Abstract
There is growing interest in the application of transition metal oxide and peroxide nanoparticles in materials science and industrial engineering. Transition metal oxide nanoparticles (TMONPs) can form materials with a wide range of unique chemical, electronic and physical properties for manufacturing various commercial products. Presently and for the foreseeable future, transition metal oxides are important constituents of energy production systems. They can be modified by various chemical, mineral, and polymeric substances, to produce nanocomposites that are suitable for the fabrication of more advanced materials. In this review article, discussion will focus on zinc oxide (ZnO) and zinc peroxide (ZnO2) which are chemically related but technologically different in their applications. The toxicity of biochemically active ZnO nanoparticles has led to the development of new analytical methods with a focus on capillary electrophoresis with UV absorption or molecular fluorescence detection. The importance of wastewater analysis is emphasized with an outlook on the future perspectives in this fast-advancing research field. Next, the unique chemical properties and physical characteristics of ZnO2 nanoparticles are introduced, followed by their latest applications in and modifications for scientific endeavors. Methods suitable for their incorporation in altering membrane properties to improve filtration performance are detailed. Major challenges and research endeavors are highlighted in the design of more effective membranes for wastewater filtration, hereby ameliorating the environmental toxicology of effluent contaminants. Polymeric membranes incorporated with composite zinc peroxide nanoparticles is proposed to be a good replacement of zinc oxide due to their strong oxidative properties involving a higher number of reactive oxygen atoms per molecule. The latest understanding of zinc oxide/peroxide nanomaterials toxicology is described, and best practices are crucial to control their environmental distribution. Last, the key research gaps that need to be addressed for future assessment of toxicological risks are unveiled.
Keywords
Biosensors, Electrochemical analysis, Engineered composites, Membrane filtration, Nanoparticles, Toxicology, Transition metal oxides, Ultrafine dust, Wastewater, Zinc oxide, Zinc peroxide
Introduction
In recent years, advanced technology with nanoparticles have greatly sparked the interest of the scientific community. Their small sizes and tunable functional properties make them appealing as unique structures for biomedical applications, ranging from bioimaging, biosensing, drug delivery and theranostics. Material scientists have focused their research on the synthesis of transition metal oxide nanoparticles (TMONPs). Transition metal oxides exhibit unique physicochemical properties including catalytic function, ferroelectricity, piezoelectricity, magnetism, and supercapacitor performance. These oxides are fascinating to work with because their electrons interact strongly with each other, giving rise to a range of phenomenal effects such as high-temperature superconductivity and magnetoresistance. A comprehensive review summarizes different methods for synthesizing (TMONPs) as catalysts in oxidation reactions, and the unique role of metal oxide substrates in anchoring metal atoms for photocatalysis is emphasized. Biosynthesis of these nanoparticles by bacteria, fungi and plants can yield desirable crystallinity, diameters and morphologies if all process parameters (concentration, pH, temperature, time, and calcination temperature) are well controlled. Different TMONPs can be combined with other transition metal oxides to form nanocomposites that offer multiple synergistic advantages. They represent an important class of semiconductors finding applications in various major industries from solar energy transformation, magnetic storage media and electronic devices to photocatalysis. The development of novel electrochemical biosensors using morphologically varied transition metal oxides and their composites has highlighted the significance of TMONPs as promising electrode modifiers for the fabrication of electrochemical and biosensors. Reactivity of the metal oxide-water interface can be understood from the viewpoint of coordination chemistry, while the reactivity of a metal ion at a nanoparticle surface is compared to the reactivity of the same metal ion dissolved in an aqueous solution [1-12].
Nanotechnology is introducing many advantages over conventional methods of food processing, extending the shelf life, reducing deterioration, maintaining quality, and adding food values. Nanoparticles and nanomaterials improve barrier properties, detect pathogens, and alert the status of food. They will reduce the wastage of post-harvest loss of agriculture and horticulture produces. A substantial review has recently been published on the incorporation of transition metal oxides in the development of intelligent food nano-packaging. Peroxides are inorganic chemicals that contain a bivalent O-O group. These compounds release nascent oxygen readily and their major industrial applications include oxidizing agents, bleaching agents, and polymerization initiators. Chemical oxidation is one of the environmental site remediation methods that have emerged lately as a better alternative to traditional technologies. Nanosized oxidizing agents increase the ratio of surface to volume and hence the biodegradation speed for contaminants in soil and ground water. Sodium peroxide (Na2O2), sodium perborate, and sodium persulfate are common inorganic salts that react with water to produce hydrogen peroxide (H2O2). Other metal peroxides, such as BaO2, CaO2, CdO2 and MgO2, are highly stable and they promote the oxidation of organic substances only at higher temperatures. BaO2, CaO2, MgO2, TiO2 and ZnO2 provide antibacterial applications in biomedicine. Tin peroxide (SnO2) transforms to SnO when exposed to orange peel extracts with reducing ability. Zinc peroxide (ZnO2) nanoparticles can be employed to prepare intelligent nano-packaging for better food preservation if they do not leach out from the packaging to the food [13-21].
Zinc Peroxide Nanoparticles
In 2021, zinc oxide and peroxide were the world’s 776th most traded product, with a total trade of US$1.87B. The top exporters of ZnO2 and ZnO were the Netherlands, Mexico, Canada, United States, and Peru. Both ZnO and ZnO2 nanoparticles can be prepared from aqueous solutions containing zinc nitrate or formate using UV irradiation. When ZnO is treated with H2O2, an interfacial ZnO2 layer forms to cover the nanoparticle surface. ZnO nanoparticles can be obtained by heat treatment of the peroxide above the transition temperature of 233°C, up to the decomposition temperature of 473°C. The weight loss due to the thermal decomposition of ZnO2 into ZnO and O2 at 250°C is considerably larger than the expected theoretical value of 16.4% just by oxygen release. ZnO2 is a stronger oxidizing agent than ZnO. Decomposition of ZnO2 to ZnO and O2 resulted in the decrease of the band gap energy from 3.75 to 3.30 eV [22-28].
ZnO2 nanoparticles were traditionally prepared by peptization of Zn(OH)2 with the aid of H2O2 aqueous solution. They could be formed by a simple oxidation-hydrolysis-precipitation procedure, using zinc acetate as a precursor, hydrogen peroxide as an oxidizer, water as a preparation medium for hydrolysis, and polyethylene glycol as a stabilizer. ZnO2 nanoparticles can facilely be synthesized from zinc acetate and H2O2 using a sol-gel method under ultrasound assistance [29-33]. Characterization by scanning electron microscopy and energy dispersive X-ray spectroscopy (Figure 1) shows an atomic ratio Zn/O of 2.01 and an average particle diameter of 304±5 nm. A green method based on the reaction between Zn5(CO3)2(OH)6 powder and H2O2 in aqueous solution at room temperature can synthesize ZnO2 nanoparticles. ZnO2 nanoparticles can be prepared by the laser ablation of zinc in 30% H2O2 as another green technique using the fundamental wavelength (1064 nm) of a pulsed Nd: YAG laser, at a repetition rate of 15 Hz and laser fluence of 22 J/cm2 for an ablation time of 10 min. Furthermore, the Leidenfrost dynamics occurring in an underwater overheated zone ensures eruption of nanoclusters towards a colder region, forming monodisperse nanoclusters of ZnO2. Nowadays, ZnO2 nanoparticles are commercially available, either bare or coated with an organic ligand shell of polyethylene glycol for stable dispersion in water, methanol, ethanol, acetone and dimethyl sulfoxide [34-38].
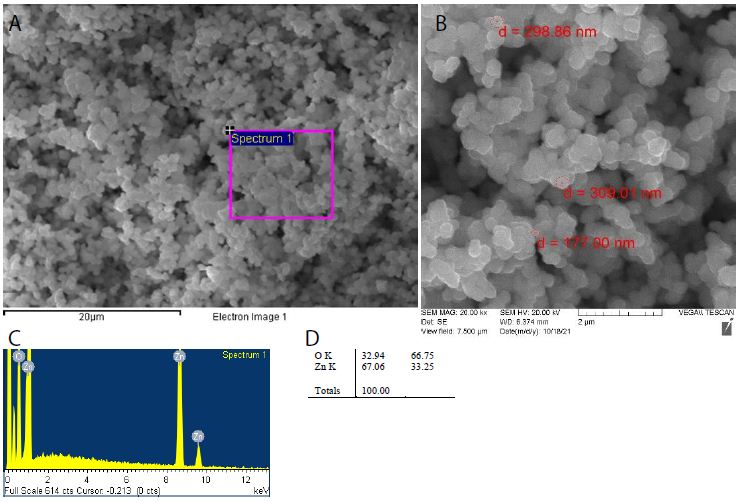
Figure 1: (a, b) Scanning electron microscopy, and (c, d) energy dispersive X-ray spectroscopy of ZnO2 nanoparticles facilely prepared in our lab by following Ramírez et al.’s sol-gel method under ultrasound assistance.
Unique Properties of Zinc Peroxide Nanoparticles
ZnO2 is much more stable in aqueous solutions (as compared to calcium and magnesium peroxides) and it retains its peroxide content down to pH 6. At lower pH levels, H2O2 release is predictable as its dissolution product, Zn2+, is highly soluble. Nanocrystalline ZnO2 can be passivated against further oxidation by the addition of sub-stoichiometric amounts of potassium permanganate, which also increases the thermal stability of ZnO2 [39,40].
ZnO2 possess unique anti-bacterial, anti-corrosion, anti-fouling, and photocatalytic properties that are considered cost effective and environment friendly. They have been studied, due to their semiconducting and oxidizing properties, for various applications in optoelectronics, photocatalysis, sensors, biomedicine, and theranostics. Due to their large nonlinear optical susceptibilities, which are enhanced by two-photon electronic resonance, metal oxides are efficient sources of coherent anti-Stokes Raman Scattering (CARS). The FTIR spectrum of ZnO2 nanoparticles shows a characteristic absorption peak at 435-445 cm−1; the Raman spectrum shows characteristic peaks at 830-840 and 420-440 cm−1. ZnO2 nanoparticles exhibit photoluminescence with one strong emission band at 400 nm, one very weak emission band at 474 nm, and at 520 nm originating from the band edge and the oxygen vacancy. Polyvinyl alcohol/ZnO2 nano-composite films have been engineered via casting. Their energy gaps decrease with increasing ZnO2 concentrations to reach 2.80 eV at 2 wt.%, which are promising in anti-ultraviolet, opto-electronic, and optical limiting applications. A correlation exists between oxygen vacancies and the magnetization for pure ZnO2 nanoparticles at room temperature. Coating of 15-20% ZnO2 nanoparticles over graphene enhances magnetization more than 30 times due to the exchange interaction between localized electron spin moments resulting from oxygen vacancies at the surface [41-47].
ZnO2 nanoparticles have reportedly oxidative stress mediated toxicity on various mammalian cell lines. Oxygen release from the biofunctionalized nanoparticles is tunable according to the solution pH. Antimicrobial tests at 37°C on bacterial species exhibiting different susceptibility to oxygen have confirmed the antimicrobial activity of ZnO2 nanoparticles against Enterococcus faecalis, Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia. Accordingly, ZnO2 showed effective antifungal activities, with a minimum inhibitory concentration (MIC) of 16 mg/L against Candida albicans. Histopathology assessment has confirmed the role of ZnO2 nanoparticles in healing skin wounds. They exhibit angiogenic activity, due to onsite production of H2O2, for rapid tissue healing. ZnO2 demonstrate antimicrobial, anti-elastase, anti-keratinase, and anti-inflammatory properties that are valuable for biomedical applications. They also inhibit bacterial biofilm formation and combat multi-drug resistant bacteria. A minimum concentration of ZnO2 nanoparticles of 1 μg/mL inhibits the production of interleukin-1-β and interleukin 6 by peripheral blood mononuclear cells in the presence of lipopolysaccharides. ZnO2 nanoparticles at a concentration of 2 μg/mL causes DNA damage in vitro; at a concentration of 5 μg/mL they promote protein aggregation and facilitate the production of protein complexes that may interfere with normal immune functions [48-54].
Applications of Zinc Peroxide Nanoparticles
Nanosized ZnO2 is an efficient oxidant for the oxidation of aromatic alcohols to the corresponding carbonyl compounds selectively in excellent yields, using dimethyl carbonate as an environmentally benign solvent. ZnO2 nanoparticles reactively adsorb chemical warfare agent surrogate of mustard gas, selectively oxidizing diethyl sulfide to diethyl sulfoxide and 2-chloroethyl ethyl sulfide to hydroxyethyl ethyl sulfide. Crosslinking of conventional/carboxylated nitrile rubber with ZnO2 achieved total peroxide decomposition at vulcanization temperatures as low as 190-200°C [55-57].
Semiconducting CuO nanoparticles, as a CO2 gas sensitive material, can with an organic binder and ZnO2 for improved gas sensitive layer quality. The Lewis acid-base reaction between oxide oxygen and CO2 has been proposed as sensing mechanism for the measurements in dry air, whereas the formation of surface barriers between nano-grains due to the reaction with CO2 has been suggested for the CO2 response under humid conditions [58].
ZnO2 is a promising adsorbent nanomaterial for the removal of Congo red dye from contaminated water. The adsorption capacity is 208 mg g-1 within 10 min at pH 2-10. The adsorbent has a unique property to adjust pH within the 6.5-7.5 range irrespective of the acidic or basic nature of water. It is highly efficient even in the absence of sunlight to remove Congo red dye from contaminated water down to the permissible limits set by the World Health Organization and the United States Environmental Protection Agency. Crystal violet dye in industrial wastewater can be removed using sodium docusate-modified ZnO2, attaining >99.5% adsorption efficiency in 5 min at pH ∼10 as the zeta potential of ZnO2 decreases from −15 mV at pH 3 to −60 mV at pH 9. The higher negative charge results in stronger electrostatic interaction with the dye. Synthetic graphite flakes can be treated with 3-mercaptopropionic acid, followed by functionalization with ZnO2 nanoparticles, to efficiently remove As(III) and As(V). The adsorption data are best fitted with pseudo second order kinetic model and Freundlich adsorption isotherm, indicating chemisorption and multilayer adsorption on heterogeneous surface respectively. ZnO2 nanoparticles, capped with polyvinyl-pyrrolidone to control the particle size, is an efficient material for the decontamination of cyanide from contaminated water by adsorption at pH 5.8-7.8 within 15 min [59-62].
As a catalyst for removal of reactive blue dye, a maximum degradation efficiency of 85% was achieved by ZnO2 nanoparticles with polyethylene glycol, and 81% without PEG, after 120 min of photocatalytic reaction. ZnO2 nanoparticles have excellent degradation efficiency of brilliant green dye, achieving 84-86% after 120 min of photocatalytic reaction at pH 6-7. Eco-friendly carbon quantum dots/ZnO2 nanocomposite has been successfully synthesized for photocatalysis applications. It has higher efficiency than carbon quantum dots/TiO2 for the removal of different dyes and high stability under UV-A light. Nitrobenzene photodegradation by ZnO2 under UV lamps of 254 nm is optimal at pH 2, reaching up to 90% degradation in 2 h at 25°C [63-66].
