DOI: 10.31038/GEMS.2023512
Abstract
The clay samples were studied using X-ray diffraction. The range of the run was between 2°and 40° and the run was 1.2°/min. Electronic Microscope also used in the study by using great magnification range from X20 up to 1000,000 to allow to examination the surfaces of fine grains and to takes photographs with great focus. Chemical analyses also carried out under the SEM, using energy-dispersive X-ray spectrometry to define their dominant elements. From the study, it emerged that the dominant clay minerals in the samples are palygorskite, followed by chlorite and illite. Based on the peak area measurements, the mean percentage of palygorskite in the clay deposits included dolomite and anhydrite is 59% (approximate percentage is 34%-79%). It is 23% for chlorite (approximate percentage is 7%-40%) and 9% for illite (approximate percentage is 4%-13%). After excluded dolomite and anhydrite from the samples, it is 65% for palygorskite (approximate percentage is 47%-85%), 25% for chlorite (approximate percentage is 8%-40%) and 10% for illite (approximate percentage is 6%-15%). Through EDX analyses of the palygorskite needles it was inferred that the P is the dominant element, followed by Si, Mg, Al, Ca, and K. Phosphate nodules of a globular shape (spherulites) in various sizes (1-<2 μ) also occur in most of the clay samples, especially that rich in palygorskite. In some samples, the phosphate nodules were present in small groups connected together as bunches.
The main conclusion of this study are:
(1) In Dukhan Sabkha, clay-rich deposits are mainly found beside the edges of the Sabkha, especially where the Sabkha is supplied with surface drainage. Later the wind contributes in distribution part of these sediments within the Sabkha. The thickness of clay-rich layers in such as these locations is between 0.5 and 1.5 cm.
(2) Palygorskite is mainly authigenic, formed inside the Dukhan Sabkha. This is because the conditions of formation this mineral within the Sabkha are widely available. Part of palygorskite is definitely detrital being, derived from shales of the Lower and Upper Dammam Formation of the Eocene age that exposed on the surface of Qatar. A small proportion of this mineral could also reach to inside Dukhan Sabkha after carried to Qatar as dust by the northern wind or by water currents from the neighboring areas, especially from the eastern part of the Arabian Peninsula.
(3) Chlorite and illite minerals are not form locally inside the Dukhan Sabkha; both of them are from pre-existing sedimentary rocks from outside the Sabkha. The main source of these two minerals is a detrital rocks derived from shales of the Tertiary of the Lower Dammam Formation and Rus Formation of Eocene age in addition to Dam Formation of Miocene age in Qatar and part of these minerals may reached to inside the Sabkha from outside Qatar.
(4) This work can be used as model for the study the possibility of formation palygorskite within the Sabkhas areas in Qatar and in the Arabian Gulf areas.
Introduction
Sabkhas are one of the important features of the Qatar Peninsula, covering about 7% of its surface area. They can be divided into coastal and inland Sabkhas. There are 42 coastal Sabkhas, covering about 590 km2 and 78 inland Sabkhas, covering about 205 km2. The surface of the Sabkhas in Qatar is mainly flat and nearly horizontal, with gradients usually less than 1:250°. Most of the Sabkhas are located at or within 1.5 m above sea level. Their interior margins, however, may be 2 or 3 m higher than sea level [1]. The study of clay minerals in the Sabkha areas of Qatar and Arabian Gulf countries are limited in number. Most studies focus on evaporite deposits. They only mention clays in the marine and Sabkha areas as fine deposits, dominant in the intertidal zone of the Sabkha and also where microbial mats and algae are found [2-8]. Further information about clay minerals in Qatar formations can be found in: Cavelier [9,10] and Cavelier [11-18].
This is the first study of clay minerals in large, famous Holocene inland Dukhan Sabkha in Qatar. The aim of the study is to identify the type, the amount and the origin of clay minerals in the Sabkha sediments, in addition to the dominant natural conditions that caused formation of these minerals in the Sabkha. Important series of science questions are answered in the study such as:
- What is the types of clay minerals that dominant in the Dukhan Sabkha.
- What is the source and the proportion of the clay minerals in the Sabkha sediments?
- What is the factors that affecting the formation of the clay in the Dukhan Sabkha?
- Is Palygorskite formed inside Dukhan Sabkha or it is detrital mineral comes from outside the Sabkha?
General Setting of the Study Area
Dukhan Sabkha is the largest inland Sabkha in the Qatar Peninsula, located in the west adjacent to the Jabal Dukhan hill and covers about 73 km2. It is situated in a depression shaped like an inverted L, one of its arms extends north-south and the second one northeast-southwest (Figures 1 and 2). The western side of the Sabkha is straighter than the other side, as it is constrained by the Dukhan anticline [1]. The surface of the Sabkha is flat, about one meter below sea level, except near the edges, is more than 1 m above sea level due to the development of nebkhas (plants trapping sand). The lowest part of the Sabkha is in the south (25° 20– E, 50° 50– N), where it reaches-6 m below sea level [19]. The deposits of the Sabkha consists of a compact, dry crust of halite (3 mm-2 cm thick), above brown or grey sand deposits, or brown sand intercalated with white and light grey gypsum-rich layers. Anhydrite and halite minerals are mainly dominant in the northeastern part of the Sabkha at shallow depth, about 20 cm. Accumulation of sand sheets and small sand dunes are found in the south and southeastern part of the Sabkha. Recent eolian sand sheets are also present in various positions in the western part of the Sabkha. Recent deposits of white salt (covering about 4.5 km2) is present in the northeastern part of the Sabkha. Limonite is found as thin subsurface beds beside the eastern edges of the Sabkha and at the surface in the northern part of the Sabkha [20]. Small areas of the Sabkha, especially around the margins, are covered by mud. Typically, mud cracks appears in different locations due to desiccation. Deposits of sand, clay and silt are present at the edges of the Sabkha [21]. The groundwater level in Dukhan Sabkha is shallow (between a few centimeters and 120 cm). The sources of groundwater in the Sabkha include: sea water, rainwater and the freshwater of the main northern aquifer [20].

Figure 1: Landsat image mosaic showing the general location of Dukhan Sabkha, in the western part of Qatar. (Produced by the Environmental Research in Statue of Michigan. Ann Arbor Michigan. Scan date 30-Jan. 1987, scale 1:200 km.

Figure 2: Landsat image mosaic of Dukhan Sabkha. (Produced by the Central for GIS-State of Qatar with Geomatics Canada. Scan date 25-Jan. 1995, scale 1:200,000).
Methodology
Field Study and Sample Collection
Field studies were carried out in Dukhan Sabkha during February and March 2020. Throughout fieldwork, observations were made for clay on the surface and at shallow depths in the sediments of the Sabkha. Information about type and size of the sediments, microbial mats dominant in the Sabkha were recorded. Sediment samples (10 samples) rich in clay were collected from different locations within the Sabkha: along the margins of the Sabkha, beside the northwestern edge, from northeastern part, from the middle of the northern part and from a surficial channels within the Sabkha (Table 1). Photographs for the common characteristics and for locations rich in clay, and fine deposits in the northern parts of the Sabkha were taken in the field (Figure 3).
Table 1: Names and locations of the studied samples in Dukhan Sabkha
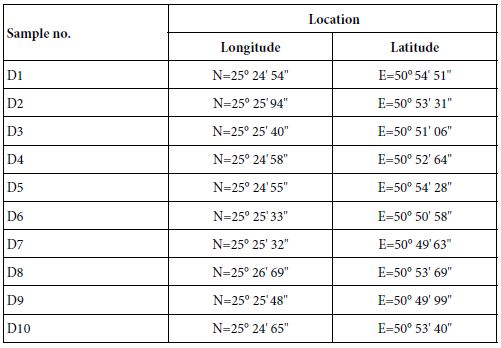

Figure 3: General view of Dukhan Sabkha: a and b showing the compact surface crust in the northern part of the Sabkha, c is thick light gray crust in the northeastern part, d is patches of sands neair the nothewestern edge of the Sabkha. Figures e and f is collection samples from northern and northwestern part of the Sabkha, g and h halite mixed with mud and anhydrite in the northeastern part. (note that mud occures as minor facies on the surface in fig. e, sand dominant beside the northwestern edge of the Sabkha in fig. f, whereas halite and anhydrite are dominent on the surface of the northeastern part in fig. g and h).
