger signs than their counterparts (AOR=2.29, 95% CI 1.56 to 3.37). Those who had three or more children were 3.8 times more likely to have a good knowledge of neonatal danger signs than those with two or fewer children (AOR=3.86, 95% CI 2.19 to 6.8). Fathers who obtained information from healthcare professionals were 2.3 times more likely to have a good knowledge of neonatal danger signs than those who received information from other sources (AOR=2.34, 95% CI 1.58 to 3.45) (Table 3).
Table 3: Regression analysis of Factors affecting Paternal knowledge about neonatal danger signs in Bishoftu town, Ethiopia 2022 (n=610).
|
Variable |
Category | Knowledgeable | Not knowledgeable | COR (95% CI) |
AOR (95% CI) |
| 20-24 |
13 (29.5%) |
31 (70.5%) | 1 | 1 | |
|
25-29 |
31 (24.5%) | 101 (76.5%) | 0.73 (0.34-1.57) |
0.67 (0.28-1.59) |
|
| Father’s age | 30-34 |
59 (47.6%) |
65 (52.4%) | 2.16 (1.04-4.52) |
1.12 (0.48-2.63) |
| ≥35 |
167 (53.9%) |
143 (46.1%) | 2.79 (1.40-5.53) |
1.52 (0.65-3.59) |
|
| No formal |
10 (23.3%) |
33 (76.7%) | 1 |
1 |
|
| education | |||||
| Father’s level of education | Primary |
37 (21.5%) |
135 (78.5%) | 0.9 (0.41-2.00) |
0.43 (0.18-1.04) |
| Secondary and above |
223 (56.5%) |
172 (43.5%) | 4.28 (2.05-8.92) |
1.92 (0.82-4.48) |
|
| Rural |
74 (28.5%) |
186 (71.5%) | 1 |
1 |
|
| Residence | Urban |
196 (56%) |
154 (44%) | 3.19 (2.27-4.50) |
2.99 (1.86- 4.8)* |
| Number of children | <=2 |
129 (37.1%) |
219 (62.9%) | 1 |
1 |
| >=3 |
141 (53.8%) |
121 (46.2%) | 1.98 (1.43-2.74) |
3.86 (2.19- 6.8)* |
|
| Mode of delivery | Normal SVD |
181 (41.6%) |
254 (58.3%) | 1 |
1 |
| Operative delivery |
89 (50.9%) |
86 (49.1%) | 1.45 (1.02-2.07) |
2.95 (1.81- 4.81)* |
|
| Accompanying wife during ANC visit | No |
102 (32.1%) |
216 (67.9%) | 1 |
1 |
| Yes |
168 (57.5%) |
124 (42.5%) | 2.87 (2.06-3.99) |
2.29 (1.57- 3.37)* |
|
| HCP as a source of information | No |
90 (33.7%) |
177 (66.3%) | 1 |
1 |
| Yes |
180 (52.5%) |
163 (47.5%) | 2.17 (1.56-3.02) |
2.34 (1.58- 3.5)* |
Notes: *Significant at p<0.001; ANC: Antenatal Care; AOR: Adjusted OR; COR: Crude Odd Ratio; SVD: Spontaneous Vaginal Delivery; HCP: Health Care Provider.
Discussion
This study was carried out in Bishoftu town to assess paternal knowledge of neonatal danger signs and pinpoint associated factors for it. According to this study, 44.3% of fathers had good knowledge about neonatal danger signs. This finding is consistent with the finding from Gurage zone, Ethiopia (40.7%). However, this finding was lower than the findings in Bungoma County; Kenya(50%), and Kathmandu; Nepal (71.8%). The possible reason for this variation could be variations in participants’ socio-demographic characteristics and availability and accessibility of health services infrastructures, sampling techniques, methods used to assess knowledge and study settings [13-15].
The discrepancy of this study from the study conducted in Kenya could be due to the difference in the sampling technique and the cut point of the measurements, the former study respondents were recruited through the convenience sampling method from those accompanying their female partners to healthcare clinics, 75.6% of the respondents had completed secondary school or higher, and those who mentioned at least one neonatal danger sign were considered to have good knowledge, while in this study, respondents were included from the selected household with fathers in the community, 64.8% of the respondents had completed secondary school or higher, and those who mentioned 3 neonatal danger signs were considered to have good knowledge. In the study conducted in Nepal, the Non-probability purposive sampling technique was used to select fathers who had newborn babies up to 7 days admitted to the Maternity Ward and Birthing Center, and the Likert scale was used to measure their level of knowledge, as a result, this led to the observed difference.