For dental implants the accumulation of anaerobic bacteria is a main reason for peri-implant inflammation that can lead to implant loss. Decorating ZnO2 by Glc-1P permits their uptake in the gram-negative oxygen-sensitive bacterial cells. ZnO2 nanoparticles can be decorated with glucose 1-phosphate (Glc-1P) due to specific interaction of the phosphate function of Glc-1P with the nanoparticle surface. The anchored glucose molecules are accessible for specific interactions with lectin concanavalin A. Generation of ROS including hydrogen peroxide, hydroxyl radical, and peroxide anion can enhance the membrane permeability, cell wall damage, internalization of nanoparticles, and uptake of toxic dissolved Zn2+ ions. A ZnO2-based theranostic nano-agent enhances oxidative damage to cancer cells by combining endogenous and exogenous reactive ROS. After uptake by cancer cells, the pH-responsive ZnO2 nanoparticles, in addition to releasing exogenous H2O2, also provide Zn2+ to facilitate the production of endogenous O2·–and H2O2 from mitochondrial electron transport chain, enabling highly effective synergistic tumor therapy [67-69].
Zinc Oxide Nanoparticles
ZnO is a white powder that has two main lattice structures: hexagonal wurtzite and cubic zincblende. The hexagonal structure is commonly found, and both structures are insoluble in water with a solubility of 0.16 mg/100 mL at 30°C. The solubility of uncoated ZnO, as determined by the Zn2+ concentration in the aqueous solution, ranges between 20 and 47 mg/L. The solubility product constant Ksp is a useful parameter for calculating the aqueous solubility of sparingly soluble compounds. A comparison of dissolution rates shows that the ZnO nanoparticles have a higher dissolution rate than the bulk oxide. A new methodology for the in-silico assessment of the solubility of ZnO based on statistical thermodynamics, combined with density functional tight binding theory for the evaluation of the free energy exchange during the dissolution process. Complete ionic dissolution of ZnO is hindered by the formation of O2− anions in solution, which are highly unstable. The dissolution rate will depend critically on the matrix with Zn ions and the mechanisms for diffusion or active transport of Zn2+ and O2-ions in biological processes. Any mass fraction of Zn2+ ions removed or washed away will lead to further dissolution and eventually complete solubilization of the particulate fraction of ZnO. The fact that zinc-rich foods are mostly animal products suggests that vegetarians and vegans may have difficulty getting enough zinc in their diet. Zinc supplements are a great way to have the recommended levels of zinc in the body for stronger immune systems and improved muscle building. They are particularly useful for older adults (especially older men), who are more likely to have zinc deficiency [70-75].
Unique Properties of Zinc Oxide Nanoparticles
A variety of industries, including the automotive, concrete, cosmetic, pharmaceutical and textile, have used ZnO nanoparticles as a major material. The annual turnover of ZnO nanoparticles is over US$ 900,000/year, and the specific cost of their production is US$20/kg. Numerous synthetic techniques have been developed to meet the increasing demand for ZnO nanoparticles. These alternatives offer environmental and financial advantages associated with their commercial production. Biological synthesis uses plant extracts or microbes as green resources for the preparation of ZnO nanoparticles. Considerable investment to improve the performance of diverse nanocomposites allows rapid development of novel photocatalytic/photooxidizing degradation technologies for removing dyes in industrial wastewater [76-78].
Nowadays, ZnO nanoparticles continue to be a great attraction to researchers in various scientific endeavors based on their unique physicochemical properties. The natural bandgap (3.37 eV) and n-type conducting behavior of ZnO can be tuned by doping with metals/metal oxides and non-metals to replace Zn2+ and O2-in the ZnO lattice, for various applications (such as solar cells, photocatalysis, medicines, light-emitting diodes, laser diodes, chemical and biosensors) owing to the direct influence of dopants on their electronic and physiochemical properties. A wide range of energy bandgaps (3-4 eV) is attained by green synthesis, indicating that ZnO nanoparticles can be employed in metal oxide semiconductor-based systems. The effectiveness of dye-sensitive solar cells is attributable to improved dye adsorption onto the nanoparticle surfaces. By adding ZnO in various amounts to a solution of polyvinylidene fluoride in 2-butanone during the fabrication process, followed by removing ZnO in an HCl bath once the organic solvent is evaporated, porous sensors can be made with different piezoelectric chains to control the piezoelectric coefficient. ZnO nanoparticles synthesized using the coprecipitation method present the best performance in catalysis, biosensing, imaging, drug delivery, and pollution absorption owing to their highest purity and crystalline phase, large Brunauer-Emmett-Teller surface area (~23 m2g-1) and pore volume in the mesoporous-macroporous structure [79-82].
ZnO nanoparticles have strong antimicrobial activity against a broad spectrum of bacteria (P. aeruginosa, E. coli, A. baumannii, K. pneumoniae and Staphylococcus aureus) and are effective against Hyalomma ticks. However, all fungal strains (P. chrysogenum, A. niger, T. citrinoviride and A. fumigatus) are resistant to ZnO nanoparticles. ZnO nanoparticles (1.5 mg/L) are protective against the detrimental effects of Clostridium perfringes type A infection in aquaculture. Their potential mechanisms of action against various kinds of viruses were discussed in a comprehensive review. The adoption of novel bio-assisted synthesis methodologies tailors the properties of ZnO nanoparticles to suit biomedical applications, underscoring their potential in cancer treatment towards MCF-7 breast cancer cell lines [83-87].
Applications of Zinc Oxide Nanoparticles
ZnO is produced synthetically for use as an additive in adhesives, antibacterials, baby powder, batteries, cement, ceramics, cigarette filters, cosmetics, ferrites, fire retardants, first-aid tapes, foods, glass, laser diodes, light emitting diodes, lubricants, ointments, paints, pigments, plastics, rubbers, sealants, semiconductors, solar cells, sun blocks, and wood products. Traditional uses of ZnO products include treating wounds following surgery and applying salves inside the mouth to treat ulcers or sores. Over 50% of ZnO is used in the rubber industry along with stearic acid for the vulcanization of rubber to produce tires, shoe soles, and even hockey pucks. ZnO nanoparticles have been added in food-packaging materials to stop food from spoiling. For use as binary/ternary composite anodes in lithium-ion batteries, ZnO has a higher theoretical capacity (978 mA.h/g) than many other transition metal oxides such as CoO (715 mA.h/g), NiO (718 mA.h/g) and CuO (674 mA.h/g). ZnO nanoparticles are extensively used in healthcare and environmental remediation applications attributable to their biodegradability. ZnO possesses unique biological properties for various antibacterial, antiinflammation, antitumor, and antiviral) applications. Addition of ZnO nanoparticles into crystal violet dye induces an alternative photoredox pathway, resulting in more generation of reactive oxygen species lethal to bacterial cells. This technique could be used to transform a wide range of bactericidal surfaces and contribute to maintaining low pathogen levels on hospital surfaces related to healthcare-associated infection. Hybrid ZnO-SiO2 nanoparticles possess favorable characteristics for antifouling purposes. Self-cleaning and anti-fouling polymeric membranes for wastewater treatment are commercially fabricable with ZnO nanocomposites [88-100].
The formation and breaking of transition metal-carbon bonds plays a pivotal role in the catalytic oxidation of organic sulfides, alcohols, olefins, and alkanes. The textile industry is environment unfriendly due to the massive use of dyes and chemicals. Discharge of untreated textile wastewaters loaded with dyes not only contaminates the soil and water resources but also threatens the public health. ZnO nanorods can be used as a photocatalyst to degrade 65% of methylene blue in 50 min. Biochar-ZnO composites obtained by pyrolysis at 600°C can degrade 90% rhodamine B in 75 min, while ZnO can degrade only 38%. ZnO nanoparticles can be doped with Ni (3%), through combustion at 550°C, to improve the photocatalytic degradation of methylene blue and tetracycline [101-103].
Sodium (15%)-doped ZnO degrades 95% of methylene blue under visible light illumination in 180 min, with a rate constant of 1.7×10-2 min−1 and tenacious photostability. Green synthesis of ZnO nanoparticles is gaining huge attention via eco-friendly protocols that reduce the destructive effect of chemical synthesis. ZnO nanoparticles synthesized from Synadium grantii leaf extract with Cu dopant exhibit superior photocatalytic activity for indigo carmine, methylene blue and rhodamine B dyes. Gynostemma plant extract can be used in a co-precipitation method to synthesize ZnO nanoparticles for the photocatalytic decolorization of malachite green dye under UV illumination within 180 min. Biogenic ZnO nanoparticles can be synthesized by using Pseudochrobactrum sp. C5 for catalytic degradation of dyes in wastewater treatment. Valorization of banana peel waste extract as the reducing and capping agents produces ZnO nanoparticles that show superior reusability and photodegradation efficiency for the removal of hazardous basic blue 9, crystal violet and cresol red dyes at pH 12 over irradiation time 90 min. Degradation of congo red by orange-peel-extract-biosynthesized ZnO nanoparticles via photocatalysis can remove 96% of the dye Photocatalytic degradation of rhodamine B dye in waste water and inhibition of butyrylcholinesterase, acetylcholinesterase and α-glycosidase enzymes are afforded by cauliflower-shaped ZnO nanoparticles synthesized using Alchemilla vulgaris leaves. Maximum photocatalytic degradation of pharmaceutical wastewater with ZnO was 40% and with TiO2 is 33% at pH 9, following pseudo-first-order kinetics. Combined use of TiO2/H2O2 is more effective than ZnO and TiO2 alone, achieving 45% degradation. ZnO and TiO2 can be used as catalysts for the degradation of dyeing factory effluents by the advanced oxidative process under UV irradiation at pH 3 for 8 h. Interestingly, a deposit of CdS nanoparticles on ZnO nanosheets provides excellent piezocatalytic efficiency for rhodamine B degradation under ultrasonic vibration. The nanocomposite of ZnO with porous hydroxyapatite (prepared from phosphate rock) improves the photodegradation of antibiotics in water and traps the by-products. An artificial neural network model can estimate the effect of different variables on AB113 dye removing decolorizing acid blue dye from textile wastewater in a sonophotocatalytic process. Reaction time, pH, ZnO dosage, ultrasonic power and persulfate dosage are optimized for maximum dye removal. After photocatalysis, if the treated water is discharged to the surface water along with the catalyst nanoparticles and degradation products, a resulting toxicity exists in the medium that can influence the lipid peroxidation and reduced glutathione in the aquatic vertebrates. Hence filtration is recommended before discharging, for separation of the catalyst nanoparticles. However, the filtration of nanoparticles from the treated water is costly and might outweigh the savings of energy [104-116].
Toxicology of ZnO Nanoparticles
Increasing production and application of transition metal oxide nanoparticles has raised concerns in regard to their environmental accumulation and toxicity in natural ecosystems. Nanoparticles are extensively studied for their chemical toxicology in aquatic microorganisms, agricultural products, fish, wildlife and humans. The uptake and accumulation of ZnO nanoparticles by aquatic organisms have considered the release of Zn2+ ions as well as the toxic mechanisms shared with other nanoparticles such as immunotoxicity, inflammation, lysosomal/mitochondrial damage, oxidative stress, programmed cell death, and redox activity. The growing usage of ZnO nanoparticles increases their release in municipal wastewater treatment plants. At 50 mg/L ZnO nanoparticles, both the granular activated sludge performance and the extracellular polymeric substances content are significantly reduced. This leads to decreases in the activities of ammonia monooxygenase and nitrate reductase. In addition, ZnO nanoparticles disrupt the cell membrane integrity and lead to bacterial cell death via intracellular ROS generation. After exposure to the nanoparticles, the bacterial community composition shifts to be dominated by Gram-positive bacteria. Antibacterial activity of ZnO nanoparticles is more pronounced with Gram-positive than Gram-negative bacteria. ZnO nanoparticles are biocompatible and effective as a food preservative against Salmonella typhi, Klebsiella pneumoniae and Shigella flexneri. They demonstrated significant antibacterial effects on various pathogenic bacteria in terms of zone-of-inhibition measured by the disc-diffusion method. When treated with ZnO nanoparticles (100-300 mg/L), significant reductions in marine microalgae C. vulgaris viable cells, LDH level, and non-enzymatic antioxidant glutathione are noticed while the activity of antioxidant enzyme superoxide dismutase and the level of lipid peroxidation significantly increase. ZnO nanoparticles possess antibacterial and antioxidant properties towards the remediation of hospital wastewater; the ones fabricated using Eriobotrya japonica leaves extract exhibit DPPH scavenging activity and are highly active against S. aureus, P. multocida, E. coli and B. subtilis strains. Redox imbalance, lignification and cell death cause reduction of root growth in wheat seedlings exposed to ZnO nanoparticles. Dietary exposure of carp to ZnO nanoparticles increases the aspartate aminotransferase activity significantly and decreases the alanine transferase activity significantly. ZnO nanoparticles act as a potent antidiabetic agent and severely elicit oxidative stress particularly at higher doses in diabetic rats (10 mg/kg). Initial exposure of human bronchial and pancreatic epithelial cells to oxidative stress sensitizes their subsequent response to cytotoxic challenge with ZnO nanoparticles. As in vitro model species human erythrocytes can be used to evaluate cytotoxicity, and human lymphocytes can be used for genotoxic studies. ZnO and TiO2 nanoparticles result in 65% and 52% hemolysis at 250 ppm respectively, indicating cytotoxicity to human red blood cells. Both nanoparticles were found to generate ROS concomitant with depletion of glutathione and glutathione S-transferase levels. ZnO nanoparticles are significantly more genotoxic than TiO2 nanoparticles at concentrations higher than 250 ppm. The nanoparticles preferentially kill cancerous cells over normal human cells. They enhance ultrasound-induced lipid peroxidation in the liposomal membrane. Two mechanisms underly the toxicity of ZnO nanoparticles: (i) generation of ROS and (ii) induction of apoptosis. The chemical toxicology of ZnO nanoparticles in adult male Wistar rats were investigated. All levels of zinc oxide nanoparticles had a significant impact on sperm quality and quantity. Significant toxicity effects of ZnO nanoparticles appeared at concentrations above 50 mg/kg body weight of animals. 200 mg/kg body weight resulted in increased total oxidant status and decreased total antioxidant capacity significantly. On the contrary, dietary supplementation of Nile tilapia with Se nanoparticles and ZnO nanoparticles induces synergistic effects that improve growth performance, blood health, and intestinal histomorphology. Seed priming with ZnO nanoparticles demonstrates beneficial effects of mitigating the phytotoxicity induced by Co stress in maize, significantly improving the plant growth, biomass, and photosynthetic machinery. Freshwater fish O. mossambicus fed with a supplemented diet of ZnO and Se nanoparticles raises the antioxidant response, boosts the immunity, and reduces the chance of getting infected by A. Hydrophilia. The entrance of ZnO or ZnS nanoparticles into freshwater systems may significantly impact the sedimentary microbial community structure and nitrogen cycling. Furthermore, they showed a strong anti-termite activity against Heterotermes indicola with a 100% mortality rate in 24 h [117-136].