Sample Preparation
The clay samples from different locations within the Sabkha were studied using X-ray diffraction First the samples were treated with acetic acid (10%) to remove all the CaCO3 in the sediment. There was variation in the reactions in the samples and after all reaction had ceased any residual acid was washed from the samples, using tap water. Tap water was later added to each sample (4 cm) in a small beaker with sodium hexametaphosphate (calgon-1 ml of 10 % per 100 ml) to disperse the clay. The samples were shaken well and left in an ultrasonic tank for about 15 minutes. Then the samples were allowed to settle, using Stokes Law (3 hours for 4 cm for the <2 µ fractions), as per Galehouse [22] and Hardy and Tucker [23,24]. The fractions of each sample were collected in a small beaker and saturated with about 5 ml of MgCl2. Then, the samples were centrifuged 3 times, and each one was carefully washed, using tap water. Finally, three smear slides were made from the paste of each sample using a spatula. One of these was left (untreated) in the laboratory to dry and then it was run as an air-dried trace. This slide was then, heated to 375°C to collapse the smectite and illite/smectite, leaving the other clays unaffected. The second slide was left in ethylene glycol (vapour) for about 24 hours at 60°C. This was to distinguish the smectite, illite/smectite and chlorite-smectite from the chlorite and illite/smectite where overlaps occurred. The third slide was heated in a furnace to a temperature of 550°C to expel the interlayer water molecules in the smectite, and this caused a contraction in the basal spacing.
X-ray Diffraction
X-ray diffractometry was carried out on the clay fractions (<2 μ) separated from a total of 10 samples of clay-rich sediments. The weight of each sample from which clay was separated was about 40 g and the percentage of clay in the samples was approximately between 25% and 40%. The range of the run was between 2° and 40° and the run was 1.2°/min. The percentage of each clay mineral was calculated by dividing the area of its main peak by the total of the main peaks of the clay minerals of the same sample and multiplying by 100.
Scanning Electronic Microscopic Study and Chemical Analyses
The clay samples were studied by using the scanning electron microscope to determine their fine compositions. The great magnification range of SEM, range from X20 up to 1000,000 is allow to examination the surfaces of fine grains and to takes photographs with great focus. For study, the samples were placed sided sticky tape fixed on surface of stub (stage) and thin coated with gold as a conductive layer. The samples were positioned in the SEM, viewed, studied and photographed by the scanning electron microscope. Chemical analyses were also carried out under the SEM, using energy-dispersive X-ray (EDX) spectrometry to define their dominant elements. Some photographs of the palygorskite and other important features present in the samples were taken.
Result of the Study
Field Study
Most the Surface of the Sabkha is covered by compact crust of fine deposits of sand, silt, clay mud, halite and very fine grains of gypsum of various color between white and beige to light gray. The crust is compact and dry, and mostly between 1 to 3 cm thick (a, b and c in Figure 3). The thickness and dominance of the crust in the northern part of the Sabkha is more than in the southern part. Sand and silt are dominant in the deposits. They found on the surface or mixed with other deposits at different depths. The type of the sediments at the edges of the Sabkha is different from those in other parts of the Sabkha and mainly consists of sand, clay and silt (d, e and f in Figure 3). Mud occurs as a minor facies in selected stations. It is generally silty in nature, some with fine sand. It is dominantly siliciclastic in composition, more rarely calcareous, and commonly contains some evaporite precipitation. It is generally structure less, sometimes with desiccation cracks at the surface. Clay is mainly present on the surface and at shallow depths (few centimeters from the surface). Clays are not as common as sand, but found in limited positions on the surface and at shallow depths. In the Sabkha, clay-rich deposits are mainly found beside the edges of the Sabkha, especially where the Sabkha is supplied by surface drainage. The thickness of clay-rich layers in locations such as these is between 0.5 and 1.5 cm. The clay deposits are also, present inside small number of elongated, narrow, shallow-dry surface channels, extending from the land inside the Sabkha. In general, the proportion of clays in the Sabkha decreases towards the center of the Sabkha and it increases at the edges of the Sabkha and, where microbial mats are present. Anhydrite presents at shallow depth (about 20 cm) as nodules and as fine grains (similar to gruel) mixed with halite, mud, silt and dark black and grey deposits of microbial mats (g, and h in Figure 3). Anhydrite and halite are mainly dominant in the northeastern part of the Sabkha. The texture of most of the clay samples in this part of the Sabkha are like soft plastic (or rubber). Accumulation of sand sheets and small sand dunes found in the south and southeastern part of the Sabkha. Recent eolian sand sheets are also present in various positions in the western part of the Sabkha. Gypsum crystals and halite decrease in abundance near the edges. Limonite founds as thin subsurface beds beside the eastern edges of the Sabkha and at the surface in the northern part of the Sabkha.
Laboratory Study
X-ray Diffraction
From X-ray study, it emerged that the clay minerals that are dominant in the samples are palygorskite, chlorite and illite (Tables 2 and 3, and Figures 4-8). Some samples included small amount of dolomite (Samples No. 3, 6 and 8 in Table 2) and other samples included anhydrite (Samples No. 1, 4 and 5 in Table 2). Based on the peak area measurements, the mean percentage of dolomite in the samples is 2% (approximate percentage is 2%-7%) and it is 7% for anhydrite (approximate percentage is 25% -49%) as shown in Table 2. The mean percentage of palygorskite in the clay deposits included dolomite and anhydrite is 59% (approximate percentage is 34%-79%) and it is 65% after excluded dolomite and anhydrite from the samples (approximate percentage is 47%-85%). The mean of the percentage of the total of the chlorite and illite minerals in the samples included dolomite and anhydrite is 23% and 9% respectively (approximate percentage is 7%-40% for chlorite and 4%-13% for illite). It is 25% for chlorite and 10% for illite (approximate percentage is 8%-40% for chlorite and 6%-15% for illite) after excluded dolomite and anhydrite from the samples (Tables 2 and 3). The main peak of chlorite is at 14.2 A° and tends not to be sharp, but slightly more rounded shape (Figures 5-8).
Table 2: Approximate percentages and mean of XRD peak areas for clay minerals in Dukhan Sabkha deposits (included dolomite and anhydrite).
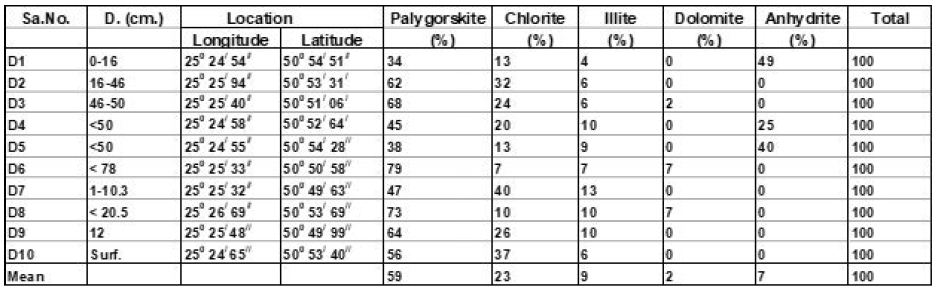
Table 3: Approximate percentage and mean of XRD peak areas for clay minerals in Dukhan Sabkha deposits (excluded dolomite and anhydrite).