Husbands who lived in urban residences, who accompanied their wives during ANC visits, and whose wives delivered babies through operative delivery were more likely to have good knowledge of neonatal danger signs. Furthermore, fathers who have three or more children and those who received the information from health professionals were significantly associated with having good knowledge.
In the current study, fathers who live in urban areas were more likely to have a good knowledge of neonatal danger signs compared to those who live in rural areas which is supported by the study conducted in Gurage zone and Chencha District [15]. This is due to the fact that most rural residents have lower access to and use certain health information sources and infrastructures relative to urban residents, including care providers, doctors, blogs, magazines, and mass media, which enable them to acquire information related to health. They also had lower use of search engines for health information and lower access to healthcare services [31,32].
Fathers whose wives had a history of operative delivery were more likely to have good knowledge compared to those whose wives had a spontaneous vaginal delivery. This is similar to the study done in Gurage zone. Due to an increasing number of days of stays in the health facility for operative delivery, the husband may have a high chance of contact with healthcare providers; thus, this allows one to seek and acquire knowledge about his newborn.Fathers who accompanied their wives during ANC visits were more likely to have good knowledge about neonatal danger signs than their counterparts. This is supported by the study conducted in Gurage zone.This may be because fathers may receive counseling from health professionals about pregnancy and newborn illnesses during accompanying ANC visits.Respondents who had three or more children were more likely to have good knowledge compared to those with no more than two children. This result is in line with a study conducted in Gurage zone. The Chance of Husbands’ exposure to different child problems increases, thus increasing their awareness about danger signs manifested by their children. Also, there is an increased likelihood of access to health institutions and postnatal services with community health workers, which can increase husbands’ interactions with healthcare professionals, providing them with more opportunities to gain knowledge about their infants’ health [15].
This study indicated that fathers who acquired information from healthcare providers were more likely to have good knowledge about neonatal danger signs than those who acquired information from other sources. This result is supported by studies done in the Gurage zone and in the town of wolkite. This could be related to the commitment of healthcare providers to provide appropriate information on newborn health issues in a memorable way compared to other sources and learners, who can concentrate better on the information given by healthcare professionals [15,33].
Level of education was not associated with the father’s level of knowledge about neonatal danger signs. This is similar with the study done in Chencha District [31] but inconsistent with the study conducted in Gurage and in the town of wolkite. The reason for this discrepancy could be due to the fact that health extension workers and other health care professionals provide health information or counseling related to neonatal health problems, regardless of the educational status of the father [15,33].
Strengths and Limitations of the Study
This community-based study was crucial to investigate factors that could predict paternal knowledge by reaching out to those who cannot access health facilities for various reasons.
However, due to the nature of the cross-sectional study design that assesses exposure and outcome at the same time, the results might not indicate reverse causality. This study is not free of recall bias because all fathers were interviewed about the content of their child who was older than 28 days of age. There is limited literature for reference.
Conclusion
In this study, the magnitude of paternal knowledge about neonatal danger signs is found to be 44.3%. Living in an urban area, having wives who have had previous operative deliveries, accompanying wives to appointments at the ANC, having three or more children and having healthcare providers as a source of information were the factors that contributed significantly to paternal knowledge of neonatal danger signs.
It is important that the stakeholders come up with specific educational and sensitization programs that can help reduce the knowledge gap of fathers who live in rural areas and with a strategy to increase husband participation in accessing maternal and child health services.
Abbreviations and Acronyms
ANC: Antenatal Care; AOR: Adjusted Odd Ratio; CI: Confidence Interval; COR: Crude Odd Ratio; HCP: Health Care Provider; IMNCI: Integrated Management of Neonatal and Child Illness; PNC: Postnatal Care; SVD: Spontaneous Vaginal Delivery; SDG: Sustainable Development Goal; VIF: Variance inflation ratio.