Biosensors Incorporating Zinc Oxide Nanoparticles
Among all the optical biosensing systems, ZnO nanoparticles formed directly atop 3-aminopropyl triethoxysilane-treated Si substrates are more adhesive. Smaller particle sizes of ZnO will increase the fluorescence emission, eliminate several emission peaks, yield higher fluorescence quantum efficiency, and require lower excitation energy for fluorescence sensing. N-doped ZnO nanoparticles exhibit fluorescence emission at 385 nm (corresponding to the exciton absorption band) under excitation of 340 nm, responding with high selectivity and a detection limit of 4.9 μM for urea in blood serum. Self-assembly of diphenylalanine nanostructures in the presence of ZnO nanoparticles display distinctive luminescent emission at 550 nm that affords sensitive detection of trypsin down to 0.1 ng mL−1. As a surface-enhanced Raman scattering substrate, ZnO tips can be decorated with gold nanoparticles to take advantage of the synergistic effect. Assay for nicotine demonstrates high sensitivity, reaching a lower detection limit of 8.9×10−12 mol/L and offering a linear dynamic range of 10−10-10−6 mol/L. A localized plasmon-based fiber optic sensor can be immobilized with ZnO nanoparticles along with Au nanoparticles for the detection of p-cresol (a water pollutant) as low as 57 μM. A field effect transistor device consisting of ZnO nanoparticles and glutathione-S-transferase in the composite channel can successfully detect and quantifies glutathione in solution and in cancerous cells. The glucose content in food samples can be determined using ZnO nanoparticles, with a correlation coefficient of 0.9812 at 3.5 mM-27.8 mM concentrations [137-143].
A novel electrochemical sensor made by drop casting zinc oxide nanoparticles and electropolymerizing glutamic acid can detect sodium dodecyl sulfate with excellent selectivity via molecular imprinting. ZnO was overlaid on the interdigitated electrode of an electrochemical DNA biosensor to detect sequence complementation from Ganoderma boninense. ZnO nanoparticles prove to be excellent for doping carbon dots in electrochemical biosensor applications. Chemical vapor deposition of ZnO nanoparticles on an aluminum foil working electrode successfully sensed cysteine electrochemically. Smartphones can be combined with screen-printed electrodes or interdigital electrodes for in-situ electrochemical detection. The electrodes are often modified with biomaterials, chemical materials, and nanomaterials (such as ZnO) for biosensing to monitor ascorbic acid, dopamine, glucose, levodopa, and uric acid in point-of-care testing. Aluminum doping can be attained by radio frequency magnetron sputtering of ZnO nanoparticles deposited on a glass substrate for biosensor applications. Four different H2O2 biosensors have been designed using ZnO nanoparticles, multiwalled carbon nanotubes, Prussian blue, ionic liquid and horseradish peroxidase. The best analytical performance offers a linear dynamic range of 9.99×10-8‒7.55×10-4 M, detection limit of 1.37×10-8 M, and sensitivity of 17.00 µA mM-1. A laser scribed graphene-ZnFe2O4 electrochemical aptasensor for acute myocardial infarction screening has been developed for detecting the cardiac troponin-I biomarker, with a limit of detection of 0.001 ng/mL and a sensitivity of 19.3 µA/(ng/mL). The Ag-ZnO-graphene oxide/glassy carbon electrode exhibits high sensitivity, detection limit of 0.02 μM, and fast response within 3 s owing to the efficient oxidation of diclofenac sodium at 0.25 V. Trimetallic Ni/Ag/Zn oxide composite-modified glass carbon electrode has good sensor sensitivity of 0.96 μA/μM cm2 and detection limit of 0.3 μM for dopamine. ZnO-reduced graphene oxide-Au nanoparticles can modify a screen-printed electrode for fast electrochemical detection of dopamine in biological samples. A uric acid biosensor was constructed with nafion/uricase/ZnO nanorods-ZnO nanoparticles on a fluorine-doped tin oxide electrode. Differential pulse voltammetry demonstrated linearity over a wide concentration range (0.01-1.5 mM) with a high sensitivity (345 μA mM−1cm−2) and low limit of detection (2.5 μM). A glassy carbon electrode modified with carbon nanotubes, cytochrome C and ZnO nanoparticles has good sensitivity for the detection of streptomycin in pharmaceutical samples. The highly sensitive interface of penicillinase@CHIT/PtNP-ZnO/ZnHCF/FTO electrode shows a linear response and good limit of detection (0.1 μM) in antibiotics in forensic samples. Biosensors based on ZnO and NiO nanostructures decorated with Au nanoparticles have opened the doors to detect volatile organic compounds using electrochemical methods. Biomass carbon derived from cassava and its composites with ZnO nanoparticles can be synthesized for biosensing due to their low cost and resource availability [144-159]. In our lab, screen-printed electrodes are modified by a deposit of ZnO nanoparticles from aqueous suspension onto the graphite working electrode surface. After drying, a sample solution containing sodium metabisulfite analyte in 1 M KCl can be placed on top for chronoamperometry using the Homianze μEA 160C electrochemical analyzer. A typical current-time curve is obtained as shown in Figure 2a, which is ready for data analysis in accordance with the Cottrell equation as shown in Figure 2b.
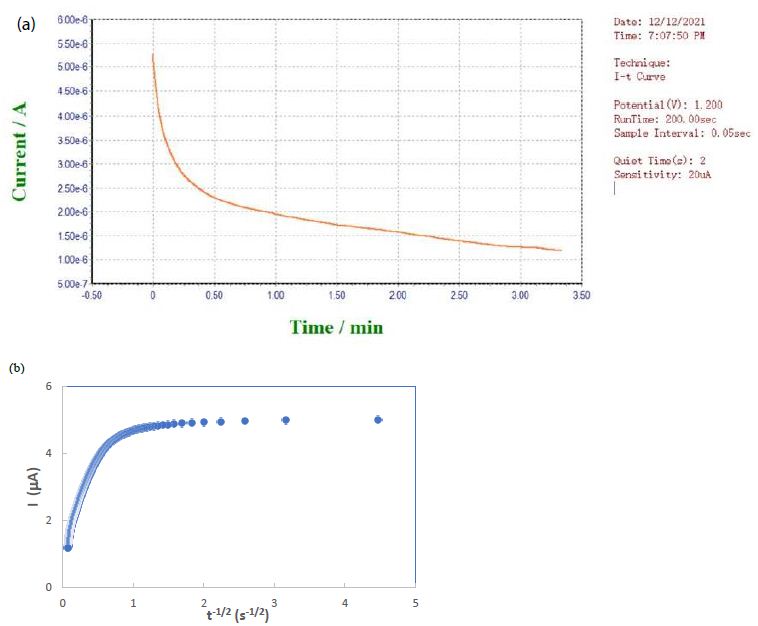
Figure 2: (a) Screen shot of current-time curve obtained in our lab from sodium metabisulfite with ZnO nanoparticles deposited on graphite electrode. (b) Plot of current vs. inverse square root of time for Cottrell analysis.
Self-cleaning and Anti-fouling Polymeric Membranes for Wastewater Treatment and Analytical Separations
A recent trend in nanotechnology shows the application of nano-based materials, such as nano-adsorbents, nano-metals, nano-membranes, and photocatalysts, in water treatment processes. Nanomaterials typically have high reactivity and a high degree of functionalization, large specific surface area, and size-dependent properties which makes them suitable for applications in wastewater treatment and for water purification. Nanostructured catalytic membranes, nanosorbents and nanophotocatalyst-based approaches to remove pollutants from wastewater are eco-friendly and efficient, but they require more energy and more investment in order to purify the wastewater. Current and potential applications of nanoparticles and nanotechnologies in wastewater treatment as well as challenges have been reviewed on the basis of bibliometric results [160-165]. Self-cleaning surfaces have attracted significant attention in both the scientific and industrial communities [166]. In the past decade, transition metal oxide nanoparticles have extensively been incorporated with polymeric membranes for water treatment. Special emphasis is given here to their anti-fouling and self-cleaning properties when used also in the preparation of wastewater samples before chemical composition analysis. Various forms of copper, titanium dioxide and zinc peroxide were tested against microbial fouling and microbiologically influenced corrosion. Their incorporation into polyethylene (high density) and fiber-reinforced plastic provides surface protection. Wastewater treatment is currently a crucial topic worldwide due to global human population growth (83 million annually), industrial downstream contamination, and weathering degradation of polymers [167-170]. Various water treatment techniques are being advanced due to the rising concern of drinking water scarcity and safety. Besides conventional water treatments, the pressure-driven water purification technology has attracted attention due to its efficiency and received substantial applications. Pressure-driven membranes can be classified into microfiltration, ultrafiltration, nanofiltration, and reverse-osmosis. These membranes are used to separate ions, macromolecules, suspended particles and nanomaterials from water. Organic polymeric membranes are extensively used for commercial purposes due to their excellent physical, chemical, and mechanical characteristics. However, membrane fouling occurs due to their hydrophobic nature plus bacterial accumulation and is limiting their sustained operation over time. Regarding membrane fouling, a combination of polymer and nanoparticles is suggested to be a practical strategy for enhancing membrane hydrophilicity. Incorporating nanoparticles into polymeric membranes is becoming a trend in membrane technology. Polymers and metal oxides are becoming popular membrane filtration materials for wastewater treatment due to their surface functionality, large surface area, and unique optical/paramagnetic properties. Under visible light conditions, the polymer-metal oxide nanocomposite membrane affords superior photodegradation activity toward organic pollutants. Transition metal oxides have been evaluated by many researchers during the last decade for wastewater reclamation, as self-cleaning and anti-fouling agents, to utilize their surface mobility, magnetic and optical properties. Recently, a review on polymer nanocomposite membranes based on metal oxide nanoparticles was published in the field of ultrafiltration membrane technology [171-194].
ZnO nanoparticles have been extensively used by scientists and researchers, known to be inorganic, hydrophilic, low-cost, and green (environment-friendly) material. Fluoride contamination of water is a serious problem in the world, and zinc oxide nanoparticles are the best adsorbent for the removal of fluoride from water and wastewater, with an adsorption capacity of 100 mg/g. In wastewater treatment processes, ZnO nanoparticles exert a negative impact on the sludge flocculation performance but do not significantly impact the sludge sedimentation behavior. A decrease of the tyrosine protein-like substance level is probably the key reason for the decreased ζ potential in the loosely bound extracellular polymeric substances, which eventually induces a decline of the sludge flocculation performance under the ZnO stress. A novel deflocculant ZnO/chitosan nanocomposite film in disperser pretreatment enhances the energy efficiency of anaerobic digestion by achieving 99% solubilization of organics. In addition to the anti-fouling performance, ZnO nanoparticles also provide photocatalytic self-cleaning ability to the polymeric membranes. Hence, ZnO-incorporated composite membranes were considered an emerging topic in membrane technology. Modification of polyvinyl chloride membrane using ZnO nanoparticles is very effective for municipal wastewater treatment in the presence of ferric chloride coagulant. The nanocomposite membrane did not adsorb the sludge inside the pores, hence substantially limiting the membrane fouling. Polyvinylidene fluoride membrane with high hydrophilicity was reported to be developed through the conglomeration of ZnO and graphene oxide. Anti-fouling properties, porosity, water flux and wettability were improved to attain a stable effluent quality (0.6 NTU). Combination of graphene oxide and ZnO nanoparticles on polysulfone membrane surface improves the membrane performances to treat petroleum refinery wastewater in terms of higher porosity, increased hydrophilicity, better mechanical strength, reduced water contact angle, increased water uptake ability, higher permeate flux, rejection of total dissolved solids, and improved antifouling properties. Unfortunately, no sustainable membrane systems are yet fully established due to their huge energy requirements for partial removal or degradation of trace organic compounds. Impregnation of ZnO-graphene also reduces polyethersulfone membrane-solute and membrane-foulant hydrophobic interactions. ZnO incorporation enhances the hydrophilicity and improves the anti-fouling property of polyether sulfone membranes. A mean pore size of 0.64 nm and good humic acid rejection make the hybrid membrane well suited for nanofiltration in wastewater treatment and water reclamation. Multifunctional nanofibrous membranes with sunlight-driven self-cleaning performance for complex oily wastewater remediation can be constructed with an Ag/ZnO layer on the porous polyacrylonitrile nanofiber substrate. The membranes demonstrate excellent mechanical strength, superhydrophilic (water contact angle = 0°), underwater superoleophobic (contact angle = 154°) properties, high permeation flux (>619 Lm-2h-1) and separation efficiency (>99.7%) for various oil-in-water emulsions [195-204].
The effect of photoactive semiconductor catalyst (TiO2 and ZnO) on the anti-fouling and self-cleaning properties of polyether sulfone composite membranes (14% by weight) was studied with different concentrations of graphene oxide. The hydrophilicity of composite membranes improved as compared to neat membrane; however, graphene oxide-TiO2 functionalized membranes showed the lowest flux. Incorporation of CuO nanoparticles in polymeric membranes for water treatment is a potential solution for biofouling formation. Promising results have been reported for antibacterial/antifouling effects, increased hydrophilicity, water flux improvement, contaminant rejection capacity, structural membrane parameters, and reduction of concentration polarization. TiO2 nanoparticles have been added to improve the self-cleaning and anti-fouling ability of ultrafiltration polymer membranes through their photocatalytic activity. Immobilization of TiO2 nanoparticles on membrane surfaces was investigated to reduce organic fouling effects in a bioreactor by increasing the membrane hydrophilicity. Cajanus cajan seed extract and carbon nanoparticles reformed the hydrophobic PVDF membrane to hydrophilic. Introduction of TiO2 (0.02% by weight) into the membrane rendered it bi-functional, thus achieving 85% rejection of Cr(VI) and 92% reduction to Cr(III) in tannery wastewater. Ag2O, Fe2O3 and ZrO2 nanoparticles can be incorporated to improve the performance of polymeric filtration membranes due to their effects on permeability, selectivity, hydrophilicity, conductivity, mechanical strength, thermal stability, antiviral, and antibacterial properties. However, they might cause membrane deterioration. Thus, careful selection is required to choose the best composition of metal oxide nanoparticles for individual polymeric membranes. The advantages and disadvantages of Ag2O, CuO, Fe2O3, TiO2, ZnO and ZrO2-incorporated polymeric membranes for water purification have been compared in a new review. Their characteristics (antibacterial property, anti-viral property, conductivity, contaminants rejection, flux permeation, hydrophilicity, mechanical strength, permeability, surface charge, and thermal stability as shown in Table 1) can help decide on the best modification towards achieving sustainable and cost-effective treatment operations. Bimetallic transition metal oxide nanoparticles have attracted many researchers due to their salient features and characteristics over mono metallic oxide nanoparticles. PES ultrafiltration membranes were fabricated using the phase inversion technique (most commonly used technique to fabricate polymeric porous membranes with a large form of structure) with a composite of Fe2O3-Mn2O3 nanoparticles as modifier. Those membranes showed an excellent porosity (74%), high water flux (398 L/m2h), and better antifouling ability. Protein-based filtration tests showed an improved flux recovery ratio in protein separation and water treatment applications [205-214].
