Abstract
In this study, we isolated five urease producing bacterial strains from coastal environment and create marine-biocement using Microbial Induced Calcium Carbonate Precipitation (MICP) procedure. It was shown that the wide range of urease producing bacteria could be isolated from coastal environment. The reinforcement effect of S1-1 strain showes the highest CaCO3 amount and Unconfined Compressive Strength (UCS) level. The SEM-EDX results showed that S1-1 definitely precipitated calcium carbonate and bound the sea sand together. Therefore, urease producing bacteria S1-1 that can be newly used for biomineralization. Germination test showed that the marine-biocement retained its form in seawater for about one month, and then collapsed along with the eelgrass grew. This indicates that the marine-biocement has the strength suitable for the germination of eelgrass. After disintegrating, the marine-biocement returned to the sea environment as sea sand, suggesting that marine-biocement may be used as an innovative seeding medium for Zostera marina bioenvironmentally friendly.
Keywords
Marine-biocement, MICP, Eelgrass meadow, Blue carbon
Introduction
Japanese coastal area has typical biodiversity in the Asian ocean cause of two big ocean currents such as Japan current from southeast asia and Oyashio current from Arctic ocean. Moreover, one of the eelgrass “Zostera marina” habits around the Japanese coastal area and provide the life of marine biodiversity. This huge ecosystem services based on the feeding place, spawning ground and habitats for shells, squid and small fishes each other. Unfortunately, eelgrass meadows in Japan were significantly damaged by development of the coastal area and factory effluent in the 1960s, a period of high economic growth, and are expected to continue declining in the future [1]. These phenomena cause the coastal areas to lose their habitat for seaweed and to reduce important fishery resources that have been deprived of habitats. Because, Zostera marina support marine life, including epiphytic organisms as well as coastal fisheries resources and contribute to marine environments by stabilizing bottom sediment and maintaining coastal water quality [2]. In addition to this, it recently has been reported that Zostera marina absorbs not only CO2 from the sea, but also about 17% from atmospheric CO2 when exposed at low [3]. Thus, Zostera marina habitats are considered one of the most valuable marine ecosystems because they has both a supportive place for marine life and a very efficient storehouse of atmosphere-derived CO2 as blue carbon [4,5]. In this study, we suggest the novel eelgrass protection technique applied with MICP technology. MICP has developed for the geotechnical applications such as liquefaction after earthquake, exchange heavy metal ions and self-repairing concrete for coastal erosion [6-8]. We modified the following MICP reaction mechanisms.
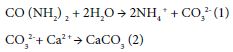
Marine bacterial urease catalyzes hydrolysis urea to ammonium and carbonate ion same as Sporosarcina pasteurii (eq1). After that, calcium carbonate precipitates from carbonate ion and calcium ion (eq2) between coastal sand particles. Then, coastal sand combines by calcium carbonate crystals and produces solidified marine-biocement (Kusube et al. 2020) [9]. The marine-biocement is harmless for several marine organisms cause of produced from marine bacteria and coastal sand. Therefore, we propose sustainable and convenience protection of eelgrass meadows with this marine-biocement.
Materials and Methods
Isolation and Identification of Urease Producing Bacteria
Urease producing bacteria were isolated from coastal sand collected from Shirahama-cho, Wakayama, Japan (33.692241°N, 135.336596°E) with Urea agar base (UAB) plate (Thermo Scientific Co. Waltham, Massachusetts, US) for screening culture media. The colonies with pinkish halo were pure cultured on a fresh UAB plates at least 5 times and were cultured at 30.0°C. After single colony culture, bacterial genomic DNA was extracted by 95.0°C boiling for 15 minutes. The 16SrDNA gene was amplified with universal primer 27F and [10] on the extracted each bacterial DNA. PCR program showed as denaturation at 94.0°C for 10 minutes and 25 cycles of denaturation (95.0°C, 30 seconds), annealing (55.0°C, 15 seconds) and extension (72.0°C, 30 seconds). And finally, gene extended at 72.0°C for 3 minutes. The elongated 16SrDNA sequences were determined by macrogen.co (Tokyo, Japan) and isolated marine bacteria was identified with Basic Local Alignment Search Tool BLASTN program [11]. A Phylogenetic tree was constructed by the neighbor-joining (NJ) method using MEGA X (ver.10.2.4) software with alimental sequenced data. An interior branch test was carried out (heuristic option and 1000 replications) to check the tree topology for robustness [12].