Acknowledgement
The authors wish to express their gratitude to the Arba Minch University School of Nursing and the Bishoftu Town Health Office for providing us the needed assistance. The authors also would like to thank the data collectors and supervisors who participated in this study.
Declarations
Ethics Approval and Consent to Participate
The studies involving human participants were reviewed and approved by the institutional research review board of the Arba Minch University School of Medicine and Health Sciences with the approval number AMU-IRB-19/2022. A formal letter from Arba Minch University was sent to the offices concerned and the Kebele Health extension workers. Permission letters were received from the Oromia Regional Health Bureau. All study participants were informed about the purpose of the study and their right to refuse to participate, and written and informed consent was obtained before the interview. The respondents were informed that the information obtained will be kept confidential and will not cause them any harm. This study was carried out in accordance with the principles of the Declaration of Helsinki. During data collection, possible COVID-19 prevention measures were implemented.
Competing Interests
The authors declare that they have no competing interests.
Funding
This study received financial support from Arba Minch University College of Medicine and Health Sciences.
Data availability Statement
Data will be made available on request.
Author Contributions
B.T.O and H.I.G. conceived the study, participated in its design and coordination, initiated the research, carried out the statistical analysis, interpreted the results, and wrote the final manuscript, critically reviewing it. N.D.M., E.N.W., and M.G.W. participated in the study’s design, guided the statistical analysis, and critically reviewed the manuscript. F.H.H., S.S.A., and H.Z.A. were involved in principal supervision, participated in the study’s design and coordination, edited the manuscript, Cover Letter and critically reviewed the manuscript. B.T.O. has main responsibility for the final content, and makes the decision to publish. The authors have read and approved the final manuscript.
References
- Robert Kliegman, editor. Nelson 19th.
- Lucia Hug DS. Levels & Trends in Child Mortality(2019) – Report 2019 [Internet]. Available from: https://www.unicef.org/media/60561/file/UN-IGME-child- mortality-report-2019.pdf
- Jhpiego (2004)Monitoring birth preparedness and complication readiness: tools and indicators for maternal and newborn 1–338. Available from: http://pdf.usaid. gov/pdf_docs/PNADA619.pdf
- Basaleem, Amin.(2011) Integrated Management of Childhood Illness in Lahej, Yemen: A qualitative analysis from the perspective of health providers East Mediterr Heal J.17(2) [crossref].
- Kantorova V, Kariuki S (2021)Levels & Trends in child mortality.
- Ethiopian Public Health Institute. Ethiopia Mini Demographic and Health Survey. EPHI AND ICF. 2019.
- Assefa N, Lakew Y, Belay B, Kedir H, Zelalem D, Baraki N, et al. (2016)Neonatal mortality and causes of death in Kersa Health and Demographic Surveillance System (Kersa HDSS), Ethiopia, 2008–2013 Matern Heal Neonatol Perinatol .2(7) Available from: http://dx.doi.org/10.1186/s40748-016-0035-8
- Nigatu SG, Worku AG, Dadi (2015)Level of mother’s knowledge about neonatal danger signs and associated factors in North West of Ethiopia: A community based study. BMC Res Notes.8(309)
- Price J, Lee J, Willcox M, Harnden A.(2019) Place of death, care-seeking and care pathway progression in the final illnesses of children under five years of age in sub- Saharan Africa: A systematic review J Glob Health9(2) [crossref]
- Syed U, Khadka N, Khan A, Wall S.(2008) Care-seeking practices in South Asia: Using formative research to design program interventions to save newborn lives. J Perinatol: S9–13. [crossref]
- Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA (2005)Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: A review of the evidence. Pediatrics.115(2) [crossref].
- Tiwari G, Thakur AK.(2018) Health care seeking behavior for common childhood illnesses in Birendranagar nepal.17(3) Available from: http://dx.doi.org/10.1371/ pone.0264676
- Dangol R, Koirala R (2020)Awareness of Fathers Regarding Newborn Danger Signs: Evidence from a Tertiary Level Hospital of Kathmandu, Open J Nurs 10(2).