Table 1: Transition metal oxide nanoparticles-incorporated polymeric membranes
|
Transition Metal Oxides |
Polymeric Membrane | Advantages |
Limitations |
| ZnO | Polysulfone, polyurethane, polyvinylidene difluoride | Antibacterial, anti-corrosion, anti-fouling, environment-friendly, hydrophilic, low-cost, mechanical strength, self-cleaning (photocatalytic activity). | Not stable (photocatalytic property), mildly toxic. |
| TiO2 | Antibacterial, anti-corrosion, anti-fouling, hydrophilic self-cleaning (photocatalytic activity). | High doses may induce cytotoxicity, not stable (photocatalytic property). | |
| Fe2O3 | Abundantly available, can remove heavy metals, magnetic properties, mechanical strength, non-toxic. | Nanoparticles tend to agglomerate easily. | |
| CuO | Antibacterial, anti-corrosion, anti-fouling, compound rejection capacity, hydrophilic, improving water flux, mechanical strength. | Low-quality nanoparticles are produced via physical synthesis, toxic chemicals are used if produced through chemical synthesis. | |
| Ag2O | Pressure retarded osmosis membranes | Almost non-toxic, anti-fouling, antimicrobial, resistant to corrosion, stable. | Membranes are sensitive to nanoparticle concentration. |
| ZrO2 | Novel membranes | Capable of treating saline water, high-temperature stability, high water retention capacity. | Fouling susceptibility, expensive raw materials. |
| Fe2O3–Mn2O3 | Polyether sulfone | Antifouling, minimal irreversible fouling, excellent water flux, improved recovery for protein separation. | Agglomeration of nanoparticles. |
| TiO2-ZnO composite membrane | Increased hydrophilicity, anti-fouling, self-cleaning properties, photocatalytic activity. | Low flux. |
Fabrication of transition metal oxide nanoparticles-modified polymeric membranes to make them operation-sustainable cost-efficient is challenging. Transition metal oxide nanoparticles have many interesting functional properties. However, integrating nanoparticles into a membrane remains a challenge. Atomic layer deposition and sequential infiltration synthesis were explored for the modification of polymeric membranes and fabrication of novel mesoporous structures. Fouling is a major problem that hinders the operation of membrane filtration processes. Bio-fouling causes performance degradation and elevates energy consumption due to blockage of membrane pores. In addition, it increases the frequency of membrane cleaning and reduces the membrane life span, thereby leading to higher maintenance and operation costs. Antibacterial membranes are considered an attractive strategy to retard biofouling. ZnO is reported to be useful as an anti-fouling agent in polymeric nanofiltration and reverse-osmosis membranes. Instead of ZnO, ZnO2 nanoparticles can be incorporated to make nanofiltration and reverse-osmosis polymeric membranes for better retardation of fouling since ZnO2 is a stronger oxidizing agent than ZnO and can produce free radicals and other reactive oxygen species to inhibit growth of microorganisms. The operating temperature of nanofiltration and reverse-osmosis is typically within 25-65°C, which is far below the transition temperature (233°C) of ZnO2. Hence, ZnO2 nanoparticles embedded in the polymeric membrane are completely stable during wastewater treatment. ZnO2 has photocatalytic self-cleaning property that would make it a strong modifier over ZnO in the fabrication of polymeric membranes. Importantly, these membranes can greatly facilitate the preparation of samples for instrumental analysis of emerging contaminants by removing microplastics (plastic particles smaller than 5 mm) that exist in wastewater and marine environments, including pharmaceuticals, personal care products, perfluoroalkyl substances, organophosphate flame retardants, illicit drugs, and isoprostanes in wastewater as biomarkers of oxidative stress during COVID-19 pandemic. A complete summary of recent advances and latest studies in the fabrication, modification, and industrial application of ZnO photocatalysts is available for further reading. Black TiO2 nanotube array can be employed as both photocatalyst and electrocatalyst to degrade dissolved organic matter in coking wastewater [215-227].
Analytical Methods for Transition Metal Oxide Nanoparticles
New developments have recently been reported concerning the chemical analysis of transition metal oxide nanoparticles in environmental water due to their biochemical toxicity. A unifying methodology for the selective detection of transition metal oxide nanoparticles in water, as well as sensitive determination of environmentally toxic and biochemically active contaminants that are bound on them, is urgently needed. In our lab, new analytical methods have undergone intensive development in the last ten years with a focus on capillary electrophoresis with UV and molecular fluorescence detection (Figure 3). The toxic effects of these emerging contaminants have already been verified by Health Canada and Environment Canada using bioassays. The methodology under intensive development in our research lab begins with a sample treatment step that encapsulates all waterborne nanoparticles/nanomaterials into lecithin liposomes. Centrifugation concentrates the loaded liposomes, and the supernatant water is withdrawn for instrumental analysis by liquid chromatography with detection by tandem mass spectrometry. Next a surfactant disintegrates the liposomes and isolates lecithin from the nanoparticles. Contaminants are desorbed from the nanoparticle surfaces using coordination chemistry, biochemical interaction, laser photo/photothermal chemistry, aerosol nebulization, and electrospray ionization. The desorbed contaminants can be analyzed, either immediately or after separation by capillary electrophoretic/gel filtration/liquid chromatography, by spectrofluorometric and mass spectrometric detection. Optical incoherent scattering and electrochemical chemistry can be adapted to detect the nanoparticles and desorbed contaminants at trace to ultratrace levels [228-238].
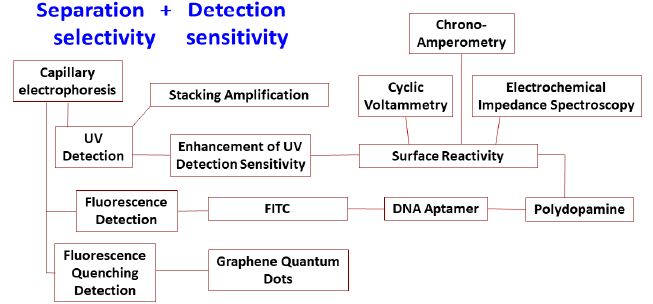
Figure 3: Development of new analytical methods for transition metal oxide nanoparticles in our lab.
Transition metal oxide nanoparticles are increasingly used as a solid carrier in the formulation of numerous drug products. They end up in waste streams, consequently infiltrating the aquatic environments and drinking water resources. Detection of nanoparticles in wastewater requires more advanced analytical methods than conventional water analysis, to prevent the ecosystem of plants and animals from unintended exposure to the released pharmaceuticals. Determination of nanoparticles in drinking water has important implications for protecting the public health sustainability. Unfortunately, many emerging contaminants are not yet stipulated in water quality regulations due to a lack of monitoring technology. Hence, there is an urgent need to develop new analytical methods that can monitor emerging contaminants in water resources. The interplay of nanoparticles, environmental pollution, and health risks is key to all industrial, environmental, and drinking water treatment regulations. A unifying analytical methodology will help scientists and engineers strengthen their control of nanoparticles in freshwater sources for drinking water treatment plants. New endeavors must challenge the traditional notion that environmental toxicological events involve only dissolved contaminants. Rather, environmental toxicology can involve a complex assortment of nanoparticles and associated contaminants whose combined effects on biological and mammalian cells are continuously evolving. The precise toxico-pathogenic effects of ZnO nanoparticles on the cardiovascular system under normal and cardiovascular disease risk factor milieu include down regulation of vascular development and elevation of oxidative stress in the heart tissue. Both endothelial nitric oxide generation and cardiac Ca2+-ATPase activity are significantly suppressed; the cardiac mitochondrial swelling is enhanced [239].
Wastewater Analysis
The nanoparticles released from different nanomaterials used in our household and industrial commodities find their way through waste disposal routes into the wastewater treatment facilities and end up in wastewater sludge. Further escape of these nanoparticles into the effluent will contaminate the aquatic and soil environment. Polyacrylic acid nanomembranes can be used as nano-filters to isolate and remove Ag and TiO2 nanoparticles in aqueous environmental samples using pressure-driven flow, with a filtration efficiency of >99%. The phytoremediation potential of Myriophyllum spicatum L. for removal of ZnO nanoparticles in tap water ranges between 29% and 70%, and slightly higher in pond water. Wastewater treatment plants are a primary source of many contaminants to the environment. Processing complex mixtures of waste, they can result in the continuous discharge of bioactive and endogenous compounds into sensitive aquatic ecosystems. Wastewater analysis has been demonstrated to be a cumulative approach for assessing the overall patterns of alcohol, drugs, tobacco and xenobiotic use by a population at the community level. Hospital wastewater, for one, is regarded as a very important source of fluoroquinolone antibiotics (ciprofloxacin, norfloxacin, and ofloxacin) in the aquatic environment. The development of analytical methods is crucial for the detection of oxidative stress biomarkers in wastewater, using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry and solid phase extraction. Mixed liquor can be collected from the secondary aeration tank while effluent wastewater is collected after the secondary settling tank in a wastewater treatment plant. Mixed liquor is the wastewater which leaves the aeration tank after biological treatment before going into the secondary settling tank for the suspended solids to sediment, while effluent wastewater is the ultimate treated wastewater which is discharged to the river from the treatment plant. Obviously, mixed liquor has much higher levels of suspended solids and relatively higher dissolved carbon content compared to effluent wastewater. Nanoparticles from thirteen different elements were determined, throughout the full-scale wastewater treatment process, by using single particle inductively coupled plasma mass spectrometry. Samples of the influent, post-primary treatment, effluent of the activated sludge process, as well as reclaimed water were analyzed. The incidence of metal-based nanoparticles decreases significantly after the conventional wastewater treatment train, and they are smaller in the effluent (<180 nm) than in the influent (<300 nm). However, anaerobic digesters store high nanoparticle concentrations. Hence, the disposal of sludge needs to take this into account to evaluate the risk of nanoparticles release to the environment [240-248].
The potential use of 8-iso-PGF2α as a sewage biomarker for assessing the status of community health was investigated by liquid chromatography-high resolution mass spectrometry coupled to immunoaffinity clean-up and β-glucuronidase treatment. Urinary excretion provides a mechanism for the entry of isoprostanes to wastewater treatment plants and subsequently the wider environment, where they may initiate a cycle of oxidative stress in aquatic biota. Additional isoprostanes may be produced within these organisms, further perpetuating this cycle of toxicity. An analytical method for their detection in wastewater, based on solid phase extraction and gas chromatography mass spectroscopy, involves a deconjugation treatment with -glucuronidase to increase the concentration of isoprostanes available for detection. The low ng/L range of concentrations of human metabolic biomarkers and the complex matrix composition pose bioanalytical challenges related to sample preparation, detection and quantification. A sensitive liquid chromatography-mass spectrometry method for the detection and analysis of opioid biomarkers has been validated according to the European Medicines Agency guidelines; Oasis HLB cartridges are useful for sample concentration. Ion pairing liquid chromatography with alkanesulfonates coupled to tandem mass spectrometry is valid for the analysis of aminoglycosides (veterinary antibiotics) in wastewater samples after addition of the ion paring salt directly into the raw or treated wastewater samples. Surface-enhanced Raman spectroscopy is good for the detection of methamphetamine based upon the assembly of Au@Ag core-shell nanoparticles on a disposable glassy nanofibrous electrospun paper matrix that gives strong scattering signals. Microplastics are generated while polishing eyeglass lenses and a huge amount of nanoplastics (<1 µm) passes through the conventional wastewater treatment process in considerable amounts. Microplastics (with adsorbed heavy metals) can be quantified in the wastewater by mass balance measurements using membrane filtration with polyaluminum chloride coagulation. The transport of nanoparticles in various wastewater treatment processes is fully discussed in another review [249-255].
Air Pollution Remediation and Quality Monitoring
One of the most favorable environmental applications of nanotechnology has been in air pollution remediation in which different nanomaterials are used. Nanoparticles have initiated the advancement in new and low-cost techniques for environmental pollution control including air pollution. Metal oxide nanofibers have demonstrated to be effective for air pollution remediation in the form of filter, catalyst, catalyst support, and photocatalyst. Fibrous metal oxide has several advantages including surface area, mechanic strength, chemical stability, thermal stability, and photocatalytic ability. In the field of selective reduction of nitrogen oxide, a catalyst with low cost, low toxicity, high activity, and good selectivity for N2 is needed to replace the high-cost and high-toxicity vanadium catalyst. Low-cost spinels MFe2O4 (M = Cu, Mn, and Zn) can be synthesized for this application, and MnFe2O4 exhibits the best activity (99.9%) and selectivity (95.7%) at 100°C [256-258].
Nanocomposites have distinctive physical and chemical properties that result in their use in the construction industry as innovative materials. Addition of nanoparticles can bring many important properties to the bulk construction and insulation materials. Unfortunately, release of ultrafine dust to the air environment has harmful impacts on human health. Nanoparticles can enter the human body through the skin, inhalation, and ingestion. Exposure to nanoparticles can cause serious respiratory, cardiovascular, skin, and nerve related diseases. It can pass through various mammalian membranes, or be absorbed in them, to cause various inflammatory reactions and fibrosis. Pneumoconiosis refers to a class of interstitial lung diseases caused by the inhalation of airborne dust and fibers. Engineered nanoparticles, owing to their high reactivity, can initiate inflammatory responses that trigger metastasis. Human exposure to nanoparticles can cause various health implications such as DNA damage and cell death. A global regulatory policy needs to be framed to assess the toxicity, risk and approval of nanoparticles used in the construction industry. The current OSHA standard for ZnO fume is 5 mg/m3 of air averaged over an eight-hour work shift. NIOSH recommends that the permissible exposure limit be changed to 5 mg/m3 averaged over a work shift of up to 10 hours per day, 40 hours per week, with a short-term exposure limit of 10 mg/m3 averaged over a 15-minute period. It would be scientifically interesting to investigate the percutaneous absorption of transition metal oxide nanoparticles following exposure to road dust powder [259-264].
Semiconducting metal oxide gas sensors have been developed for environmental gases including CO2, O2, O3 and NH3; highly toxic gases including CO, H2S and NO2; combustible gases such as CH4, H2, and liquefied petroleum gas; and volatile organic compound gases. Nanomaterial enabled sensors are applied for the detection of harmful gases such as H2S, SO2, and NO2 . An ultrafast sensor has been developed for trace-level detection of NH3 gas using ZnO nanoparticles, with ultra-fast response (5 sec) and recovery time (8 sec) at 5 ppm. Dopants can enhance the performance of semiconductor metal oxides for gas sensing applications by changing their microstructure morphology, activation energy, electronic structure, and band gap of the metal oxides. In some cases, dopants create defects in semiconductor metal oxides by generating oxygen vacancy or by forming solid solutions. To date, very little is known about the magnitude, patterns, and associated risks of human exposure to microplastics, particularly in the indoor environment. This is a significant research gap given that people spend most of their time indoors, which is exacerbated over the past year by COVID-19 lockdown measures [265-269].