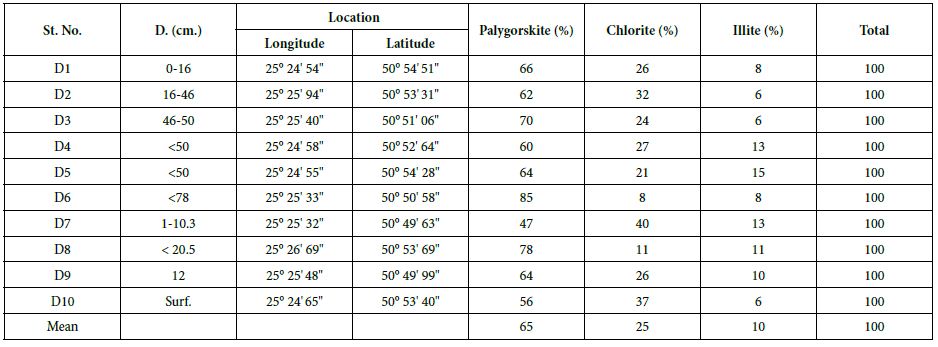
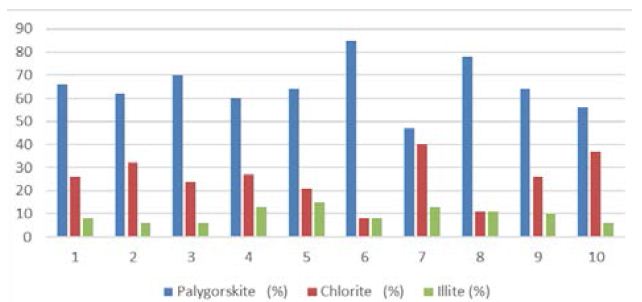
Figure 4: Mean total percentage of clay minerals (peak areas) dominant in Dukhan Sabkha
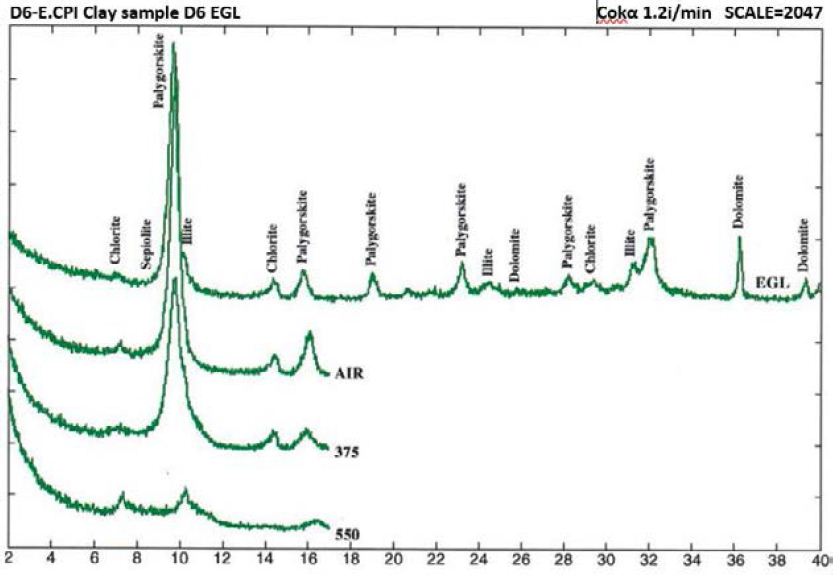
Figure 5: Clay mineral peaks, Dukhan Sabkha, Sample D6 (XRD analyses)
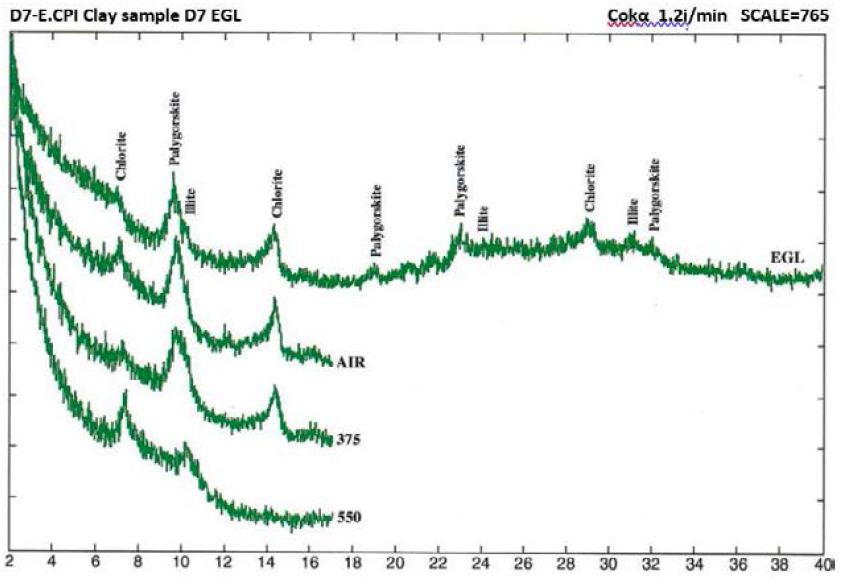
Figure 6: Clay mineral peaks, Dukhan Sabkha, Sample D7 (XRD analyses)
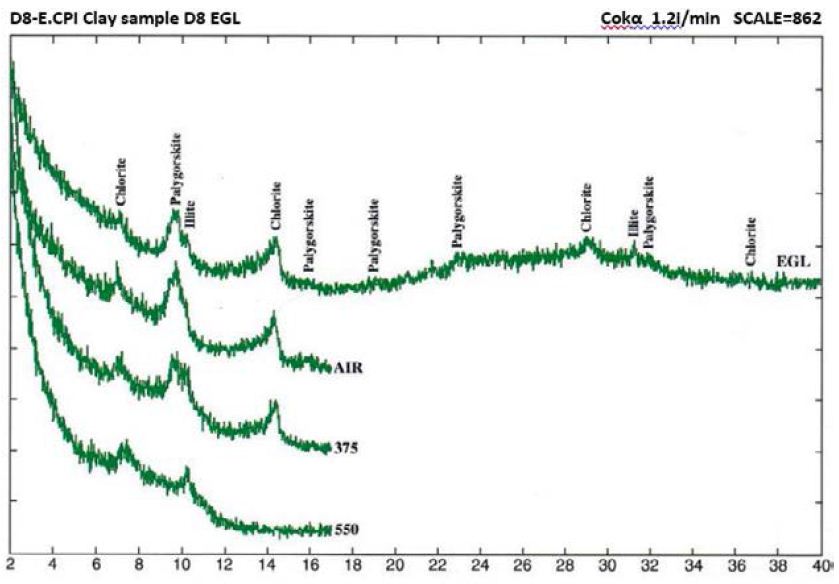
Figure 7: Clay mineral peaks, Dukhan Sabkha, Sample D8 (XRD analyses)
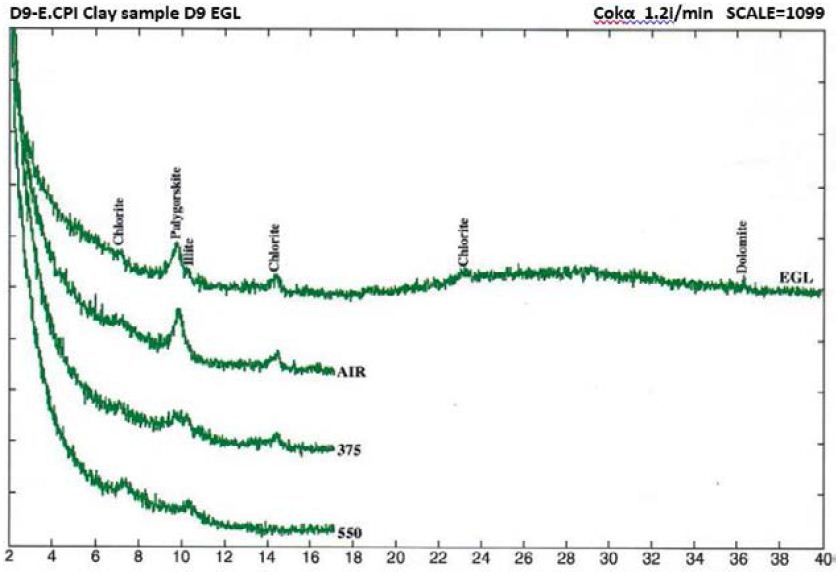
Figure 8: Clay mineral peaks, Dukhan Sabkha, Sample D9 (XRD analyses)
EDX Analyses
The EDX analyses of the palygorskite needles shows that the Si is the dominant element, followed by P, Mg, Al, Ca, and K (Figures 9 and 10). Because the size of phosphate nodules is extremely minute (<2 μm) and the spot used in the analyses is 2-3 μm diameter area and 2-3 μm deep, the X-ray beam covers the surrounding clays in addition to the phosphate nodule. P is the main peak and then Si, Mg, Ca, Al and K in that order.