MICP Mechanism Applied to Marine-Biocement
Coastal sand 40.0 g was mixed for the making marine-biocement with isolated marine bacterial pellet from 100 mL culturing media and ionic solution 10 mL containing 0.75 M calcium chloride and 1.5 M urea [13-15] in the plastic tube (27 mm diameter and 50 mm height) with 2 mm mesh holes on the side of tubes to easy penetrate ionic solutions. The soaking materials placed at 25°C for 1 day to fully enzymatic reaction on the surface of the sand particles. To increase hardness of the marine-biocement, this treatment was repeatedly 2 more times. After this enzymatic reaction, marine-biocement was washed out excess ionic solution in distilled water and completely dried up at 110°C for 3 days to prevent hardening by quenching.
Measurement of Unconfined Compressive Strength of Marine-Biocement
In this study, Unconfined Compressive Strength (UCS) measurement was adopted to assess the strength for the purpose of determining the physical strength properties imparted on MICP. The UCS measurement have been used in most of the experimental programs reported in the literature in order to evaluate the effectiveness of the stabilization of marine-biocement [16] (Cheng, Shahin and Ruwisch 2014). UCS measurement is a compression test of a cylindrical rock specimens under confining pressure where the loading path is followed by a computer. After MICP curing, specimens were extruded from the mold, and it was sized 27 mm in diameter and 40 mm in height. And the strength properties of each marine-biocement were measured by UCS measurement, and shear strength were determined. Shear strength is a measure of how much stress (force/area) can be applied before the material undergoes shear failure.
Scanning Electron Microscopic (SEM) Observation and Energy Dispersible X-ray Spectrometry (EDX) Measurement of Marine-Biocement
The binding precipitate morphology and chemical component was analyzed with SEM-EDX analysis. Marine-biocement was dewatering treated with 50% acetone solution stepwise to 100% acetone in order to reduce the water molecules in the marine-biocement and completely vacuum drying with evaporator. After drying, marine-biocement for SEM-EDX specimens were stored in desiccator at room temperature. The binding precipitate morphology was observed by SEM (FlexSEM1000Ⅱ, HITACHI, Tokyo, Japan). The SEM specimens were coated 17 nm thickness platinum with AUTO FINE COATER (JFC-1600H) and SEM analysis was carried out at 15 kV and 62 mA. The chemical components of binding precipitates were analyzed by EDX system (AZtecOne, HITACHI, Tokyo, Japan). Kα spectra was used to analyze the surface precipitates.
Quantitative analysis of CaCO3 in marine-biocement
To dissolve calcium carbonate, a calcite-acid reactor chamber was used [17]. The device consists of a reactor chamber, pressure meter and valve for the exhaust gas.
The ratio of binding calcium carbonate C is defined as:
![]()
The 1.0 g of marine-biocement was placed into reactor chamber, and binding calcium carbonate include the marine-biocement was measured under standard conditions (25.0°C, 1 atm). The 10 mL of 1.0 M hydrochloric acid added to generate the CO2 gas from the calcium carbonate. The calibration curve was made with standard pure CaCO3 reagent (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The reactor chamber was sealed and gently shake to facilitate dissolve CaCO3 in the reactor chamber. The binding calcium carbonate in marine-biocement was calculated from the calibration curve and the blank as non-treated coastal sand.
Germination test from Marine-Biocement with Zostera marina
In the creation process, a hole of φ 3 mm H 15 mm was dented on the upper part of the marine-biocement and three seeds of Zostera marina from Eiga jima, Hyogo prefecture provided by the NPO corporate seed of Zostera marina bank were embedded in marine-biocement of S1-1 which was most hardest of all specimens in UCS test. Only the seeds that showed high density in saturated saline were selected. Selected three seeds were planted each marine-biocement hole and covered with sea-sand and placed in a water tank (35 L) as germination condition irradiated for 10 hours for a day with LED light to accelerate germination, circulation of natural seawater controlled at 10°C constant [18]. As a control, seeds were sowed at 10 mm deep under the seasand and germinated spontaneously and compared with the germination rate from marine-biocement.