Conclusion
This scientific field possesses immense potential that may provide incredible technological advances soon. The research findings covered in this review article could open many doors to new endeavors. Having more reactive oxygen atoms per molecule, ZnO2 can be considered as a stronger oxidizing agent than ZnO. This unique property makes its nanoparticles an excellent candidate for potential breakthroughs in analytical, biomolecular, food, material, and separation sciences. In addition, mono-dispersed ZnO2 nanoparticles could be coupled with a magnetic core to produce nanocarriers for in-situ disruption of cancer cells. High-performance nano-filtration and reverse-osmosis could be developed with ZnO2 for fouling remediation with self-cleaning feature. For future applications, intelligent antibacterial food nano-packaging could undergo new developments through incorporation of ZnO2 nanoparticles to the packaging film. It will become more and more important that the presence of ZnO/ZnO2 in wastewater is detected and quantitated for the protection of environmental sustainability and public health. The knowledge gap in this dynamic field, as highlighted in this review, will require novel research work.
Acknowledgement
Financial support from NSERC Canada (grant number RGPIN-2018-05320) is gratefully acknowledged.
Competing Interest Statement
The authors have no competing interests to declare.
Data Availability Statement
The raw/processed data required to reproduce our findings in Figure 2a/2b cannot be shared at this time as the data also forms part of an ongoing study.
References
- Schiattarella C (2020) Photoemissive inorganic nanomaterials: characterization and their application in biophotonics. Ph.D. thesis, Università degli Studi di Napoli Federico II.
- Abdah MAAM, Azman NHN, Kulandaivalu S, Sulaiman Y (2020) Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Materials & Design 186: 108199.
- Oka H, Okada Y, Kaminaga K, Oka D, Hitosugi T, et al. (2020) Width-induced metal-insulator transition in SrVO3 lateral nanowires spontaneously formed on the ultrathin film. Applied Physics Letters 117: 051603.
- Akbari A, Amini M, Tarassoli A, Eftekhari-Sis B, Ghasemian N, et al. (2018) Transition metal oxide nanoparticles as efficient catalysts in oxidation reactions. Nano-Structures & Nano-Objects 14: 19-48.
- Li R, Luo L, Ma X, Wu W, Wang M, et al. (2022) Single atoms supported on metal oxides for energy catalysis. Journal of Materials Chemistry A 5717-5742.
- Drummer S, Madzimbamuto T, Chowdhury M (2021) Green synthesis of transition metal nanoparticles and their oxides: a review. Materials (Basel) 14: 2700. [crossref]
- Khaldakar M, Butala D (2017) The synthesis and characterization of metal oxide nanoparticles and its application for photocatalysis. International Journal of Scientific and Research Publications 7: 499-504.
- Lousada CM, LaVerne JA, Jonsson M (2013) Enhanced hydrogen formation during the catalytic decomposition of H2O2 on metal oxide surfaces in the presence of HO radical scavengers. Physical Chemistry Chemical Physics 15: 12674-12679.
- Agnihotri AS, Varghese A, Nidhin M (2021) Transition metal oxides in electrochemical and bio sensing: a state-of-art review. Applied Surface Science Advances 4: 100072.
- George JM, Antony A, Mathew B (2018) Metal oxide nanoparticles in electrochemical sensing and biosensing: a review. Microchimica Acta 2018, 185, 358. [crossref]
- Han X, Liu R, Xu Z, Chen W, Zheng Y (2005) Room temperature deposition of nanocrystalline cadmium peroxide thin film by electrochemical route. Electrochemistry Communications 7: 1195-1198.
- Avena M (2021) The reactivity of the metal oxide-water and mineral-water interfaces-an inorganic/coordination viewpoint. European Journal of Inorganic Chemistry.
- Ningthoujam R, Jena B, Pattanayak S, Dash S, Panda MK, et al. (2022) Nanotechnology in food science. In Bio-Nano Interface, Springer, Singapore.
- Amir AA, Hamed A, Maryam A (2021) Antimicrobial properties of food nanopackaging: a new focus on foodborne pathogens. Frontiers in Microbiology 12: 690706.
- Khodaveisi J, Banejad H, Afkhami A, Olyaie E, Lashgari S, et al. (2011) Synthesis of calcium peroxide nanoparticles as an innovative reagent for in situ chemical oxidation. Journal of Hazardous Materials 192: 15, 1437-1440.
- Jakob H, Leininger S, Lehmann T, Jacobi S, Gutewort S (2007) Peroxo Compounds, Inorganic. Ullmann’s Encyclopedia of Industrial Chemistry.
- Ahmad S, Kharkwal M, Gupta G, Nagarajan R (2011) Journal of Physical Chemistry C, 115: 10131-10139.
- Liu Y, Zhang YC, Zhang M (2010) Green hydrothermal synthesis and characterization of CdO2 Materials Letters 64: 1779-1781.
- Hu H, Yu L, Qian X, Chen Y, Chen B, et al. (2020) Chemoreactive nanotherapeutics by metal peroxide-based nanomedicine. Advanced Science 8: 2000494.
- Gnanasekaran L, Priya AK, Gracia F (2022) Orange peel extract influenced partial transformation of SnO2 to SnO in green 3D-ZnO/SnO2 system for chlorophenol degradation. Journal of Hazardous Materials 424: 127464.
- Anvar AA, Ahari H, Ataee M (2021) Antimicrobial properties of food nanopackaging: a new focus on foodborne pathogens. Frontiers in Microbiology 12: 690706.
- Zinc oxide and peroxide. https://oec.world/en/profile/hs/zinc-oxide-and-peroxide
- Gbur T, Čuba V, Múčka V, Nikl M, Knížek K, et al. (2011) Photochemical preparation of ZnO nanoparticles. Journal of Nanoparticle Research 13: 4529-4537.
- Lee PHY, Wu BK, Chern MY (2014) Study on the formation of zinc peroxide on zinc oxide with hydrogen peroxide treatment using X-ray photoelectron spectroscopy. Electronic Materials Letters 10: 51-55.
- Uekawa N, Mochizuki N, Kajiwara J, Mori F, Wu YJ, et al. (2003) Nonstoichiometric properties of zinc oxide nanoparticles prepared by decomposition of zinc peroxide. Physical Chemistry Chemical Physics 5: 929-934.
- Bocharov D, Chesnokov A, Chikvaidze G, Gabrusenok J, Ignatans R, et al. (2022) A comprehensive study of structure and properties of nanocrystalline zinc peroxide. Journal of Physics and Chemistry of Solids 160: 110318.
- Escobedo-Morales A, Esparza R, García-Ruiz A, Aguilar A, Rubio-Rosas E, et al. (2011) Structural and vibrational properties of hydrothermally grown ZnO2 Journal of Crystal Growth 316: 37-41.
- Sebőka D, Szabóa T, Dékány I (2009) Optical properties of zinc peroxide and zinc oxide multilayer nanohybrid films. Applied Surface Science 255: 6953-6962.
- Zinc peroxide. https://en.wikipedia.org/wiki/Zinc_peroxide.
- Naofumi U, Jyunichi K, Naomi M, Kazuyuki K, Yoshinori S (2001) Synthesis of ZnO nanoparticles by decomposition of zinc peroxide. Chemistry Letters 30: 606-607.
- Rosenthal-Toib L, Zohar K, Alagem M, Tsur Y (2008) Synthesis of stabilized nanoparticles of zinc peroxide. Chemical Engineering Journal 136: 425-429.
- Ramírez JIDL, Villegas VAR, Sicairos SP, Guevara EH, Brito Perea MDC, et al. (2020) Synthesis and Characterization of Zinc Peroxide Nanoparticles for the Photodegradation of Nitrobenzene Assisted by UV-Light. Catalysts 10: 1041.
- Wang K, Lai EPC (2023) Electrochemical sensing of zinc oxide and peroxide nanoparticles: Modification with meso-tetrakis(4-carboxyphenyl) porphyrin. Chemosensors 11: 369.
- Yang LY, Feng GP, Wang TX (2010) Green synthesis of ZnO2 nanoparticles from hydrozincite and hydrogen peroxide at room temperature. Materials Letters 64: 1647-1649.
- Palma-Palma HE, Camacho-López M, Camacho-López MA, Vilchis-Néstor AR (2015) Preparation of zinc peroxide nanoparticles by laser ablation of solid in liquids. Superficies vacío 28: 74-77.
- Elbahri M, Abdelaziz R, Disci-Zayed D, Homaeigohar S, Sosna J, et al. (2017) Underwater Leidenfrost nanochemistry for creation of size-tailored zinc peroxide cancer nanotherapeutics. Nature Communications 8: 15319.
- Ana Industries. Zinc peroxide. http://anaindustries.in/product/zinc-peroxide/.
- Zinc peroxide nanoparticles coated with oPEG ligands. https://nanoxo.eu/product/zinc-peroxide-nanoparticles-zno2-nps-coated-with-opeg-ligands/.
- Wolanov Y, Prikhodchenko PV, Medvedev AG (2013) Zinc dioxide nanoparticulates: a hydrogen peroxide source at moderate pH. Environmental Science and Technology 47: 15. [crossref]
- Shames AI, Lev O, Mikhaylov AA, Medvedev AG, Gun J, et al. (2019) Unusual stabilization of zinc peroxide by manganese oxide: mechanistic understanding by temperature-dependent EPR studies. Journal of Physical Chemistry C 123: 20884-20892.
- Tatarchuk VV, Gromilov SA, Maksimovskii EA, Plyusnin PE (2021) Zinc peroxide nanoparticles: micellar synthesis and preparation of films. Russian Journal of Inorganic Chemistry 66: 1748-1760.
- Moger J, Johnston BD, Tyler CR (2008) Imaging metal oxide nanoparticles in biological structures with CARS microscopy. Optics Express 16: 3408-3419. [crossref]
- Drmosh QA, Gondal MA, Yamani ZH, Saleh TA (2010) Spectroscopic characterization approach to study surfactants effect on ZnO2 nanoparticles synthesis by laser ablation process. Applied Surface Science 256: 4661-4666.
- Bai H, Liu X (2010) Green hydrothermal synthesis and photoluminescence property of ZnO2 nanoparticles. Materials Letters 64: 341-343.
- El-Shamy AG (2021) The optical anatomy of new polyvinyl alcohol/zinc peroxide nanocomposite films for promising optical limiting applications. Progress in Organic Coatings 150: 105981.
- Gao D, Zhang J, Yang G, Qi J, Si M, et al. (2011) Ferromagnetism induced by oxygen vacancies in zinc peroxide nanoparticles. Journal of Physical Chemistry C 115: 16405-16410.
- Ganguly P, Kotnala RK, Singh S, Pant RP, Singh N (2015) Graphene functionalized with 3-mercatopropionic acid capped zinc peroxide nanoparticles: a potential ferromagnetic material at room-temperature. Carbon 95: 428-433.
- Syama S, Reshma SC, Sreekant PJ, Varma HK, Mohanan PV (2013) Effect of zinc oxide nanoparticles on cellular oxidative stress and antioxidant defense mechanisms in mouse liver. Toxicological & Environmental Chemistry 95: 495-503.
- Bergs C, Brück L, Rosencrantz RR, Conrad G, Elling L, et al. (2017) Biofunctionalized zinc peroxide nanoparticles as active oxygen sources and antibacterial agents. RSC Advances 7: 38998-39010.
- Hussein HM, Ghafoor DD, Omer KM. (2021) Room temperature and surfactant free synthesis of zinc peroxide nanoparticles in methanol with highly efficient antimicrobials. Arabian Journal of Chemistry 14: 103090.
- Ahtzaz S, Nasir M, Shahzadi L, Amir W, Anjum A, et al. (2017) A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Materials & Design 132: 409-418.
- Ali SS, Morsy R, El-Zawawy NA, Fareed MF, Bedaiwy MY (2017) Synthesized zinc peroxide nanoparticles: a novel anti-microbial, anti-elastase, anti-keratinase, and anti-inflammatory approach toward polymicrobial burn wounds. International Journal of Nanomedicine 12: 6059-6073.
- El-Shounya WA, Moawad M, Haider AS, Ali S, Nouh S (2019) Antibacterial potential of a newly synthesized zinc peroxide nanoparticles to combat biofilm-producing multi-drug resistant Pseudomonas aeruginosa 59: 657-666.
- Makumire S, Chakravadhanula VSK, Köllisch G, Redel E, Shonhai A (2014) Immunomodulatory activity of zinc peroxide and titanium dioxide nanoparticles and their effects on DNA and protein integrity. Toxicology Letters 227: 56-64.
- Verma S, Jain SL (2014) Nanosized zinc peroxide: a novel inorganic oxidant for the oxidation of aromatic alcohols to carbonyl compounds. Inorganic Chemistry Frontiers 1: 534-539.
- Giannakoudakis DA, Florent M, Wallace R, Secor J, Karwacki C, et al. (2018) Zinc peroxide nanoparticles: Surface, chemical and optical properties and the effect of thermal treatment on the detoxification of mustard gas. Applied Catalysis B: Environmental 226: 429-440.
- Ibarra L, Alzorriz M (2002) Effect of temperature on the crosslink densities of nitrile rubber and carboxylated nitrile rubber with zinc peroxide. Journal of Applied Polymer Science, 86: 335-340.
- Tanvir NB, Yurchenko O, Laubender E, Pohle R, Sicard OV, et al. (2018) Zinc peroxide combustion promoter in preparation of CuO layers for conductometric CO2 Sensors and Actuators B: Chemical 257: 1027-1034.
- Chawla S, Uppal H, Yadav M, Bahadur N, Singh N (2017) Zinc peroxide nanomaterial as an adsorbent for removal of Congo red dye from wastewater. Ecotoxicology and Environmental Safety 135: 68-74.
- Sachin, Joishar D, Singh NP, Varathan E, Singh N (2021) Sodium docusate surface-modified dispersible and powder zinc peroxide formulation: an adsorbent for the effective and fast removal of crystal violet dye, an emerging wastewater contaminant. ACS Omega 6: 22570-22577.
- Uppal H, Hemlata, Tawale J, Singh N (2016) Zinc peroxide functionalized synthetic graphite: an economical and efficient adsorbent for adsorption of arsenic (III) and (V) Journal of Environmental Chemical Engineering 4: 2964-2975.
- Uppal H, Tripathy S, Chawla S, Sharma B, Dalai MK, et al. (2017) Study of cyanide removal from contaminated water using zinc peroxide nanomaterial. Journal of Environmental Sciences 55: 76-85.
- Kale PC, Chaudhari PL (2017) Removal of reactive blue 221 dye from textile wastewater by using zinc peroxide nanoparticles. International Journal of Scientific Research and Management 5: 5700-5709.
- Chaudhari PL, Kale PC (2017) Synthesis and characterization of nano zinc peroxide photocatalyst for the removal of brilliant green dye from textile wastewater. International Journal of ChemTech Research 10: 477-486.