- Roney E, Morgan C (2021)Men’s and women’s knowledge of danger signs relevant to postnatal and neonatal careseeking: A cross sectional study from Bungoma County, PLoS One Available from: http://dx.doi.org/10.1371/journal.pone.0251543
- Solomon shitu hai Greenspan JA, Chebet JJ, Mpembeni R, Mosha I, Mpunga M, Winch PJ, et (2019)Men’s roles in care seeking for maternal and newborn health: A qualitative study applying the three delays model to male involvement in Morogoro Region, Tanzania. BMC Pregnancy Childbirth. 19(293)
- Yogman M, Garfield CF (2016)Fathers’ roles in the care and development of their children: Pediatrics. 138(1) [crossref]
- RM, R. S (2013)A study on father’s knowledge and attitude towards their role in child care in selected areas of Mangalore with a view to develop an informational booklet. J Heal Allied Sci NU. 03(02)
- Greenspan JA, Chebet JJ, Mpembeni R, Mosha I, Mpunga M, Winch PJ, et (2019) Men’s roles in care seeking for maternal and newborn health: A qualitative study applying the three delays model to male involvement in Morogoro Region, Tanzania. BMC Pregnancy Childbirth.19(1)
- Osamor P, Grady C. (2018)Factors associated with women’s health care decision- making autonomy: Empirical evidence from Nigeria J Biosoc Sci.50(1)[crossref]
- Nigatu D, Gebremariam A, Abera M, Setegn T, Deribe (2014) Factors associated with women’s autonomy regarding maternal and child health care utilization in Bale Zone: A community based cross-sectional study BMC Womens Health. 2014(1)
- Asabu MD, Altaseb DK.(2021) The trends of women’s autonomy in health care decision making and associated factors in Ethiopia: evidence from 2005, 2011 and 2016 DHS BMC Womens Health . 21(1) Available from: https://doi.org/10.1186/ s12905-021-01517-9
- Ridgwell A, Flintan F.(2007)Gender and Pastoralism, Livelihood and income generation in Ethiopia. V(2)
- Bishoftu town health office report of 2022.
- World Health Organization (WHO)(2013) WHO recommendations on postnatal care of the mother and newborn. Geneva: World Health Organization. 1–72 p.
- Kibaru EG, Otara AM (2016)Knowledge of neonatal danger signs among mothers attending well baby clinic in Nakuru Central District, Kenya: cross sectional descriptive study. BMC Res Notes.9(1)
- Kebede AA, Cherkos EA, Taye EB (2020)Mother ’ s Knowledge of Neonatal Danger Signs and Health-Seeking Practices and Associated Factors in Debretabor, Northwest Ethiopia : A Community-Based Cross-Sectional Study.Research and Reports in Neonatology.v( 2020);
- Ismail SA, McCullough A, Guo S, Sharkey A, Harma S, Rutter P (2019)Gender- related differences in care-seeking behaviour for newborns: A systematic review of the evidence in South Asia. BMJ Glob Heal. 4(3) [crossref]
- Assessment of Mothers Level of Knowledge About Neonatal Danger Signs and Its Associated Factors in St Paul’s Hospital Millenium Medical College Addis Ababa Ethiopia (2020)J Med Physiol Biophys.vol 67,Available from: https://pdfs. org/6467/5493dfd2ebb451cb0e591d0a5632579f23a9.pdf
- Dida G.(2020) Knowledge and attitude of postnatal mothers on essential newborn care practices at Marsabit county referral Available from: http://erepository. uonbi.ac.ke/handle/11295/154065
- Oscar Shea, Kiersten J.(2004) the DHS wealth index.
- Mersha A, Assefa N, Teji K, Bante A, Shibiru (2017) Mother ’ s Level of Knowledge on Neonatal Danger Signs and Its Predictors in Chencha District , Southern Ethiopia.6(5) Available from: https://www.researchgate.net/publication/320796153_ Mother’s_Level_of_Knowledge_on_Neonatal_Danger_Signs_and_Its_Predictors_ in_Chencha_District_Southern_Ethiopia
- Schofield E, Li Y, Kiviniemi MT (2020)Differences in Rural and Urban Health Information Access and UseJ Rural Health.35(3) .[crossref].
- Town W, Zone G (2017) Mother’s knowledge and Practice about Neonatal Danger Signs and Associated Keywords 1-7.