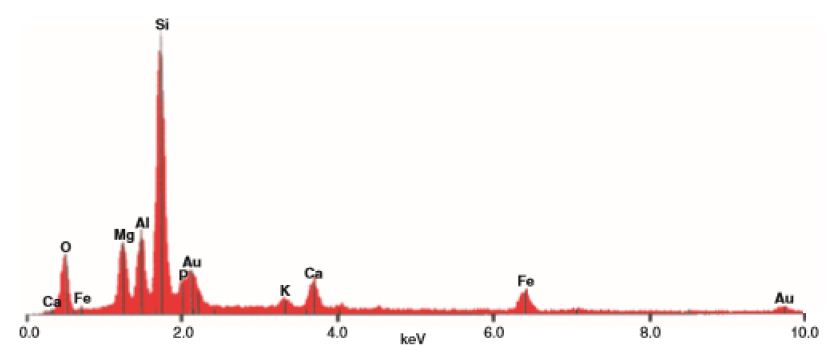
Figure 9: EDX analyses of clay sample (D6) from Dukhan Sabkha (See Figure 11). Au: from the gold coating
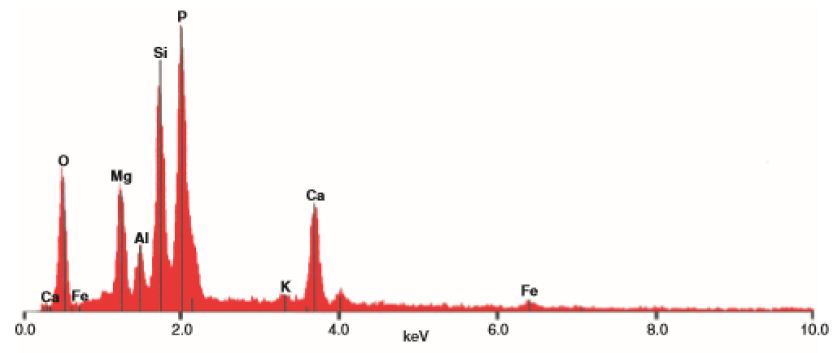
Figure 10: EDX analyses of main elements in minute sphere plus surrounding area, Sample D6 from Dukhan Sabkha (see Figure 11). Note the size of the phosphorous peak in the clay sample D6 above.
SEM Survey
The SEM study of the clay samples from Dukhan Sabkha shows various proportions of long, fibrous palygorskite crystals (Figures 11-14). Phosphate nodules of a globular shape (spherulites) in various sizes (1-<2 μ) also occur in most of the clay samples (Figures 11-14). Some of the samples include a high proportion of extremely minute phosphate nodules of spherical shape and less than 2 μm diameter. A small protuberance appears on one side of some of the relatively large spheres (Figures 13 and 14). In some samples, the phosphate nodules were present in small groups connected together as bunches (Figures 1 and 2).

Figure 11: Palygorskite fibers in various sizes. (1-<2 μm), Dukhan Sabkha, sample D6

Figure 12: Palygorskite fibers and illite with minute spheres of phosphate (1 μm), Dukhan Sabkha, sample D6
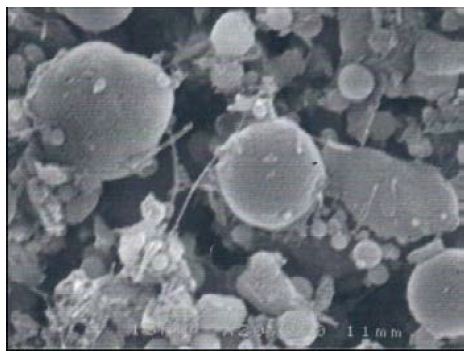
Figure 13: Minute spheres of phosphate with some palygorskite and illite, Dukhan Sabkha, sample D8
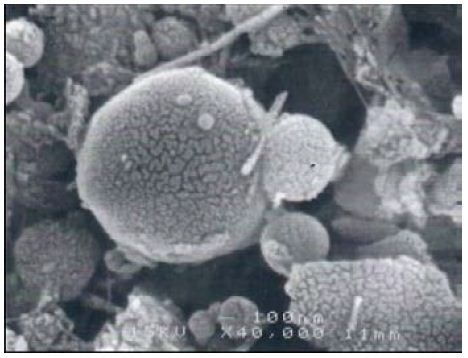
Figure 14: Minute spheres of phosphate, Dukhan Sabkha, D8
Discussions
Sabkha Sediments
Most of the surface of the Dukhan Sabkha is covered with compact firm crust of sand, silt, clay mud, halite and fine grains of gypsum with fine carbonate sediments. The thickness of the crust is mostly between 1 to 3 cm thick. It dominant in the northern part of the Sabkha than in the southern part and not presents at the edges of the Sabkha. There are several natural factors prevailing in the area have played a major role in formed such as this crust within the Sabkha. The first important factor is the surface of the Sabkha is flat, about one meter below sea level and this leads to feeding Sabkha basin by great rate of groundwater. This water reaches to the Sabkha through subsurface water drainage that feed Sabkha with seawater, mainly from the western coast of the Qatar Peninsula. Because of the hot, dry climate, prevailing in Qatar during of the year seasons the water exposed to high evaporation process within the Sabkha, accordingly the previous compact crust formed. The second factor is the groundwater level in the northern part of the Sabkha is shallower than that in the southern part and this is why the crust is dominant in the northern part of the Sabkha than in the southern part. This reason also helped in increase the thickness of crust in the northern part of the Sabkha more than in the southern part. The third factor is the accumulation of sand by the prevalent northern and northwestern winds worked to prevent the formation of the crust at the edges of the Sabkha. The dominant sediment facies in the Dukhan Sabkha are sands with variable amounts of evaporite precipitates. Of secondary importance are sandy silts, muds and algal/microbial mat deposits. Mud occurs as a minor facies in selected areas. It is generally silty in nature, some with fine sand. It is dominantly siliciclastic in composition, more rarely calcareous, and commonly contains some evaporite precipitation. It is generally structureless, sometimes with desiccation cracks at the surface. The main types are mud with evaporite, mud with little or no evaporite and mud with phosphate nodules. On the other hand, the proportion of clay and silt in the sediments is high. The silt and clay facies increase in abundance towards the Sabkha margins, particularly along the eastern and northwestern edges. The surface deposits of these edges are different from those in the other parts of the Sabkha; the amount of gypsum and halite decreases, while the proportion of silt and clay increases. This is probably because the presence of surface water drainage brings rainwater and fine sediment to inside the Sabkha from outside especially during the rainy period. We cannot ignore the presence of another source supply the Sabkha with clay deposits. Probably the clays and shales of the exposed deposits of Midra (and Sila) shale of the Lower Dammam Formation and the Lower beds of the Simsima Member of the Upper Dammam Formation in Qatar forms a secondary source. In the northeastern part of the Sabkha, the clay is mainly present on the surface and at shallow depths (few centimeters from the surface). The texture of most of the clay samples is like soft plastic (or rubber). Undoubtedly there are more than factor worked in combined to give this texture for clay in this part of the Sabkha, such as the porosity and permeability of the deposits, percentage of silica, pressure on the sediments and percentage of the salinity of water. Al- Yousef [20] found that the TDS of the brine in the northeastern part of Dukhan Sabkha is 112.8 ppt and it increases from east to west. She believed that this is because the eastern part of the Sabkha is receiving amount of fresh groundwater as drainage from the main northern aquifer and from small ground lenses located to the east, whereas the western part of the Sabkha is more affecting by seawater. However, this recent study believed that such as this type of clay (soft plastic) is need special, independent study, especially it has not previously been studied or referred to in the previous studies of the clay minerals of the geological formations of the State of Qatar.
Palygorskite (Attapulgite)
Palygorskite is an aluminum-Magnesian silicate (2MgO3 SiO2. 4H2O-Al2O3 5SiO2. 6H2O) [25], in which the ratio of Mg: Al is varies from approximately 3:1 to 1:3 [26]. Palygorskite is not common in most clay-rich deposits, but does form authigenically in arid-region soils rich in Mg and Si, in alkaline lakes and in hypersaline sediments such as Sabkhas. It is well known throughout the Arabian Peninsula region, and occurs as a windblown component of sediments in the Arabian Gulf and Indian Ocean. It can also form authigenically in volcanic sediments and in calcareous soils. Waver [27] and Isphording [28]. It can transported by rivers as a detrital mineral and as windblown dust to other continental or marine environments [29]. There is no studies give details about the palygorskite in the deposits of the formations in Qatar or even about its presence in the Sabkhas deposits of Qatar. All the studies that dealt with clay deposits indicated only the presence of this mineral without gave details about it. Cavelier [9,10] observed that the Midra (and Saila) Shales Member of the Lower Dammam Formation mainly consist of fibrous clays of the attapulgite family. The shales have varying proportions of carbonate (calcite and dolomite) with numerous lenticular or nodule of phosphates and secondary black iron oxide. Cavelier [10] also stated that the deposits at the bottom (over 5 m) of the Simsima Member (lower part of the Upper Dammam Formation) in Qatar included marls and attapulgite shales (palygorskite) of red color, quite rich in marine fossils overlain by reddish granular limestone. Alkuwari [16] from her study of the Miocene argillaceous rocks in the southwestern part of Qatar found that the clay minerals are illite, kaolinite, Ca, Mg-montmorillonite and attapulgite. Aba-Husayn and Sayegh [30], from their mineralogical study of Al-Hasa desert soil in Saudi Arabia (20 soil horizons and beds of adjacent outcrops) found that it includes palygorskite (between 37% and 65%). They also found that palygorskite not only occurs in Al-Hasa, but also widely distributed throughout the Arabian Peninsula.