- El-Sham AGy (2020) New carbon quantum dots nanoparticles decorated zinc peroxide nanocomposite with superior photocatalytic efficiency for removal of different dyes under UV-A light. Synthetic Metals 267: 116472.
- Ramírez JIDL, Villegas VAR, Sicairos SP, Guevara EH, Perea MDCB, et al. (2020) Synthesis and characterization of zinc peroxide nanoparticles for the photodegradation of nitrobenzene assisted by UV light. Catalysts 10: 1041.
- Fröber K, Bergs C, Pich A, Conrad G (2020) Biofunctionalized zinc peroxide nanoparticles inhibit peri-implantitis associated anaerobes and Aggregatibacter actinomycetemcomitans pH-dependent. Anaerobe 62: 102153.
- Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, et al. (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Letters 7: 219-242.
- Lin LS, Wang JF, Song J, Liu Y, Zhu G, et al. (2019) Cooperation of endogenous and exogenous reactive oxygen species induced by zinc peroxide nanoparticles to enhance oxidative stress-based cancer therapy. Theranostics 9: 7200-7209.
- Zinc oxide. https://en.wikipedia.org/wiki/Zinc_oxide.
- https://arthurscience.weebly.com/uploads/5/0/9/2/5092096/3u_unit_4_day_5__1.pdf
- Université Laval. Solubility product constants.
http://www2.chm.ulaval.ca/gecha/chm1903/6_solubilite_solides/solubility_products.pdf - European Commission. Zinc oxide (nano form)
https://ec.europa.eu/health/scientific_committees/opinions_layman/zinc-oxide/de/l-3/3.htm. - Escorihuela L, Fernández A, Rallo R, Martorell B (2018) Molecular dynamics simulations of zinc oxide solubility: from bulk down to nanoparticles. Food and Chemical Toxicology 112: 518-525.
- Ranking the best zinc supplements of 2021. https://bodynutrition.org/zinc/
- Youssef FS, Ismail SH, Fouad OA, Mohamed GG (2024) Green synthesis and biomedical applications of zinc oxide nanoparticles-review. Egyptian Journal of Veterinary Sciences 55: 287-311.
- Alsaiari NS, Alzahrani FM, Amari A, Osman H, Harharah HN, et al. (2023) Plant and microbial approaches as green methods for the synthesis of nanomaterials: applications and future perspectives. Molecules 28: 463.
- Sani GD, Yakubu A, Saidu A, Aati R, Sahabi S, et al. (2023) A review on industrial applications of zinc oxide nanoparticles. International Journal of Advances in Engineering and Management 5: 1031-1041.
- Vinitha V, Preeyanghaa M, Vinesh V, Dhanalakshmi R, Neppolian B, et al. (2021) Two is better than one: catalytic, sensing and optical applications of doped zinc oxide nanostructures. Emergent Materials 4: 1093-1124.
- Degefa A, Bekele B, Jule LT, Fikadu B, Ramaswamy S, et al. (2021) Green synthesis, characterization of zinc oxide nanoparticles, and examination of properties for dye-sensitive solar cells using various vegetable extracts. Journal of Nanomaterials 3941923.
- Danley M (2021) Characterization of spongelike porous polyvinylidene fluoride for use as a biosensor. M. Eng. thesis, University of Minnesota-Duluth.
- Baharudin KB, Abdullah N, Derawi D (2021) Synthesis of raspberry-like structure zinc oxide nanoparticles via glycol-solvothermal, low-temperature solvothermal and coprecipitation methods. Comptes Rendus. Chimie 24: 33-42.
- Thakur S, Shandily M, Guleria G (2021) Appraisement of antimicrobial zinc oxide nanoparticles through Cannabis Jatropha curcasa Alovera and Tinosporacordifolia leaves by green synthesis process. Journal of Environmental Chemical Engineering 9: 104882.
- Zaheer T, Imran M, Pal K, Sajid MS, Abbas RZ, et al. (2021) Synthesis, characterization and acaricidal activity of green-mediated ZnO nanoparticles against Hyalomma ticks. Journal of Molecular Structure 1227: 129652.
- Ibrahim RE, Fouda MMS, Younis EM, Abdelwarith AA, Salem GA, et al. (2024) The anti-bacterial efficacy of zinc oxide nanoparticles synthesized by Nelumbo nucifera leaves against Clostridium perfringes challenge in Oreochromis niloticus. Aquaculture 578: 740030.
- Alavi M, Kamarasu P, McClements DJ, Moore MD (2022) Metal and metal oxide-based antiviral nanoparticles: properties, mechanisms of action, and applications. Advances in Colloid and Interface Science 306: 102726.
- Shubha P, Ganesh S, Shyamsundar S (2024) Anti-proliferative activity of biosynthesized zinc oxide nanoparticles against breast cancer MCF-7 cells. Materials Chemistry and Physics 128900.
- Talodthaisong C, Plaeyao K, Mongseetong C, Boonta W, Srichaiyapol O, et al. (2021) The decoration of ZnO nanoparticles by gamma aminobutyric acid, curcumin derivative and silver nanoparticles: synthesis, characterization and antibacterial evaluation. Nanomaterials 11: 442.
- Shilova OA, Tsvetkova IN, Vlasov DY, Ryabusheva YV, Sokolov GS, et al. (2022) Microbiologically induced deterioration and environmentally friendly protection of wood products. In Micro and Nano Technologies, Biodegradation and Biodeterioration At the Nanoscale, 283-321.
- Levy J. Zinc oxide benefits for protecting your skin from the sun + more. https://draxe.com/health/zinc-oxide-benefits/.
- CCL Mineral & Chemical. Zinc oxide formula-properties & manufacturing.
- Sharma N, Gupta PC, Upadhyay S, Rai S, Mishra P (2024) Antimicrobial applications of zinc oxide nanoparticles in food packaging industry. In: R.K. Bachheti, A. Bachheti, A. Husen (eds) Metal and Metal-Oxide Based Nanomaterials. Smart Nanomaterials Technology.
- Bui VKH, Pham TN, Hur J, Lee YC (2021) Review of ZnO binary and ternary composite anodes for lithium-ion batteries. Nanomaterials 11: 8.
- Verma R, Pathak S, Srivastava AK, Prawer S, Tomljenovic-Hanic S (2021) ZnO nanomaterials: green synthesis, toxicity evaluation and new insights in biomedical applications. Journal of Alloys and Compounds 876: 160-175.
- Xie J, Li H, Zhang T, Song B, Wang X, et al. (2023) Recent advances in ZnO nanomaterial-mediated biological applications and action mechanisms. Nanomaterials (Basel) 13: 1500.
- Khan M, Ahmad B, Hayat K, Ullah F, Sfina N, et al. (2024) Synthesis of ZnO and PEG-ZnO nanoparticles with controlled size for biological evaluation. RSC Advances 14: 2402-2409.
- Hwang GB, Stent J, Noimark S, Heo KJ, MacRobert AJ, et al. (2024) White light-activated bactericidal coating using acrylic latex, crystal violet, and zinc oxide nanoparticles. Materials Advances 5: 259-266.
- Ghattavi S, Homaei A (2024) Synthesis and characterization of ZnO-SiO2 hybrid nanoparticles as an effective inhibitor for marine biofilm and biofouling. Journal of Molecular Liquids 396: 123974.
- Shen L, Huang Z, Liu Y, Li R, Xu Y, et al. (2020) Polymeric membranes incorporated with ZnO nanoparticles for membrane fouling mitigation: a brief review. Frontiers in Chemistry 8: 224.
- Sahu A, Dosi R, Kwiatkowski C, Schmal S, Poler JC (2023) Advanced polymeric nanocomposite membranes for water and wastewater treatment: a comprehensive review. Polymers (Basel) 15: 540.
- Roy N, Chakraborty S (2021) ZnO as photocatalyst: An approach to wastewater treatment. Materials Today: Proceedings 2021, 46: 6399-6403.
- Hu I, Ma W, Pan Y, Chen Z, Zhang Z, et al. (2022) Resolving the tribo-catalytic reaction mechanism for biochar regulated zinc oxide and its application in protein transformation. Journal of Colloid and Interface Science 607: 1908-1918.
- Shkir M, Palanivel B, Khan A, Kumar M, Chang JH, et al. (2021) Enhanced photocatalytic activities of facile auto-combustion synthesized ZnO nanoparticles for wastewater treatment: An impact of Ni doping. Chemosphere 132687.
- Sharma SS, Palaty S, John AK (2021) Band gap modified zinc oxide nanoparticles: an efficient visible light active catalyst for wastewater treatment. International Journal of Environmental Science and Technology 18: 2619-2632.
- Karthik KV, Raghu AV, Reddy KR, Ravishankar R, Sangeeta M, et al. (2022) Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 287: 132081.
- Park JK, Rupa EJ, Arif MH, Li JF, Anandapadmanaban G, et al. (2021) Synthesis of zinc oxide nanoparticles from Gynostemma pentaphyllum extracts and assessment of photocatalytic properties through malachite green dye decolorization under UV illumination-a green approach. Optik 239: 166249.
- Siddique K, Shahid M, Shahzad T, Mahmood F, Nadeem H, et al. (2021) Comparative efficacy of biogenic zinc oxide nanoparticles synthesized by Pseudochrobactrum sp. C5 and chemically synthesized zinc oxide nanoparticles for catalytic degradation of dyes and wastewater treatment 28: 28307-28318.
- Abdullah FH, Abu Bakar NHH, Bakar MA (2021) Comparative study of chemically synthesized and low temperature bio-inspired Musa acuminata peel extract mediated zinc oxide nanoparticles for enhanced visible-photocatalytic degradation of organic contaminants in wastewater treatment. Journal of Hazardous Materials 406: 124779.
- Yashni G, Al-Gheethi A, Mohamed RMS, Dai-Viet NV, Al-Kahtani AA, et al. (2021) Bio-inspired ZnO nanoparticles synthesized from Citrus sinensis peels extract for congo red removal from textile wastewater via photocatalysis: optimization, mechanisms, techno-economic analysis. Chemosphere, 281: 130661.
- Rajendrachari S, Taslimi P, Karaoglanli AC, Uzun O, Alp E, et al. (2021) Photocatalytic degradation of Rhodamine B dye in wastewater and enzymatic inhibition study using cauliflower shaped ZnO nanoparticles synthesized by a novel one-pot green synthesis method. Arabian Journal of Chemistry 14: 103180.
- Khan WZ, Najeeb I, Ishtiaque S, Jabeen S (2016) Photodegradation of real pharmaceutical wastewater with titanium dioxide, zinc oxide, and hydrogen peroxide during UV treatment. IOSR Journal of Engineering 6: 36-46.
- Domingues FS, de Souza Freitas TKF, de Almeida CA, de Souza RP, Ambrosio E, et al. (2019) Hydrogen peroxide-assisted photocatalytic degradation of textile wastewater using titanium dioxide and zinc oxide. Environmental Technology 40: 1223-1232. [crossref]
- Li X, Wang J, Zhang J, Zhao C, Wu Y, et al. (2022) Cadmium sulfide modified zinc oxide heterojunction harvesting ultrasonic mechanical energy for efficient decomposition of dye wastewater. Journal of Colloid and Interface Science 607(1), 412-422. [crossref]
- El Bekkali C, Labrag J, Oulguidoum A, Chamkhi I, Laghzizil A, et al. (2022) Porous ZnO/hydroxyapatite nanomaterials with effective photocatalytic and antibacterial activities for the degradation of antibiotics. Nanotechnology for Environmental Engineering 7: 1.
- Reddy BS, Maurya AK, Narayana PL, Pasha SKK, Reddy MR, et al. (2022) Knowledge extraction of sonophotocatalytic treatment for acid blue 113 dye removal by artificial neural networks. Environmental Research, 204: 112359. [crossref]
- Banerjee P, Das D, Mitra P, Sinha M, Dey S, et al. (2014) Solar photocatalytic treatment of wastewater with zinc oxide nanoparticles and its ecotoxicological impact on Channa punctatus-a freshwater fish. Journal of Materials and Environmental Science 5: 1206-1213.
- Attarilar S, Yang J, Ebrahimi M, Wang Q, Liu J, et al. (2020) The toxicity phenomenon and the related occurrence in metal and metal oxide nanoparticles: a brief review from the biomedical perspective. Frontiers in Bioengineering and Biotechnology, 8: 822. [crossref]
- Falfushynska H, Sokolova I, Stoika R (2022) Uptake, biodistribution, and mechanisms of toxicity of metal-containing nanoparticles in aquatic invertebrates and vertebrates. In Biomedical Nanomaterials,
- Daraei H, Toolabian K, Thompson I, Qiu G (2022) Biotoxicity evaluation of zinc oxide nanoparticles on bacterial performance of activated sludge at COD, nitrogen, and phosphorus reduction. Frontiers of Environmental Science & Engineering 16: 19.
- Venkatasubbu GD, Baskar R, Anusuya T, Seshan CA, Chelliah R (2016) Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloids and Surfaces B: Biointerfaces 148: 600-606.
- Rambabu K, Bharath G, Banat F, Show PL (2021) Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. Journal of Hazardous Materials 402: 123560.
- Suman TY, Radhi Rajasree SR, Kirubagaran R (2015) Evaluation of zinc oxide nanoparticles toxicity on marine algae chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicology and Environmental Safety 113: 23-30.
- Tatagar AM, Moodi JI, Abbar JC, Phaniband MA (2022) Photocatalytic activity and anti-microbial application of synthesized zinc oxide nanoparticles towards remediation of hospital wastewater. Materials Today: Proceedings 49: 699-702.
- Nazir A, Akbar A, Baghdad HB, ur Rehman S, Al-Abbad E, et al. (2021) Zinc oxide nanoparticles fabrication using Eriobotrya japonica leaves extract: photocatalytic performance and antibacterial activity evaluation. Arabian Journal of Chemistry 14: 103251.
- Prakash MG, Chung IM (2016) Determination of zinc oxide nanoparticles toxicity in root growth in wheat seedlings. Acta Biologica Hungarica 67: 286-296. [crossref]
- Chupani L, Niksirat H, Velíšek J, Stará A, Hradilová S, et al. (2018) Chronic dietary toxicity of zinc oxide nanoparticles in common carp (Cyprinus carpio L.): tissue accumulation and physiological responses. Ecotoxicology and Environmental Safety, 147: 110-116. [crossref]
- Nazarizadeh A, Asri-Rezaie S (2016) Comparative study of antidiabetic activity and oxidative stress induced by zinc oxide nanoparticles and zinc sulfate in diabetic rats. AAPS PharmSciTech 17: 834-843. [crossref]
- Heng BC, Zhao X, Xiong S, Ng KW, Boey FYC, et al. (2010) Toxicity of zinc oxide nanoparticles on human bronchial epithelial cells is accentuated by oxidative stress. Food and Chemical Toxicology 48: 1762-1766.