Results and Discussion
Phylogenetical Relation of Isolated Urease Producing Bacteria
Five isolated strains were identified from approximately 1,200 bp of 16SrDNA sequences. The results shown that they were closely related as well as to bacteria genus belonging to the Cupriavidus, Bacillus and Pseudomonas. These 16SrDNA sequences submitted on DDBJ and BLAST results suggested that the closest relatives of Cupriavidus basirensis S1-1 (Accession No. LC760339), Bacillus cereus S1-2 (Accession No. LC760340), Pseudomonas ceruminis S1-4 (Accession No. LC760341), Pseudomonas nitroreducens S1-5 (Accession No. LC760342) and Priestia megaterium S1-8 (Accession No. LC760343) cause of E value 0.0, 0.0,0.0 and 0.0, identified value 99.24%, 99.50%, 98.24% and 98.86% respectively. These sequences have been deposited in DDBJ are available under accession numbers LC760339-43). These strains included the urease producing bacteria isolated from marine environment previously reports (Figure 1). The relationship between isolates and previously reported marine urease producing bacteria showed in Figure 1. Phylogenetic analysis shows that the no specific species need for producing marine-biocement, it means that is able to isolate in every marine environment. Moreover, these bacterial species were already applied for industrial bio-remediation such as oil degradation, clean up for soil and water contaminated with heavy metals and/or chlorinated organic compounds [19,20].
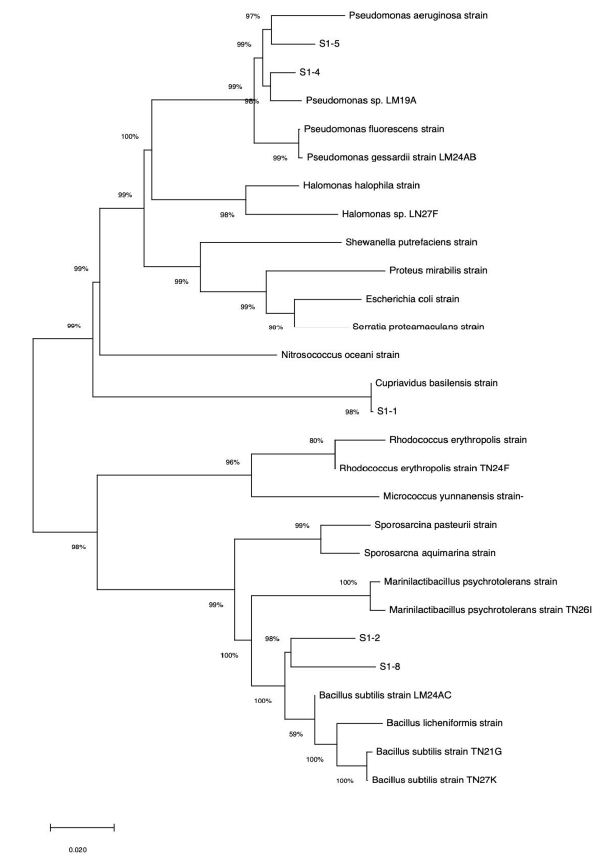
Figure 1: Neighbor-joining tree based on bacterial 16S rRNA gene sequence data from different isolates of the current study along with sequences available in the GenBank database. Bootstrap values calculated from 1000 resamplings using neighbor-joining are shown at the respective nodes when the calculated values were 50% or greater. The phyla to which the strains belong are presented on the right.
Characterization of Marine-Biocement
The relationship between bacterial CaCO3 (weight%) and UCS level (kPa) of the marine-biocement was shown at Figure 2. Isolated bacteria produced 1.7%-3.9% of CaCO3 crystalline and then hardness showed 36-449 kPa in UCS level. Strain S1-1 showed the highest CaCO3 amount and UCS level were 3.9% and 449 kPa, respectively. On the other hand, bacterial-free blank included 1.8% of CaCO3 content and showed 106 kPa hardness. In addition, marine-biocement with strain S1-1 shows CaCO3 content is 2.1 times higher than blank, and 4.2 times higher UCS level than that of blank. This result shows that was good related CaCO3 content and UCS levels. Because CaCO3 crystalline could be binding with each sea-sand particles to be strength enhancement (Figure 2). Moreover, CaCO3 crystalline grew up on the surface of sand particles by urease producing bacteria. The important point is the urease producing bacteria was adsorbed on the surface of the sea-sand particles through the bacterial membrane electrostatic interactions. Previous studies have shown that bacterial cell surfaces are negatively charged and adsorb onto the particle surface [21,22]. The marine-biocement S1-1 induced ideal MICP system and was ocean-friendly materials cause of natural sea-sand and isolated bacterial species from the ocean. Biomineralization with S1-1 never reported. Therefore, it was suggested that S1-1 may be a urease producing bacteria that can be newly used for biomineralization.