This recent study shows that palygorskite is the dominant clay mineral in the Dukhan Sabkha sediments. From XRD analyses, it appeared that the mean percentage of this mineral in the clay deposits included and excluded dolomite and anhydrite from the deposits is 59% and 65% respectively and the approximate percentage is between 34%-79% and 47%-85% respectively (Tables 2 and 3, and Figures 4-8). The EDX analyses of the palygorskite needles showed that the Si is the dominant element, followed by P, Mg, Al, Ca, and K (Figures 9 and 10). In indeed, P is more dominant than S, but because the size of the phosphate nodules extremely minute (<2 μm) and the analyses spot is 2-3 μm diameter area and 2-3 μm deep, the X-ray beam covers the surrounding clays in addition to the phosphate nodules, and this is why S appeared more dominant than P. Phosphate nodules of a globular shape (spherulites) in various sizes (1-<2 μm) also occur in most of the clay samples that rich in palygorskite (Figures 11-14). In some samples, the phosphate nodules were present in small groups connected together as bunches (Figure 12). With the presence of this high percentage of palygorskite in all the analyzed samples, the most likely the origin of palygorskite in the Dukhan Sabkha sediments is authigenic. This interpretation supported by the following: 1). the suitability of the Sabkha environment for palygorskite formation. It is an inland Sabkha, situated in flat depression of about one meter below sea level, feeding by subsurface seawater of shallow level, includes various types of deposits (evaporite, silica and carbonates), and chemical reaction within the Sabkha is active due to the hot climate conditions. These conditions probably provide the appropriate proportions for the elements for formation palygorskite mineral in the Sabkha. This saying can be supported by the results that reached by many of researchers. Al-Yousef [20] from XRD analyses of 36 sediment samples from Dukhan Sabkha found the mean percentage of gypsum and halite after excluded carbonate and clastic minerals from the samples is 46.1% and 46.8% respectively. With carbonate and clastic minerals, the main percentage of gypsum is 22% and it is 15.6% for halite. The mean percentage of dolomite in the samples is 10.4% and 17.2% included and excluded evaporite from the calculation respectively. She also found there is a good relationship between TDS and Mg2+, R2= 0.98 and good relationship between TDS with Mg2+/Ca2+; R2= 0.7. From XRF study of geochemical composition she found that the major elements oxides in the sediments is SiO2 and it various from trace amount to 75% and its respective mean value is 37%. The next most common major oxides present are MgO and AL2O3. The mean values for MgO is between 4.93% and 7.34% and it is between 2.228-3.04% for Al2O3. The overall mean percentage of CaO is 15.51% and reach to 20.08% in the northeastern part of the Sabkha.
Al-Youssef, 2015 [31] from her study of 22 shallow brine samples from various locations in Dukhan Sabkha at depth between 25-120 cm (temperature range for the brine was 21°C-23°C) found that the main of pH is 6.8 (range between 6.4 and 7.3). The mean for Na+ is 38.6 ppt, 62.1 ppt for Cl– and 3.8 ppt for Mg2+. Khademi and Mermut [28] in their study of the source of palygorskite in gypsiferous aridisols and associated sediments from central Iran mentioned that palygorskite probably formed after the initial precipitation of gypsum that created a high pH and Mg/Ca ratio. Hassouba and Shaw [33] mentioned that the formation of gypsum rise the Mg/Ca ratio of the water which could in turn encourage palygorskite formation. Maurice, E. Tucker [29] believed that shallow saline lakes during the Tertiary were chemically favorable for the formation of fibrous silicate clays. This is because formation of gypsum increase the Mg/Ca ratio, which under an evaporative environment brought about the authigenic formation of large amount of palygorskite. He believed that palygorskite could precipitated directly from water or pore water in surficial sediments in Mg rich alkaline lake. Khormali and Abtahl [35] in their study of origin and distribution of clay minerals in calcareous arid and semi-arid soils of Fars Province, southern Iran found that the percentage of palygorskite in soils, significantly related to the gypsum content and P/ETº of the soils. Singer [36] believed that an increase in gypsum or decrease in the P/ETº, or increase in aridity would lead to higher levels of palygorskite in soils. He add that the presence of shallow saline and alkaline groundwater could also have favor formation of palygorskite from soil solution and under such conditions palygorskite may form from smectite. Callen [26] mentioned that Shallow hyper-saline water bodies appear to have been chemically suitable for formation of palygorskite and sepiolite. No doubt that all of these are good indications for the possibility of the formation of palygorskite mineral inside Dukhan Sabkha. 2). the clear complete sharp and delicate needle-like crystalline structure observed by SEM, this gives good evidence that the needle crystals of palygorskite not carried from outside the Sabkha and if it is, they will broke. 3). the clear sharp peak present on all x-ray diffraction traces. 4). there is no smectite mineral recording in the samples and this is may be because the elements of this mineral utilized in forming the palygorskite mineral within the Sabkha. 5). scientifically it is proven that palygorskite can forms authigenically in arid-region soils rich in Mg and Si, in alkaline lakes and in hypersaline sediments as the condition in the Dukhan Sabkha.
However, in this study, we cannot ignore the sediments that transport to inside the Sabkha by the wind or by the surface water discharges, they inevitably forming second source for this mineral inside the Sabkha. This is because palygorskite is present in the shales of Midra (and Sila) shale of the Lower Dammam Formation and in the Lower beds of the Simsima Member of the Upper Dammam Formation that exposed in different locations on the surface of Qatar. A small proportion of palygorskite could be also, carried to Qatar as dust by the northern wind or by water currents from the neighboring areas, especially from the eastern part of the Arabian Peninsula. With regard to phosphate nodules in Sabkha deposits, there is several reasons may have played a role in formed these nodules such as: Microorganisms in the Sabkha; limestone sediments within the Sabkha deposits (by replace the element of preexisting limestone rock). It possible also some of these nodules came with the clay sediments from outside the Sabkha by wind and by surface drainage during heavy rains.
Chlorite
Chlorite is a second clay mineral dominant in the Dukhan Sabkha sediments. Chlorites (Mg, Fe, Al)6 (Si, Al)4 O10 (OH)8 are a group of hydrous silicates of magnesium, aluminum and iron in widely varying proportions. The main source of chlorites is metamorphic rocks, but many of them appear to be either authigenic or diagenetic [37]. Chlorites are less common in igneous rocks, but very common in shales and muddy sandstones. Most of the chlorite in sediments is detrital in origin from metamorphic rocks or from pre-existing sedimentary rocks [33]. Weaver [37] observed that Mg-rich chlorites are found in evaporite deposits, such as dolomite and halite. Under evaporitic alkaline conditions, Mg concentrations and Mg/Ca are high and Mg-chlorite is stable. Maurice [34] mentioned that chlorite forms during intermediate stages of leaching in temperate soils but it is more easily oxidized and so occurs preferentially in acid soils. It also forms in soils of arid regions, both high and low latitude where chemical processes are minimal. Weaver [37] believed that chlorite is form by-product of the conversion of smectite to illite. He add that the chlorite packets consist of interstratified 7 Å and 14 Å layers. The 14 Å phase has the following structural formula: (Al1.5 Fe3.1 Mg1.1 (Si2.8Al1.2) O10 (OH)8. The 7 Å chlorite is normally a low-temperature phase that converts to the 14 Å polymorph at higher temperatures. Khormali and Abtahl [35] mentioned that, the transformation of chlorite into smectite was rarely been reported in contrast to many reports about illite to smectite transformation, which seems rather more likely. Chester et al., [39] considered that the high concentration of chlorite in the sediments of the northern Arabian Sea and the Gulf of Oman are carried to the area as dust by the northeast monsoon during the winter from the adjacent arid landmasses. They found that in all seven of the eolian dust the average is approximately 30%. Wilson [40] and Khormali and Abtahl [35] mentioned that chlorite and illite are the major clay minerals of both the parent rocks and soils of southern Iran. Venkatarathnam, et al. [41] reported chlorite values in the sediments of the northern Arabian Sea and the Gulf of Oman as all being greater than or approximately equal to 30% with general decrease westwards towards the Indian coast. The authors postulated that the source may be an eolian one and that an input of atmospheric dust from the surrounding arid regions of Iran-Makran, the Arabian Peninsula and Somalia might be significant source of chlorite in the northwest Arabian Sea.