- Khan M, Naqvi AH, Ahmad M. (2015) Comparative study of the cytotoxic and genotoxic potentials of zinc oxide and titanium dioxide nanoparticles. Toxicology Reports 2: 765-774.
- Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G (2011) Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine: Nanotechnology, Biology and Medicine, 7: 184-192.
- Abbasalipourkabir R, Moradi H, Zarei S, Asadi S, Salehzadeh A, et al. (2015) Toxicity of zinc oxide nanoparticles on adult male Wistar rats. Food and Chemical Toxicology 84: 154-160.
- Ghazi S, Diab AM, Khalafalla MM (2022) Synergistic effects of selenium and zinc oxide nanoparticles on growth performance, hemato-biochemical profile, immune and oxidative stress responses, and intestinal morphometry of Nile tilapia. Biological Trace Element Research 200: 364-374.
- Salam A, Khan AR, Li Liu, Yang S, Azhar W, et al. (2022) Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. Journal of Hazardous Materials 423: 127021.
- Yazhiniprabha M, Gopi N, Mahboob S, Al-Ghanim KA, Al-Misned F, et al. (2022) The dietary supplementation of zinc oxide and selenium nanoparticles enhances the immune response in freshwater fish Oreochromis mossambicus against aquatic pathogen Aeromonas hydrophila. Journal of Trace Elements in Medicine and Biology 69: 126878.
- Bao S, Xiang D, Xue L, Xian B, Tang W, et al. (2022) Pristine and sulfidized ZnO nanoparticles alter microbial community structure and nitrogen cycling in freshwater lakes. Environmental Pollution 294: 118661.
- Din MI, Rani A, Hussain Z, Khalid R, Aihetasham A, et al. (2021) Biofabrication of size-controlled ZnO nanoparticles using various capping agents and their cytotoxic and antitermite activity. International Journal of Environmental Analytical Chemistry 101: 821-837.
- Hazzazi F, Young A, O’Loughlin C, Daniels-Race T (2021) Fabrication of zinc oxide nanoparticles deposited on 3-aminopropyltriethoxysilane-treated silicon substrates by an optimized voltage-controlled electrophoretic deposition and their application as fluorescence-based sensors. Chemosensors 9: 5.
- Soundharraj P, Dhinasekaran D, Rajendran AR, Prakasarao A, Ganesan S (2021) N-Doped zinc oxide as an effective fluorescence sensor for urea detection. New Journal of Chemistry 45: 6080-6090.
- Swaminathan N, Sharma N, Nerthigan Y, Wu HF (2021) Self-assembled diphenylalanine-zinc oxide hybrid nanostructures as a highly selective luminescent biosensor for trypsin detection. Applied Surface Science 554: 149600.
- Cao J, Zhai Y, Tang W, Guo X, Wen Y, et al. (2021) ZnO tips dotted with Au nanoparticles — advanced SERS determination of trace nicotine. Biosensors 11: 465.
- Wang Y, Zhu G, Li M, Singh R, Marques C, et al. (2021) IEEE Transactions on NanoBioscience 20: 377-384.
- Barman U, Goswami N, Ghosh SS, Paily RP (2021) Fabrication of glutathione-S-transferase-ZnO nanoconjugate ensemble FET device for detection of glutathione. IEEE Transactions on Electron Devices 68: 1242-1249.
- Nadaroglu H, Alayli GA (2020) Highly sensitive glucose sensor based on ZnO nanoparticles as a biomimetic enzyme. Bioscience Research 17: 775-785.
- Faradillaa P, Setiyanto H, Manurung RV, Saraswaty V (2022) Electrochemical sensor based on screen printed carbon electrode-zinc oxide nano particles/molecularly imprinted-polymer for detection of sodium dodecyl sulfate. RSC Advances 12: 743-752.
- Thivina V, Hashim U, Gopinath SCB, Ayoib A, Nordin NKS, et al. (2019) Distinct detection of Ganoderma boninense on metal oxides-gold nanoparticle composite deposited interdigitated electrode DNA sensor. Journal of Physics: Conference Series 012050.
- Gugoasa LAD, Negut CC, Stefanov C (2021) Electrochemical applications of inorganic material-doped quantum dots. Electroanalytical Applications of Quantum Dot-Based Biosensors 12: 395-425.
- Kulkarni MB, Enaganti PK, Amreen K, Goel S (2021) Integrated temperature controlling platform to synthesize ZnO nanoparticles and its deposition on Al foil for biosensing. IEEE Sensors Journal 21: 9538-9545.
- Ji D, Low SS, Zhang D, Liu L, Lu Y, et al. (2022) Smartphone-Based Electrochemical System for Biosensors and Biodetection. In: Ossandon MR, Baker H, Rasooly A. (eds) Biomedical Engineering Technologies. Methods in Molecular Biology, 2393: 493-514.
- García-Salinas F, Vázquez-Durán A, Yáñez-Limón JM (2021) Comparative study of Al-doped ZnO films deposited by sol-gel and by sputtering using a sintered target from ZnO nanoparticles synthesized by sol-gel. Boletín de la Sociedad Española de Cerámica y Vidrio 62: 134-144.
- Tunca K, Öztürk F, Erden P (2021) A Comparison of four different electrode matrices on the performance of amperometric hydrogen peroxide biosensors. Electroanalysis 34: 1092-1102.
- Rauf S, Mani V, Ait A, Saravanan L, Tutku Y, et al. (2021) Binary transition metal oxide modified laser-scribed graphene electrochemical aptasensor for the accurate and sensitive screening of acute myocardial infarction. Electrochimica Acta 386: 138489.
- Naz S, Nisar A, Qian L, Hussain S, Karim S, et al. (2021) Graphene oxide functionalized with silver nanoparticles and ZnO synergic nanocomposite as an efficient electrochemical sensor for diclofenac sodium. Nano 16: 2150139.
- Zhang W, Sharma G, Kumar A, Shekh MI, Stadler FJ. (2021) Fabrication and characterization of Ni/Ag/Zn trimetal oxide nanocomposites and its application in dopamine sensing. Materials Today Communications 29: 102726.
- Gu M, Xiao H, Wei S, Chen Z, Cao L (2021) A portable and sensitive dopamine sensor based on Au nanoparticles functionalized ZnO-rGO nanocomposites modified screen-printed electrode.
- Nagal V, Kumar V, Khan M, Alomar SY, Tripathy N, et al. (2021) A highly sensitive uric acid biosensor based on vertically arranged ZnO nanorods on a ZnO nanoparticle-seeded electrode. New Journal of Chemistry 45: 18863-18870.
- Chokkareddy R, Redhi GG, Thangavel K (2021) Cytochrome c/multi-walled carbon nanotubes modified glassy carbon electrode for the detection of streptomycin in pharmaceutical samples. Analytical Sciences 37: 1265-1273.
- Chauhan N, Balayan S, Gupta S, Singh J, Jain U (2021) Enzyme-based sensing on nanohybrid film coated over FTO electrode for highly sensitive detection of antibiotics. Bioprocess and Biosystems Engineering 44: 2469-2479.
- Almog R, Shashar E, Sverdlov Y, Shacham-Diamand Y (2021) Decorating metal oxide nanostructures with noble metal nanoparticles for bio-sensing applications. ECS Meeting Abstracts 01: 1424.
- de J. Godoi MS, Faria AM, Mazon T (2020) Síntese de estruturas de carbono a partir de biomassa de mandioca fibra e para aplicação em biossensores. I Jornada de Iniciação Científica do CTI Renato Archer.
- Ahmad I, Ahmad A, Tabassum H, Kuddus M (2017) Applications of nanoparticles in the treatment of wastewater. In L.M.T. Martínez et al. (eds.), Handbook of Ecomaterials 2017.
- Andrade-Guel M, Cabello-Alvarado C, Cano-Salaza L, Ávila-Orta C, Cruz V (2023) Recent Developments in Wastewater Treatments.
- Bora T, Dutta J (2014) Applications of nanotechnology in wastewater treatment-a review. Journal of Nanoscience and Nanotechnology 14: 613-626. [crossref]
- Gordano A (2024) Chapter 6-Hybrid inorganic membranes. Editors: A. Basile, F. Lipnizki, M.R. Rahimpour, V. Piemonte. Current Trends and Future Developments on Bio-Membranes 131-174.
- Yaqoob AA, Parveen T, Umar K, Mohamad Ibrahim MN (2020) Role of nanomaterials in the treatment of wastewater: a review. Water 12: 495.
- Jiang M, Qi Y, Liu H, Chen Y (2018) The role of nanomaterials and nanotechnologies in wastewater treatment: a bibliometric analysis. Nanoscale Research Letters 13: 233.
- Behera A (2022) Self-cleaning materials. Advanced Materials.
- Hunt E. (2019) Zinc-based additives for biofouling and MIC protection: Fabrication method for long-term efficacy. Master’s thesis. Department of Engineering & Computer Science, West Texas A&M University.
- Eliasson J (2015) The rising pressure of global water shortages. Nature 517: 6.
- Schaep J, Vandecasteele C (2001) Evaluating the charge of nanofiltration membranes, Journal of Membrane Science 188: 129-136.
- Zaharescu T, Lungulescu EM (2016) Weathering degradation of polymers. In: Rosu D, Visakh P. M. (eds) Photochemical Behavior of Multicomponent Polymeric-based Materials. Advanced Structured Materials, 26.
- Chong MN, Jin BC, Chow WK, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Research 44: 2997-3027.
- Pendergast MM, Hoek EMV (2011) A review of water treatment membrane nanotechnologies. Energy & Environmental Science 4: 1946-1971.
- Strathmann H (1981) Membrane separation processes. Journal of Membrane Science 9: 121-189.
- Baker RW (2002) Future directions of membrane gas separation technology. Industrial & Engineering Chemistry Research 41: 1393-1411.
- Padaki M, Surya MR, Abdullah MS, Misdan N, Moslehyani A, et al. (2015) Membrane technology enhancement in oil-water separation-a review. Desalination 357: 197-207.
- Ang WL, Mohammad AW, Hilal N, Leo CP (2015) A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination, 363: 2-18.
- Subramani A, Jacangelo JG (2015) Emerging desalination technologies for water treatment: a critical review. Water Research 75: 164-187.
- Tran NH, Ngo HH, Urase T, Gind KYH (2015) A critical review on characterization strategies of organic matter for wastewater and water treatment processes. Bioresource Technology 193: 523-533. [crossref]
- Van Der Bruggen B, Vandecasteele C, Van Gestel T, Doyen W, Leysen R (2003) A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environmental Progress 22: 46-56.
- Anderson MA, Gieselmann MJ, Xu Q (1988) Titania and alumina ceramic membranes. Journal of Membrane Science 39: 243-258.
- Meng F, Chae SR, Drews A, Kraume M, Shin HS, Yang F (2009) Recent advances in membrane bioreactors: membrane fouling and membrane material. Water Research 43: 1489-1512. [crossref]
- Kim J, Van der Bruggen B (2010) The use of nanoparticles in polymeric and ceramic membrane structures: review of manufacturing procedures and performance improvement for water treatment. Environmental Pollution 158: 2335-2349. [crossref]
- Kang GD, Cao YM (2014) Application and modification of poly(vinylidene fluoride) membranes-a review. Journal of Membrane Science 463: 145-165.
- Lin H, Peng W, Zhang M, Chen J, Hong H, et al. (2016) A review on anaerobic membrane bioreactors: applications, membrane fouling and future perspectives. Desalination 314: 169-188.
- She Q, Wang R, Fane AG, Tang CY (2016) Membrane fouling in osmotically driven membrane processes: a review. Journal of Membrane Science 499: 201-233.
- Cuiming W, Tongwen X, Weihua Y (2003) Fundamental studies of a new hybrid (inorganic-organic) positively charged membrane: membrane preparation and characterizations. Journal of Membrane Science 216: 269-278.
- Wu C, Xu T, Yang W (2003) A new inorganic-organic negatively charged membrane: membrane preparation and characterizations. Journal of Membrane Science 224: 117-125.
- Zunita M, Makertihartha IGBN, Saputra FA, Syaifi YS, Wenten IG (2018) Metal oxide based antibacterial membrane. IOP Conference Series: Materials Science and Engineering 395: 012021.
- Saif AA (2018) Engineering Nanocomposite Membranes: Fabrication, Modification and Application. Doctoral Thesis. Chemical Engineering.
- Opoku F, Kiarii EM, Govender PP, MA. Mamo MA (2017) Metal oxide polymer nanocomposites in water treatments. Descriptive Inorganic Chemistry Researches of Metal Compounds.
- Taiba N, Tayyiba D (2021) The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: a review. Environmental Chemistry and Ecotoxicology 3: 59-75.
- Kumar S, Ye F, Dobretsov S, Dutta J (2021) Nanocoating is a new way for biofouling prevention. Frontiers in Nanotechnology 3: 2673-3013.
- Sadamichi M, Takami T, Stewart EB, Sumio I, Wataru K, et al. (2004) Physics of Transition Metal Oxides. Springer Science & Business Media, Technology & Engineering,
- Meenakshi SSAS, Alagumalai N, Dipak R.||(2019) Tailored polymer nanocomposite membranes based on carbon, metal oxide and silicon nanomaterials: a review. Journal of Materials Chemistry A, 7: 8723-8745.
- Ghosh S, Malloum A, Igwegbe CA, Ighalo JO, Ahmadi Set al. (2022) New generation adsorbents for the removal of fluoride from water and wastewater: a review. Journal of Molecular Liquids 346: 118257.
- Ye J, Gao H, Wu J, Yu R (2021) Effects of ZnO nanoparticles on flocculation and sedimentation of activated sludge in wastewater treatment process. Environmental Research 192: 110256. [crossref]
- Banu JR, Sharmila VG, Kannah RY, Kanimozhi R, Elfasakhany A, et al. (2022) Impact of novel deflocculant ZnO/chitosan nanocomposite film in disperser pretreatment enhancing energy efficient anaerobic digestion: parameter assessment and cost exploration. Chemosphere 286: 131835. [crossref]
- Shen L, Huang Z, Liu Y, Li R, Xu Y, et al. (2020) Polymeric membranes incorporated with ZnO nanoparticles for membrane fouling mitigation: a brief review. Frontiers in Chemistry 8: 224. [crossref]
- Taherizadeh H, Hashemifard SA, Izadpanah AA, Ismail AF (2021) Investigation of fouling of surface modified polyvinyl chloride hollow fiber membrane bioreactor via zinc oxide-nanoparticles under coagulant for municipal wastewater treatment. Journal of Environmental Chemical Engineering 9: 105835.
- Kusworo TD, Dalanta F, Aryanti N, Othman NH (2021) Intensifying separation and antifouling performance of PSF membrane incorporated by GO and ZnO nanoparticles for petroleum refinery wastewater treatment. Journal of Water Process Engineering 41: 102030.
- Ayyaru S, Dinh TTL, Ahn YH (2020) Enhanced antifouling performance of PVDF ultrafiltration membrane by blending zinc oxide with support of graphene oxide nanoparticle. Chemosphere 241: 125068.