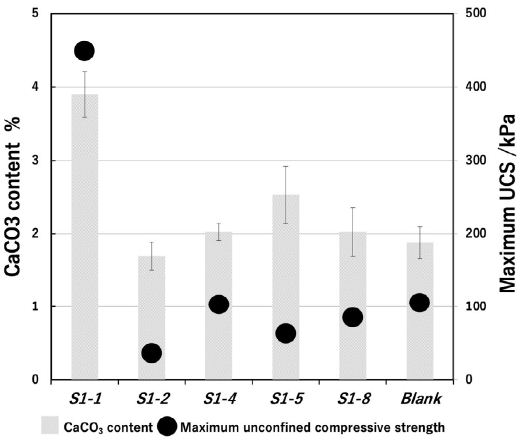
Figure 2: Relationship between CaCO3 content of the marine-biocement and UCS. (S1-1: Cupriavidus basirensis, S1-2: Bacillus cereus, S1-4: Pseudomonas putida, S1-5: Psedomomas nitroreducens. S1-8: Bacillus megaterium).
Observation of Surface of Marine-Biocement Using SEM-EDX
SEM allows a direct and closer look at the CaCO3 bonds developed at the interparticle soil particles and the EDX analyzer records the counts of representative elements from the elements’ spectrum for provides insights the MICP mechanism with coastal sand with local marine-bacteria [23]. The hardest marine-biocement with isolated strain S1-1 surface characteristics was shown in Figure 3. Figure 3(a) shows a SEM image of specimen of marine-biocement S1-1, and b, c, e and f show calcium and silica elemental mapping results by EDX analysis. EDX analysis was used to observe the elements included in marine-biocement. Figure 3(d) shows precipitated short rod like crystals on surroundings of the sand particle surfaces. The average of crystalline size was 10-20 μm in length and 5 mm in diameter, and this rod like shape agree with aragonite crystals [24]. The crystal phase (Figure 3(a)) and calcium phase (b) were overlapped in these SEM-EDX images, but silica phase was detected at the other region in Figure 3. Because of calcium from bacterial CaCO3 and sand main silica compounded chemicals such as SiO2 and Al2O3 for about 70% of the total in Japanese coastal sand. Furthermore, this is the evidence of CaCO3 crystal layer could be bind another sand particle on the same frame of the sand particle surface. SEM image of bacterial free specimen was shown in Figure 3(g) and 3(h).

Figure 3: SEM images of marine-biocement with strain S1-1. CaCO3 crystals on the surface of sand particle (a) and Enlargement of crystal region (d). Chemical composition analysis on the sand surface by Energy Dispersive X-ray (EDX) system (b, c, e) and (f). Blank as Marine-biocement surface without urease producing bacteria surface SEM image (a). And chemical composition by EDX analysis of blank.
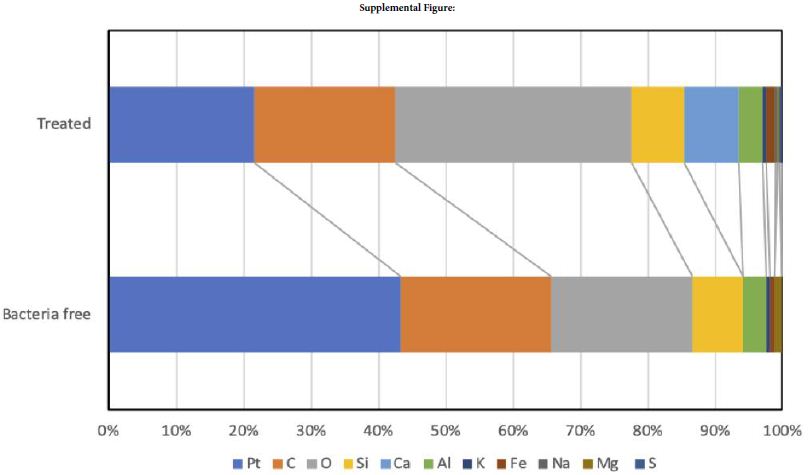
There were observed flat phase on all surfaces and never confirmed rod like crystals as aragonite. Quantitative EDX results were provided to supplemental Figure 1 and shows that the composition ratio of marine-biocement that is mainly contained carbon (48%), oxygen (34%), silicon (7%) and calcium (4.3%) in bacterial one. In contrast that, no calcium detected in bacterial free specimen. Therefore, isolated urease producing bacteria begin to the MICP process in marine-biocement same as the other applied MICP materials (Supplemental Figure).