In Qatar, chlorite is widespread as a detrital clay mineral both in the more clay-rich sedimentary rocks and in soil samples [5, 10, 30, 42]. Unfortunately, there is no detailed studies of chlorite minerals in the sediments of Qatar. All the previous studies that dealt with the geological formations and sediments of Qatar only referred the presence of this mineral within the clay minerals of Qatar without give any details. In the present study, the X-ray analyses of clay samples showed that the proportions of chlorite in the samples is smaller than the amount of palygorskite (Figure 4).
Based on the peak area measurements, the main percentage of chlorite in the samples, included dolomite and anhydrite is 23% and the range is between 7%-40%. After excluded dolomite and anhydrite minerals from the samples, the mean percentage is 25% and the range is between 8%-40%. The main peak of chlorite is 14.2 Å and tends not to be sharp, but a slightly more rounded shape (Tables 2 and 3, and Figures 5-8). For several important reasons this study is exclude the formation of chlorite minerals within the Dukhan Sabkha. The most consideration of these reasons are: 1). the providers suitable conditions for formation chlorite minerals in the Dukhan Sabkha are not appeared in this study. Such as suitable conditions for transformation, presence of metals that usually contain this mineral like schist, fillet or silicate minerals containing aluminum, iron, and magnesium as: pyroxenes, amphiboles, biotite, garnet, and or even hot solutions. 2). the pattern of the main peak of chlorite mineral is not sharp as appeared in Figures 5-8.
This pattern is commensurate with a detrital origin either as windblown dust or by floods. 3). no evidence was record in this study indicate that there is changes was happened to the chlorite that would affect its percentage in the deposits. For example there is no smectite minerals found in the samples to say there is part of the chlorite transformed to smectite. In addition to that, we did not recorded any present of kaolinite to say there is replacement process happened and affected on the formation of chlorite minerals in Dukhan Sabkha deposits. 4). even though, chlorite usually common in shales and muddy sandstones, we found in Dukhan Sabkha it is not form a main clay mineral in the Sabkha sediments. Because of the previous conditions of formation of chlorite, this study strongly believed that the chlorite mineral in the Dukhan Sabkha is detrital in origin from pre-existing sedimentary rocks outside the Sabkha area, especially detrital rocks derived from shales of the Tertiary (Dammam, Dam and Rus Formations) in Qatar. Some of the chlorite may reached to inside the Sabkha from outside Qatar by sea currents and blown winds after mixed with the sediments of Qatar. Accordingly, the study can confirm that the chlorite minerals does not form within the Dukhan Sabkha.
Illite
Illite is a hydrous potassium dominant-silicate. It consists of K0.8-0.9 (Al, Fe, Mg)2 (Si, Al)4 O10 (OH2). The mineral has two tetrahedral and one octahedral layer, with an interlayer ion of potassium holding the layers together [25]. Half or more of the clay minerals in the earth crust is illite. Illite can be formed in the oceans or on the continents. At the present time more illite appears to be formed on the land from the weathering of K-feldspar than in the oceans [38]. Deer and others [43] mentioned that illite is formed where silica is abundant and potassium is present. It is usually formed from igneous and metamorphic rocks, also by weathering of feldspar in cool climate. Weaver [37] mentioned that the presence of excess Na, Ca or Mg in solution may have inhibited Al3+ uptake from solution. In nature, illite might form by either solid-state reorganization or dissolution- re-precipitation. Weaver believed that the acid conditions created by the CO2 is increase the solubility of K-feldspar and facilitate the growth of illite layers and variations in the organic content could have an effect on the rate of conversion of smectite to illite. Maurice [34] mentioned, where the degree of leaching is limited, as with many soils in temperate areas, then illite is the typical clay mineral formed. Weaver [37] mentioned that, in the Gulf Coast where smectite and illite coexist in direct contact, as individual phases and over a wide range of temperature, smectite + illite cannot be an equilibrium assemblage. The degree of the reaction of smectite to illite must be controlled by kinetic factors, such as: temperature, time, activities of chemical components and rock/water ratio. Khormali and Abtahl [35] from their study of clay minerals of Fars Province, southern Iran found there is simple transformation of illite to other clay minerals (mainly smectite). They mentioned that this might play a major role in the decrease in illite content with the depth. Kolla et al. [44] mentioned that illite is the dominant clay mineral (>40-50%) in most sediments of the western Arabian Sea, including Gulf of Oman and Murray ridge off the Iran-Makran region and the Owen Basin and Owen Ridge of the Arabian Peninsula. They postulated that the source may be an eolian one, and that an input of atmospheric dust from the surrounding arid regions of Iran-Makran, Arabian Peninsula and Somalia might be significant in the northwest Arabian Sea. Chester, R. et al. [39] found in all seven of the eolian dusts, illite is the dominant clay mineral in the sediments of the northern Arabian Sea and the Gulf of Oman, with an average of greater or approximately equal to 50%. Hilmy et al [16,17] mentioned that that the Precambrian rocks in western Saudi Arabia represented the only possible source for the detrital fraction of the Miocene argillaceous rocks. Consequently, it is most probable that the illite originated from feldspars and alumino-silicates in these rocks under mild to strongly acidic conditions and favorable potassium equilibrium. These conditions involved considerable leaching with the subsequent removal of Na+ and the divalent cations as Ca2+, Mg2+ and Fe2+. Ecclestone et al., [42] found that the deposits of the depressions in Qatar contain 40% illite and 10% illite-smectite. Alkuwari [16] from the study of the Miocene argillaceous rocks in the southwestern part of Qatar stated that illite and kaolinite are present in nearly all the rocks. Cavelier [11] from his analyses of 11 clay samples of the Lower Dam Formation from near Abu Samra in the southwestern part of Qatar found that they included illite. Abu-Zeid and Alkuwari [15] mentioned that the clay mineral composition of argillaceous rocks of Dam Formation of Miocene stratigraphic sequences, located in western and southwestern Qatar are consist of illite, kaolinite, attapulgite and Ca, Mg-montmorillonite. They found illite mineral is present in the oldest rocks while, it disappears in the overlying younger sediments. They believed that illite and kaolinite formed by detrital inheritance from weathering horizons and / or soils on the Precambrian rocks in western Saudi Arabia and transported by freshwater and/or wind to the basin of deposition in Qatar. They add that the suitable condition for formed this mineral is not found in Qatar, such as deep burial involving high temperatures and pressures. Aba-Husayn and Sayegh [30] from X-ray study of Al-Hasa desert soil found that it included 6% to 31% illite. The Attapulgite and illite are the most abundant and common clay minerals in the soils and strata, but are most pronounced in the strata. Sander [45] mentioned that the surface Eocene rocks in Qatar Peninsula are a part of the Al-Hasa Group. The Eocene rocks cover more than 80% of the surface of Qatar Peninsula [46].
In this recent study, it appeared that illite is a third clay mineral dominant in the Dukhan Sabkha sediments (Tables 2 and 3, and Figures 5-8). It forms around 10% in all analyzed samples for this study, with a somewhat ragged peak occurring at 10 Å. Based on the peak area measurements, the main percentage of illite in the samples included dolomite and anhydrite is 9% (range between 4%-13%( and it is 10% after excluded dolomite and anhydrite minerals from the samples (range between 6%-15%). This study excluded the possibility of formation illite mineral in the Dukhan Sabkha sediments for several reasons, which are: 1). the suitable conditions for formation illite mineral are not dominant in the Dukhan Sabkha such as present of feldspars, acidic conditions, the divalent cations as Ca2+, Mg2+ and Fe2+, high temperatures, and suitable pressure. 2). the study did not record any smectite minerals in the studied samples to say that the source of this small amount of illite formed because of alteration smectite to illite. 3). the study also, did not record any presence of kaolinite to say that there is replacement process happened and affected the amount and process of formation illite mineral in the Dukhan Sabkha deposits. 4). no igneous or metamorphic rocks in the Dukhan Sabkha deposits, even within its original rocks at great depths, which also reinforces the statement that the possibility of formation this mineral inside the Sabkha is excluded. Logically the illite mineral carried to inside Dukhan Sabkha from its inheritance formations parent rocks that containing this metal, whether from inside or outside Qatar.