- Mahlangu OT, Nackaerts R, Mamba BB, Verliefde ARD (2017) Development of hydrophilic GO-ZnO/PES membranes for treatment of pharmaceutical wastewater. Water Science & Technololgy 76: 501-514.
- Purushothaman M, Arvind V, Saikia K, Vaidyanathan VK (2022) Fabrication of highly permeable and anti-fouling performance of poly(ether ether sulfone) nanofiltration membranes modified with zinc oxide nanoparticles. Chemosphere 286: 131616.
- Cao W, Ma W, Lu T, Jiang Z, Xiong R, et al. (2022) Multifunctional nanofibrous membranes with sunlight-driven self-cleaning performance for complex oily wastewater remediation. Journal of Colloid and Interface Science 608: 164-174. [crossref]
- Dizge N, Gonuldas H, Ozay Y, Ates H, Ocakoglu K, et al. (2017) Synthesis and performance of antifouling and self-cleaning polyethersulfone/graphene oxide composite membrane functionalized with photoactive semiconductor catalyst. Water Science & Technology 75: 670-685. [crossref]
- García, Andreina, Rodríguez B, Giraldo H, Yurieth Q, et al. (2021) Copper-modified polymeric membranes for water treatment: a comprehensive preview. Membranes 11: 93. [crossref]
- Mohamad S, Idris M, Abdullah H, Ismail A (2013) Short review of ultrafiltration of polymer membrane as a self-cleaning and antifouling in the wastewater system. Advanced Materials Research 795: 318-323.
- Tae HB, Tae MT (2005) Preparation of TiO2 self-assembled polymeric nanocomposite membranes and examination of their fouling mitigation effects in a membrane bioreactor system. Journal of Membrane Science 266: 1-5.
- Arif Z, Sethy NK, Mishra PK, Verma B (2020) Development of eco-friendly, self-cleaning, antibacterial membrane for the elimination of chromium (VI) from tannery wastewater. International Journal of Environmental Science and Technology (Tehran) 17: 4265-4280. [crossref]
- Meenakshi SSAS, Alagumalai N, Dipak R (2019) Tailored polymer nanocomposite membranes based on carbon, metal oxide and silicon nanomaterials: a review. Journal of Materials Chemistry A, 7: 8723-8745.
- Lavisha B, Rasmeet S, Jonita V (2020) Metal/metal oxide nanocomposite membranes for water purification. Materials Today: Proceedings 2020, 44 538-545.
- Rabajczyk A, Zielecka M, Cygańczuk K, Pastuszka Ł, Jurecki L (2021) Nanometals-containing polymeric membranes for purification processes. Materials (Basel, Switzerland), 14: 513.
- Xiaofang C, Yaoxin H, Zongli X, Huanting W (2018) Chapter 3-Materials and Design of Photocatalytic Membranes. In Current Trends and Future Developments on (Bio-) Membranes, 71-96.
- Khan SB, Alamry KA, Bifari EN, Asiri AM, Yasir M, et al. (2015) Assessment of antibacterial cellulose nanocomposites for water permeability and salt rejection. Journal of Industrial and Engineering Chemistry 24: 266-275.
- Waldman RZ (2020) Metal oxide growth on polymer surfaces and within polymer volumes: applications in novel membrane materials. Ph.D. thesis, University of Chicago, USA.
- Balta S, Sotto A, Luis P, Benea L, Van der Bruggen B. et al. (2012) A new outlook on membrane enhancement with nanoparticles: the alternative of ZnO. Journal of Membrane Science 389: 155-161.
- Zinc peroxide nanoparticles. Marcina Kasprzaka 44/52. 01-224 Warszawa, Poland.
- Dupont Water Solutions.
- Manttari M, Pihlajamaki A, Kaipainen E, Nystrom M (2002) Effect of temperature and membrane pretreatment on the filtration properties of nanofiltration membranes. Desalination 145: 81-86.
- Liora RT, Zohar K, Tsur Y (2008) Synthesis of stabilized nanoparticles of zinc peroxide. Chemical Engineering Journal 2008, 136: 425-429.
- Hsu CC, Wu NL (2005) Synthesis and photocatalytic activity of ZnO/ZnO2 Journal of Photochemistry and Photobiology A: Chemistry 172: 269-274.
- Halfar J, Brožová K, Čabanová K, Heviánková S, Kašpárková A, et al. (2021) Disparities in methods used to determine microplastics in the aquatic environment: a review of legislation, sampling process and instrumental analysis. International Journal of Environmental Research and Public Health 18: 7608. [crossref]
- Gaw S, Glover CN (2016) A case of contagious toxicity? Isoprostanes as potential emerging contaminants of concern. Science of The Total Environment 560-561: 295-298.
- Ofrydopoulou A, Nannou C, Evgenidou E, Lambropoulou D (2021) Sample preparation optimization by central composite design for multi class determination of 172 emerging contaminants in wastewaters and tap water using liquid chromatography high-resolution mass spectrometry. Journal of Chromatography A 1652, 462369. [crossref]
- Bowers I, Subedi B (2021) Isoprostanes in wastewater as biomarkers of oxidative stress during COVID-19 pandemic. Chemosphere 271: 129489. [crossref]
- Abdullah FH, Abu Bakar NHH, Abu Bakar M (2022) Current advancements on the fabrication, modification, and industrial application of zinc oxide as photocatalyst in the removal of organic and inorganic contaminants in aquatic systems. Journal of Hazardous Materials 424: 127416.
- Liu X, Yu X, Sha L, Wang Y, Zhou Z, et al. (2021) The preparation of black titanium oxide nanoarray via coking fluorinated wastewater and application on coking wastewater treatment. Chemosphere 270: 128609.
- Sheikh M, Pazirofteh M, Dehghani M, Asghari M, Rezakazemi M, et al. (2020) Application of ZnO nanostructures in ceramic and polymeric membranes for water and wastewater technologies: a review. Chemical Engineering Journal 391: 123475.
- Kalaivizhi R, Danagody B, Yokesh A (2022) ACs@ZnO incorporated with a PSF/PU polymer membrane for dye removal. Materials Advances 3: 8534-8543.
- van den Berg T, Ulbricht M (2020) Polymer nanocomposite ultrafiltration membranes: the influence of polymeric additive, dispersion quality and particle modification on the integration of zinc oxide nanoparticles into polyvinylidene difluoride membranes. Membranes 10: 197. [crossref]
- Kim HG, Kim R, Kim S, Choi C, Kim B. et al. (2018) Propylene carbonate-derived size modulation of water cluster in pore-filled Nafion/polypropylene composite membrane for the use in vanadium redox flow batteries. Journal of Industrial and Engineering Chemistry 2 60: 401-406.
- Shabbir S, Kulyar MF, Bhutta ZA, Boruah P, Asif M (2021) Toxicological consequences of titanium dioxide nanoparticles and their jeopardy to human population. BioNanoScience 11: 621-632. [crossref]
- Ghaemi N, Madaeni SS, Daraei P, Rajabi H, Zinadini S, et al. (2015) Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe 3O 4 Chemical Engineering Journal 263: 101-112.
- Zhang A, Zhang Y, Pan G, Xu J, Yan H, et al. (2017) In situ formation of copper nanoparticles in carboxylated chitosan layer: Preparation and characterization of surface modified TFC membrane with protein fouling resistance and long-lasting antibacterial properties. Separation & Purification Technology 176: 164-172.
- Liu X, Foo LX, Li Ye, Lee JY, Cao B, et al. (2016) Fabrication and characterization of nanocomposite pressure retarded osmosis membranes with excellent anti-biofouling property and enhanced water permeability. Desalination 389: 137-148.
- Goh PS, Matsuura T, Ismail AF, Hilal N (2016) Recent trends in membranes and membrane processes for desalination. Desalination 391: 43-60.
- Thanigaivelan A, Mariam O, Rambabu K, Abdul H, Nirmala G, et al. (2022) Surface-engineered polyethersulfone membranes with inherent Fe-Mn bimetallic oxides for improved permeability and antifouling capability. Environmental Research 204: 112390.
- Arumugham T, Ouda M, Krishnamoorthy R, Hai A, Gnanasundaram N, et al. (2021) Surface-engineered polyethersulfone membranes with inherent Fe-Mn bimetallic oxides for improved permeability and antifouling capability. Environmental Research 204: 112390. [crossref]
- Nagarajan M, Maadurshni GB, Tharani GK, Udhayakumar I, Kumar G et al. (2022) Exposure to zinc oxide nanoparticles induces cardiovascular toxicity and exacerbates pathogenesis-role of oxidative stress and MAPK signaling. Chemico-Biological Interactions 351: 109719. [crossref]
- Brar SK, Verma M, Tyagi RD, Surampalli RY (2010) Engineered nanoparticles in wastewater and wastewater sludge-evidence and impacts. Waste Management 30: 504-520. [crossref]
- Sadik OA, Du N, Yazgan I, Okello V (2014) Chapter 6-Nanostructured Membranes for Water Purification, Editors: A. Street, R Sustich, Jeremiah Duncan, Nora Savage, In Micro and Nano Technologies, Nanotechnology Applications for Clean Water, William Andrew Publishing, 95-108.
- Ergönül MB, Nassouhi D, Çelik M, Atasağun S (2021) A comparison of the removal efficiencies of Myriophyllum spicatum L. for zinc oxide nanoparticles in different media: a microcosm approach. Environmental Science and Pollution Research 28: 8556-8568. [crossref]
- Afonso-Olivares C, Sosa-Ferrera Z, Santana-Rodríguez JJ (2017) Occurrence and environmental impact of pharmaceutical residues from conventional and natural wastewater treatment plants in Gran Canaria (Spain) Science of The Total Environment 599-600: 934-943. [crossref]
- Ryu Y, Gracia-Lor E, Bade R, Baz-Lomba JA, Bramness JG, et al. (2016) Increased levels of the oxidative stress biomarker 8-iso-prostaglandin F2α in wastewater associated with tobacco use. Scientific Reports 6: 39055.
- Ajibola AS, Amoniyan OA, Ekoja FO, Ajibola FO (2021) QuEChERS approach for the analysis of three fluoroquinolone antibiotics in wastewater: concentration profiles and ecological risk in two Nigerian hospital wastewater treatment plants. Archives of Environmental Contamination and Toxicology 80: 389-401. [crossref]
- Sims N, Rice J, Kasprzyk-Hordern B (2019) An ultra-high-performance liquid chromatography tandem mass spectrometry method for oxidative stress biomarker analysis in wastewater. Analytical and Bioanalytical Chemistry 411: 2261-2271.
- Azodi M, Ghoshal S, Stephan C (2015) Measurement and analysis of silver nanoparticles in wastewaters with single particle ICP-MS. PerkinElmer Application Note
- Cervantes-Avilés P, Keller AA (2021) Incidence of metal-based nanoparticles in the conventional wastewater treatment process. Water Research 189: 116603.
- Ryu Y, Reid MJ, Thomas KV (2015) Liquid chromatography-high resolution mass spectrometry with immunoaffinity clean-up for the determination of the oxidative stress biomarker 8-iso-prostaglandin F2alpha in wastewater. Journal of Chromatography A 1409: 146-151.
- Kovacs-Wilks K (2016) Agents of unknown concern: isoprostanes as potential emerging contaminants. M.Sc. thesis, University of Canterbury, 2016.
- Boogaerts T, Quireyns M, Covaci A, De Loof H, van Nuijs ALN (2021) Analytical method for the simultaneous determination of a broad range of opioids in influent wastewater: Optimization, validation and applicability to monitor consumption patterns. Talanta 232: 122443. [crossref]
- Guironnet A, Sanchez-Cid C, Vogel TM, Wiest L, Vulliet E (2021) Aminoglycosides analysis optimization using ion pairing liquid chromatography coupled to tandem mass spectrometry and application on wastewater samples. Journal of Chromatography A 1651: 462133.
- Mao K, Yang Z, Zhang H, Li X, Cooper JM (2021) Paper-based nanosensors to evaluate community-wide illicit drug use for wastewater-based epidemiology. Water Research 189: 116559. [crossref]
- Lee J, Choi YJ, Jeong J, Chae KJ (2021) Eye-glass polishing wastewater as significant microplastic source: microplastic identification and quantification. Journal of Hazardous Materials 403: 123991.
- Yu C, Kim S, Jang M, Park CM, Yoon Y (2022) Occurrence and removal of engineered nanoparticles in drinking water treatment and wastewater treatment processes: a review. Environmental Engineering Research 10: 134-152.
- Mubarak SM (2022) Nanotechnology for air remediation. In Research Anthology on Emerging Techniques in Environmental Remediation. IGI Global.
- Kang CS, Evans EA, Chase GG (2022) Metal oxide nanofiber for air remediation via filtration, catalysis, and photocatalysis. Elsevier, 191-211.
- Ding S, Li C, Bian C, Zhang J, Xu Y et al. (2022) Application of low-cost MFe2O4 (M = Cu, Mn, and Zn) spinels in low-temperature selective catalytic reduction of nitrogen oxide. Journal of Cleaner Production, 330: 129825.
- Santhosh G, Nayaka GP. Nanoparticles in construction industry and their Toxicity. In: Ecological and Health Effects of Building Materials, J.A. Malik, S. Marathe (eds) Springer.
- Gupta AD, Patil SZ (2022) Potential environmental impacts of nanoparticles used in construction industry. In Ecological and Health Effects of Building Materials, J.A. Malik, S. Marathe (eds) Springer, Cham.
- Srivastava S, Bhargava A (2022) Toxicity aspects of biologically synthesized nanoparticles. In: Green Nanoparticles: The Future of Nanobiotechnology. Springer, Singapore.
- Shet VB, Navalgund L, Joshi K, Yumnam S (2022) Application of nanoparticles in construction industries and their toxicity. In Ecological and Health Effects of Building Materials.
- American Welding Society. Metal fume fever. Safety and Health Fact Sheet No. 25. 2014.
- Magnano GC, Marussi G, Pavoni E, Adami G, Filon FL, et al. (2022) Percutaneous metals absorption following exposure to road dust powder. Environmental Pollution 292: 118353.
- Chavali MS, Nikolova MP (2019) Metal oxide nanoparticles and their applications in nanotechnology. SN Applied Sciences 1: 607.
- Saleem H, Zaidi SJ, Ismail AF, Goh PS (2022) Advances of nanomaterials for air pollution remediation and their impacts on the environment. Chemosphere 287: 132083.
- Nagar A, Kumar A, Tyagi U, Dhasmana H, Majeed Khan MA, et al. (2022) Ultrafast, trace-level detection of NH3 gas at room temperature using hexagonal-shaped ZnO nanoparticles grown by novel green synthesis technique. Physica B: Condensed Matter 626: 413595.
- Dey A. (2018) Semiconductor metal oxide gas sensors: a review. Materials Science and Engineering B 229: 206-217.
- Ageel HK, Harrad S, Abou-Elwafa Abdallah M (2022) Occurrence, human exposure, and risk of microplastics in the indoor environment. Environmental Science: Processes & Impacts 24: 17-31.