Zostera marina Germination Test with Marine-Biocement for the Blue Carbon Systems
Figure 4(a) shows the germinated two white coleoptiles from marine-biocement after 23 days after seed sowing. Green leaves started photosynthetic for some bubbles come from leaves surface in 30 days. After 2 months, green leaves growing up to 4.7 cm length and leaf vein formation was confirmed. This final germination rate was 26.7% of marine-biocement S1-1 and 23.3% from sea-sand. The maximum leaf length from the marine-biocement and sea-sand were 4.7 and 4.9 cm, respectively. The lack of difference in germination results between marine-biocement and sea sand conditions indicate that marine-biocement can be used to germinate Zostera marina. From these results, it means that this marine-biocement can be applied to the marine environment and ecosystem protection.
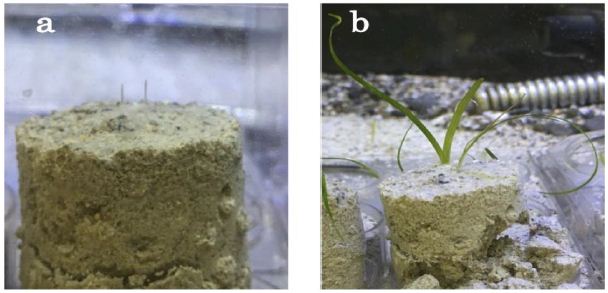
Figure 4: Germination image of Z. marina from seeds. (a)Two white coleoptiles from marine-biocement after 23 days after the germination test (b) 2 months after the test, they were grown green leaves up to 4.7 cm. The formation of parallel veins was confirmed.
Acknowledgment
This paper is a summary of work that under the Marine Challenge Program 2017 and Marine Tech grand prix 2018 andsupported by JSPS KAKENHI (JP18K05695), “Innovation inspired by Nature” Research Support Program from Sekisui chemical Co., Ltd, 20th ESPEC for Global Environment Research and Technology and Mitsumasa Ito Memorial Research Grant. We would like to thank Mitsui Chemicals, Inc. for providing the technology for the mass cultivation of marine bacteria.
Data Availability
The data underlying this article are available in the GenBank Nucleotide Database at https://www.ncbi.nlm.nih.gov/nucleotide/ and can be accessed with following accession number as LC760339-760343.
Author’s Contribution
Yuki Nakashima, Momoka Miyasaka and Masataka Kusube were involved in study design and data interpretation. Koki Kusumoto, Keizo Kashihara, Kazuyuki Hayashi and Shinya Maki were involved in the data analysis. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
Funding Statement
This work was supported by the Marine Challenge Program 2017 Leave a Nest Co., Ltd, JSPS KAKENHI under Grant JP18K05695 and 20th ESPEC for Global Environment Research and Technology (Charitable Trust) and Mitsumasa Ito Memorial Research Grant.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- Iqbal M, Nishimura M, Haider M, Masayoshi S, Minoru I, et al. (2021) Diversity and Composition of Microbial Communities in an Eelgrass (Zostera marina) Bed in Tokyo Bay, Japan. Microbes Environ 36. [crossref]
- Nakaoka M, Aioi K (2001) Ecology of seagrasses Zostera spp. (Zosteraceae) in Japanese waters: A review. Otsuchi Marine Science 26: 7-22.
- Watanabe K, Kuwae T (2015) Radiocarbon isotopic evidence for assimilation of atmospheric CO2 by the seagrass Zostera marina. Biogeosciences 12: 6251-6258.
- Poppe K, Rybczyk J (2018) Carbon Sequestration in a Pacific Northwest Eelgrass (Zostera marina) Meadow. Northwest Sci 92: 80-91.