Conclusion
Dukhan Sabkha is the largest inland Sabkha located in the western part of Qatar, covers about 73 Km2 and occupies a synclinal area. The surface of the Sabkha is flat, about one meter below sea level, except near the edges is more than 1 m above sea level because the accumulation of blown sand by Nebkhas (plants trapping sand). The lowest part of the Sabkha is 6 m below sea level and found in the south (25° 20– E, 50° 50– N). Most of the Surface of the Sabkha is covered by crust of fine deposits of sand, clay, silt, mud, halite and very fine grains of gypsum of various color between white and beige to light gray. The crust is compact and dry, and mostly between 1 to 3 cm thick. The thickness and dominance of the crust in the northern part of the Sabkha is more than in the southern part. Sand and silt are dominant in the deposits. They found on the surface or mixed with other deposits at different depths. Mud occurs as a minor facies and it is generally silty in nature, some with fine sand. It is dominantly siliciclastic in composition, more rarely calcareous, and commonly contains some evaporite precipitation. Clays are found in limited positions on the surface and at shallow depths (few centimeters from the surface). The proportion of clays decreases towards the center of the Sabkha and increases at the edges, especially where the Sabkha is supplied by surface drainage, and where microbial mats are present. It also, presents inside small number of elongated, narrow and shallow surface channels, extending from the land to inside the Sabkha. The thickness of clay-rich layers in locations such as these is between 0.5 and 1.5 cm. X-ray diffractometric was carried out for 10 clay samples, collected from the northern part of the Sabkha. The weight of each sample is about 40 g and the percentage of clay is between 25% and 40%. The range of the run was between 20 and 400 and the run was 1.20/min. The percentage of each clay mineral was calculated by dividing the area of its main peak by the total of the main peaks of the clay minerals of the same sample and multiplying by 100.
Scanning electron microscope were used to study the clay samples and to determine their fine compositions (the range of SEM is from X20 to 1000,000). Chemical analyses were also, carried out under the SEM, using energy-dispersive X-ray (EDX) spectrometry to define their dominant elements. Photographs were taken for the palygorskite and other important features present in the samples. From the study, it emerged that the dominant clay minerals in the samples are palygorskite, chlorite and illite. Based on the peak area measurements, the mean percentage of palygorskite in the clay deposits included dolomite and anhydrite is 59% (approximate percentage is 34%-79%) and it is 23% for chlorite (approximate percentage is 7%-40%), and 9% for illite (approximate percentage is 4%-13%). After excluded dolomite and anhydrite from the samples, it is 65% for palygorskite (approximate percentage is 47%-85%), 25% for chlorite (approximate percentage is 8%-40%) and 10% for illite (approximate percentage is 6%-15%). The main peak of chlorite is at 14.2 Å and tends not to be sharp, but slightly more rounded shape. Phosphate nodules of a globular shape (spherulites) in various sizes (1-<2 m) also occur in most of the clay samples, in some cases grouped into bunches. The analysis of the palygorskite needles showed that the Si is the dominant element, followed by P, Mg, Al, Ca, and K. This is because the size of phosphate nodules is extremely minute (<2 μm) and the spot used in the analyses is 2-3 μm diameter area and 2-3 μm deep, the X-ray beam covers the surrounding clays in addition to the phosphate nodule. In indeed, P is the main peak and then Si, Mg, Ca, Al and K in that order.
Most likely, the origin of palygorskite in the Dukhan Sabkha sediments is authigenic. This is because: 1). the suitability of the Sabkha environment for palygorskite formation. It is an inland Sabkha, situated in flat depression of about one meter below sea level, feeding by subsurface seawater and the chemical reaction within the Sabkha is active due to hot climate conditions. These conditions probably provide appropriate proportions for elements for formation palygorskite mineral in the Sabkha. Palygorskite can does form authigenically in arid-region soils rich in Mg and Si, in alkaline lakes and in hypersaline sediments (such as Sabkhas). In addition, formation of gypsum rise the Mg/Ca ratio of the water, which could in turn encourage palygorskite formation. 2). the clear complete sharp delicate needle-like crystalline structure observed by SEM, this gives good evidence that the needle crystals of palygorskite not carried from outside the Sabkha and if it is, they will broke. 3). the clear sharp peak of palygorskite, present on all x-ray diffraction traces. 4). there is no smectite mineral recording in the samples and this is may be because the elements of this mineral utilized in forming the palygorskite mineral within the Sabkha. 5). scientifically it is proven that palygorskite can forms authigenically in arid-region soils rich in Mg and Si, in alkaline lakes and in hypersaline sediments as the condition in the Dukhan Sabkha. Definitely, the sediments that transport to inside the Sabkha by the wind or by the surface water discharges are forming a second source for palygorskite inside the Sabkha. This is because palygorskite is present in the shales of Midra (and Sila) shale of the Lower Dammam Formation and in the Lower beds of the Simsima Member of the Upper Dammam Formation that exposed in different locations on the surface of Qatar. A small proportion of palygorskite could also reach to inside Dukhan Sabkha after carried to Qatar as dust by the northern wind or by water currents from the neighboring areas, especially from the eastern part of the Arabian Peninsula. Regard to the phosphate nodules in the Sabkha deposits, there is several reasons for formed these nodules such as: 1). Microorganisms in the Sabkha deposits. 2). Replacement the elements of preexisting limestone rock within the Sabkha deposits. 3). Some of these nodules came with the clay sediments from outside the Sabkha (specially Midra and Saila shales in Qatar) by wind and by surface drainage during heavy rains. However, a separate study is inevitably required to verify this. Chlorite and illite minerals both are interpreted as detrital in origin probably from within Qatar and possibly part of them are bring by windblown from Arabian Peninsula. This is based on several evidences such as: 1). the suitable conditions for formation these two minerals are not dominant in the Dukhan Sabkha such as: presence of metals that usually contain these minerals, suitable pressure, high temperatures and hot solutions for transformation. 2). There is no any evidences was recorded by this recent study for formation chlorite and illite within the Sabkha sediments or any proof indicate that there is changes was happened to the chlorite or illite that would affect their percentage in the deposits. 3). the study also, did not recorded any evidence for replacement process happened in the Sabkha and affected the amount and formation of these two minerals in the Sabkha deposits. 4). the pattern of the main peak of chlorite mineral is not sharp it is commensurate with a detrital origin either as windblown dust or by floods. 5). no igneous or metamorphic rocks in the Dukhan Sabkha deposits, even within its original rocks at great depths, which also reinforces the statement that the possibility of formation these mineral inside the Sabkha. According to the previous results, this study confirm that both of chlorite and illite minerals in the Dukhan Sabkha is detrital in origin from pre-existing sedimentary rocks outside the Sabkha area; especially detrital rocks derived from shales of the Tertiary (Dammam, Dam and Rus Formations) in Qatar. Some of these minerals may reached to inside the Sabkha from outside Qatar by sea currents and blown winds after mixed with the sediments of Qatar.
Recommendation
- This study has highlighted the necessity for further research to address many specific questions and gaps in our knowledge to the origin and formation of clay minerals in Dukhan Sabkha soils, in addition to do further study of the chemical characteristics and nature of the clay minerals in the geological formations deposits of Qatar. There is an urgent need also, to make detailed study and comparison for the types, percentages and chemical properties of clay minerals in the sediments of depressions (Rawdat) in addition to the deposits of the other Sabkhas areas in Qatar.
- A wide-ranging study to identify the reasons of formation and dominant of palygorskite within Dukhan Sabkha sediments is need to be undertaken. In addition to that, the formation of phosphate nodules of a globular shape and various sizes (1-<2 μ), also need more study to focus on the main reason for formation and dominance of these nodules, especially in the locations where palygorskite prevalent.
- Focus study need to be make on the clay that distinguished by plastic (or rubber) texture that dominant in the northeastern part of the Sabkha.
References
- Ashour MM, Abdul Mogeath MS, Metwelly AA, Al-Ghzally AJ, Abdul Gaor AS, et al. (1991) El-Sabkhat in Qatar Peninsual (geomorpholgical study-vitality-geological). Humanities and Documentation research centre, University of Qatar- Doha. Pg: 514 (in Arabic).
- Illing LV, Wells AJ, Taylor JCM (1965) Pencontemporary dolomite in the Persian Gulf. In Dolomitization and Limestone Diagenesis. Symposium. Edited by Pray, L. C. and Murray, R.C. Society of Economic Paleontologists and Mineralogists. Special Publication. No. 13. Tulsa, Oklahoma, U.S.A., pg: 89-111.
- Kinsman DJJ (1966a) Gypsum and anhydrite of recent age, TrucialCoast, Persian Gulf. Reprinted from second syymposium on salt. 1: 302-326. Northern Ohio Geological Society Cleveland, Ohio.
- Wood GV, Wolfe MJ (1969) Sabkha cycles in the Arab/Darb Formation of the Trucial coast of Arabia. Sedimentology 12: 165-191.
- FAO (1973) Reconnaissance soil survey and land classification. Hydro-Agricultural resources survey, Qatar. AGL: DP/Qat./71/501, technical report No.1, UNDP/FAO, Rome, 52 pp.