- Dahl M, Asplund M, Bjork M, Diana D, Eduardo I, Martin I, et al. (2020) The influence of hydrodynamic exposure on carbon storage and nutrient retention in eelgrass (Zostera marina ) meadows on the Swedish Skagerrak coast. Sci Rep 10.
- Aoki M, Noma T, Yonemitsu H, Nobuo A, Takashi Y, et al. (2018) A Low-Tech Bioreactor System for the Enrichment and Production of Ureolytic Microbes. Pol J Microbiol 6: 59-65. [crossref]
- Yasuhara H, Neupane D, Hayashi K, Mitsu O (2012) Experiments and predictions of physical properties of sand cemented by enzymatically-induced carbonate precipitation. Soils Found 52: 539-549.
- Mujah D, Shahin M, Cheng L (2016) State-of-the-Art Review of Biocementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiol J 34: 524-537.
- Muynck W, Verbeken K, Belie N, Willy V (2010) Influence of urea and calcium dosage on the effectiveness of bacterially induced carbonate precipitation on limestone. Ecol Eng 36: 99-111.
- Kusube M, Kyaw T, Tanikawa K, Roger AC, Kevin MH, et al. (2017) Colwellia marinimaniae nov., a hyperpiezophilic species isolated from an amphipod within the Challenger Deep, Mariana Trench. Int J Syst Evol Microbiol 67: 824-831. [crossref]
- Thawadi S (2011) Ureolytic Bacteria and Calcium Carbonate Formation as a Mechanism of Strength Enhancement of Sand. J Adv Res 1: 98-114.
- Santos O, Pontes P, Santos J, Guilherme M, Marcia GD, et al. (2010) Isolation, characterization and phylogeny of sponge-associated bacteria with antimicrobial activities from Brazil. Res Microbiol 161: 604-612. [crossref]
- Dilrukshi R, Watanabe J, Kawasaki S (2016) Strengthening of sand cemented with calcium phosphate compounds using plant-derived urease. Int J GEOMATE 11: 2461-2467.
- Acunna S, Jimenez M, Zambrano J, Hugo ARS (2018) Soil bacteria that precipitate calcium carbonate: mechanism and applications of the process, Biological. Chem Phys Soil Function 67: 277-288.
- Omoregie A, Siah J, Pei B, Stephenie PJY , Luke SW, et al. (2018) Integrating Biotechnology into Geotechnical Engineering: A Laboratory Exercise. Trans Sci Technol 5: 76-87.
- Fonseca A, Cruz R, Consoli N (2009) Strength Properties of Sandy Soil-Cement Admixtures. Geotech Geol Eng 27: 681-686.
- Fukue M, Ono S, Sato Y (2011) Cementation of sands due to microbiologically-induced carbonate precipitation. Soils Found 51: 83-93.
- Morita T, Miyamatsu A, Fujii M, Hideki K, Mahiko A, et al. (2011) Germination in Zostera japonica is determined by cold stratification, tidal elevation and sediment type. Aquat Bot 95: 234-241.
- Coenye T, Goris J, Vos P, Peter V, John JL, et al. (2003) Classification of Ralstonia pickettii-like isolates from the environment and clinical samples as Ralstonia insidiosa nov. Int J Syst Evol Microbiol 53: 1075-1080.
- Goris J, Vos P, Coenye T, Hoste B, Janssens D, et al. (2001) Classification of metal-resistant bacteria from industrial biotopes as Ralstonia campinensis nov., Ralstonia metallidurans sp. nov. and Ralstonia basilensis Steinle et al. 1998 emend. Int J Syst Evol Microbiol 51: 1773-1782. [crossref]
- Dhami KN, Reddy MS, Mukherjee A (2013) Biomineralization of Calcium Carbonate Polymorphs by the Bacterial Strains Isolated from Calcareous Sites. J Microbiol Biotechnol 23: 707-714. [crossref]
- Hammes F, Verstraete W (2002) Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev Environ Sci Biotechnol 1: 3-7.
- Achal V, Mukherjee A, Basu P, Sudhakara MR, et al. (2009) Strain improvement of Sporosarcina pasteurii for enhanced urease and calcite production. J Ind Microbiol Biotechnol 36: 981-988. [crossref]
- Hongxia G, Zhenping Q, Peng Q, Yu Peng, Cui Suping, et al. (2011) Crystallization of aragonite CaCO3 with complex structures. Adv Powder Technol 22: 777-783.