- Warren JK (1989) Evaporite sedimentology. Importance in hydrocarbon accumulation. Prentice Hall, Englewood Cliffs, New Jersey 07632.
- Al-Hargan AAK (1997) Creation of coastal zone information system for Qatar using remote sensing and GIS. Ph.D thesies. Centre for Environmental Sciences. Faculty of Science. Pg: 268.
- Al-Saafin Adly KH (1996) The characterization of sabkhas in the eastern part of Saudi Arabia and its implications for engineering. PhD. thesis. University of London, Queen Mary and Westfield College, Department of Engineering, pg: 300.
- Cavelier (1970a) Geological description of the Qatar Peninsula. Department of Petroleum Affairs. Bureau de Recherches Geologiques et Minieres, Paris, France, pg: 39.
- Cavelier C (1970b) Geological survey and mineral substances exploration in Qatar, Arabian Gulf. Government of Qatar, Department of Petroleum Affairs, pg: 100.
- Cavelier C (1975) Lexique Stratigraphique International Tertiary in Qatar Peninsula Asie, tome III, fasc. 10 b 3, (CNRS), Paris, pg: 90-120.
- FAO (1974) Water resources and use. Report prepared for the Government of Qatar. Hydro-Agricultural Resources Survey. United Nations Development Programme, p g: 9-122. Goldberg ED and Griffin JJ (1970) The sediments of the Northern Indian Ocean: deep sea research,17: 513-537.
- Seltrus Engineering Ltd (1979) Investigation of the development potential of mineral occurrences in Qatar. Final report. Vol. 7. Building and ornamental stone. Industrial Developments, Qatar. (Unpublished report).
- Abu-Zeid MM, Khalifa H (1983) Sedimentological and Paleoenvironmental aspects of the Miocene succession in Gebel Al-Nikhsh, Qatar, Arabian Gulf. N Jb Geo Palaont Mh. Pg: 385-399.
- Abu-Zeid MM, Alkuwari AJ (1989) Distribution, genesis and thermal behavior of clay minerals in the Miocene argillaceous rocks in Qatar, Arabian Gulf. Qatar Univ. Pg: 245-264.
- Hilmy ME, Abu-Zeid MM, Alkuwari AJ (1987) Petrography and sedimentology of the Miocene argillaceous rocks in Qatar, Arabian Gulf. M.E.R.C. Ain Shams University. Earth Sci Ser 1: 169-179.
- Al-Hitmy HH (1987) Geological, mineralogical and geochemical studies on the Sabkha deposits of Umm Said Area, east of Qatar. M.Sc. theses. Department of Geology, Faculty of Science, Ain Shams University, Cairo, Egypt, pg: 135.
- Al-Kuwari AJ (1987) Petrological, mineralogical and geochemical studies on the Miocene argillaceous rocks in Qatar, Arabian Gulf. M.Sc. thesis, Geology Dept, Faculty of Science, Ain Shams University, Cairo, Egypt, pg: 339.
- Batanouny KH (1980) Ecology and flora of Qatar. Alden Press, Oxford, U.K., Centre for Scientific and Applied Research, University of Qatar, Qatar, pg: 245.
- Al-Youself MM (2003) Mineralogy, geochemistry and origin of Quaternary Sabkhas in the Qatar Peninsula, Arabian Gulf. PhD. thesis. University of Southampton, School of Ocean and Earth Science, Faculty of Science. UK. Pg: 437.
- Perthuisot JP (1977) La sebkha de Doukane, Qatar et la transformation: gypse = anhydrite + eau, Bull. Geol. Soc. France, 7 (XIX,5), pg: 1145-1149.
- Galehouse JS (1971) Sedimentation analysis. In: procedures in sedimentary petrology (Ed. By R. E. Carver), Pg: 65-94. Wiley-Interscience, New York.
- Hardy R, Tucker M (1988) X-ray powder diffraction of sediments. In Techniques in sedimentology. Edited by Tucker, M. Blackwell Scientific Publications. Oxford, pg: 191-228.
- Tucker ME (1981) Sedimentary Petrology. An introduction to the Origin of Sedimentary Rocks. Second edition. Oxford, Blackwell Scientific Publications. London, pg: 260.
- Velde B (1995) Origin and mineralogy of clays. Clays and the environment. Springer. Borlin Heidelberg, pg: 334.
- Brindley GW (1980) Quantitative X-ray mineral analysis of clays. In Crystal structures of clay minerals and their X-ray identification. Edited by Brindley GW and Brown G. Mineralogy Society Monograph 5: 411-438.
- Waver CE (1984) Origin and geologic implications of the palygorskite deposits of S.E. United States. Pg: 39-58. In Singer A and Galan E (1984) Palygorskite-Sepiolite Occurrences, Genesis and Uses. Developments in Sedimentology, Elsevier 37.
- Isphording WC (1984) The clays of Yucatan, Mexico: a contrast in genesis. Pg: 59-73. In: Singer A and Galan E (1984). Developments in Sedimentology, Palygorskite-Sepiolite Occurrences, Genesis and Uses. Elsevier 37.
- Callen RA (1984) Clays of the palygorskite-sepiolite group: depositional environment, age and distribution. Pg: 1-37.
- Aba-Husayn MM, Sayegh AH (1975) Mineralogy of Al-Hasa desert soils in Saudi Arabia. Clays and clay minerals. Vol. 25: 138-147. Pergamon Press: Oxford New York, Paris, and Frankfort.
- Al-Youself MM (2015) Gypsum crystals formation and habits, Dukhan Sabkha, Qatar. Journal of Earth Science and Climatic Change 6: 1-12.
- Khaddmi H, Mermut AR (1998) Source of palygorskite in gypsiferous aridisols and associated sediments from central Iran. Clay Minerals 33: 561-575.
- Hassouba H, Shaw HF (1980) The occurrence of palygorskite in Quaternary sediments of the coastal plain of northwest Egypt. Clay Minerals 15: 77- 83.
- Maurice E Tucker (2009) Sedimentary Petrology, Anintroduction to the origin of sedimentary rocks (second edition). Blackwell Scientific Publications, London, Edinburgh Boston, Melbourne Paris Berlin Vienna.
- Khormali F, Abtahl A (2003) Origin and distribution of clay minerals in calcareous arid and semi-arid soils of Fars Province, southern Iran. Clay Minerals 38: 511-527.
- Singer A (1989) Palygorskite and sepiolite group minerals. Pg: 829-872. In: Minerals in Soil Environment, eds: Dixon JB, Weed SB. Soil Science Society of America. Madison, Wisconsin, USA.
- Weaver CE (1989) Clays, muds and shales. Developments in Sedimentology No. 44. Elsevier, Oxford, pg: 819.
- Weaver C, Pollard L (1973) The Chemistry of Clay Minerals. Developments in Sedimentology, 15, Elsevier Scientific Publishing Company. Amesterdam, Oxford and New York, pg: 213.
- Chester R, Sharples EJ, Sanders GS (1985) The concentrations of particulate aluminum and clay minerals in aerosols from the northern Arabian Sea. J Sed Petrol 55: 37-41.
- Wilson MJ (1999)The origin and formation of clay minerals in soils: past, present and future perspectives. Macaulay Land Use Research Institute, Craigiebuckler, Aberdeen AB15 8QH, UK (Received 23 September 1997; revised 15 January 1998.Clay Minerals (1999) 34: 7-25.
- Venkatarathnam K, Kostecki JA, Robinson F, Biso PE (1981) Distributions and origins of clay minerals and quartz surface sediments of the Arabian Sea. Jour Sed Petrology 51: 563-569.
- Eccleston B, Pick I, Harhash I (1981) The water resources of Qatar and their development. Technical note, Vol.1, no. 5, Doha Qatar, Ministry of Industry and Agriculture, Water Resources and Agricultural Development Project, Doha Qatar, 1981: 390.
- Deer WA, Howie RA, Zussman J (1975) An introduction to rock-forming minerals: Longman Group Ltd., London, 528.
- Kolla Venkatarthnam JA, Kostecki F, Biscaye PE (1981) Distribution and origins of clay minerals and quartz in surface sediments of the Arabian Sea. Journal of sedimentary petrology 51: 0563-o569.
- Sander NJ (1962) Apercu, Paleontologique et strattigraphique de Paleogene en Arabic Secudite orientale. Rev de Micro-Pleontologic 5: 3-40
- Hewaidy A, Al-Saad H (1993) Surface Eocene Stratigraphy of Qatar Peninsula. Al-Azhar Bull Sci 4: 165-193.