Abstract
This short paper shows the excessive enrichment of Cs in a volatile-rich evolved pegmatitic silicate melt. This water-rich melt (plus B and F) with about 30% bulk water was, with high probability, created by an input of a supercritical fluid from the earth’s mantle. The water content of a granitic melt is at ~900°C, and crustal pressures (<5 kbar) are too low to create such a volatile-rich evolved melt. Furthermore, the quartz contains nanodiamonds and graphite. These are strong hints to the participation of supercritical fluids.
Keywords
H2O-B2O3-F-rich melt, Cs-rich melt inclusions, Multistage liquid-liquid immiscibility, Supercritical fluids
Introduction
During the study of melt inclusions in quartz of a pegmatite body related to the Variscan Ehrenfriedersdorf tin deposit in the Central Erzgebirge/Germany, we often found high concentrations of Cs in different inclusion types: water-poor melt inclusions rich in Cs, water-rich melt inclusions with a moderate Cs content, and extreme Cs-pentaborate rich inclusions trapped near the crest of the main solvus. The primary solvus curve (B vs. H2O) results from the simultaneous enrichment of water, boron, and fluorine and forms a pseudo-binary solvus (H2O + B2O3 + F + Silicate melt) versus temperature (Figure 1a and 1b).
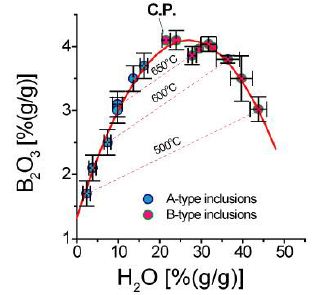
Figure 1a: Boron versus water concentration in conjugate type-A (blue) and type-B melt inclusions (red) in the Ehrenfriedersdorf pegmatite quartz. Both compounds portray a solvus curve (melt-water). Included are the corresponding isotherms (500, 600, and 650°C) – see Thomas et al. (2003) [1].
From these curves (Figures 1a and 1b), we see that at the critical point (solvus crest), a high concentration of H2O, B2O3, and F are present: about 27.5, 4.2, and 9.0%, respectively. The data are from published and unpublished data from Veksler and Thomas (2002) [1-4].
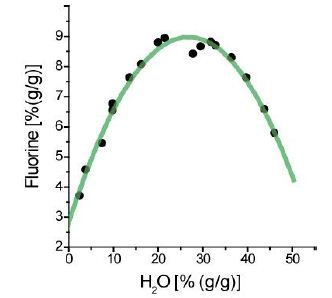
Figure 1b: Fluorine versus water (H2O) concentration obtained from melt inclusions from the same Ehrenfriedersdorf pegmatite quartz.
From unpublished hydrothermal diamond anvil cell (HDAC) experiments performed in 2002 together with Ilya Veksler and Christian Schmidt, we know that using synthetic pegmatite melts similar to the Ehrenfriedersdorf pegmatite with about 50 [% (vol/vol)] water in the temperature range from 840 down to 300°C multistage liquid-liquid immiscibility processes happens. Each main phase ever formed tends to liquid immiscibility. Such compartments are very contrasting and show extreme enrichment of rare elements like boron, fluorine, cesium, beryl, tin, and others (e.g., Thomas et al. 2019 and 2022) [5,6]. This specific experiment, unfortunately, ended short before the total homogenization at about 900°C by the crash of a diamond of the HDAC. This experiment shows, however, clearly that the formation of such water-rich melt is only possible at very high temperatures, and consistently, with cooling, multi-phase separation happens steadily down to low temperatures around 160°C (Figures 2 and 3). If we look at Figure D at Schröcke’s contribution (1954) [7], it follows that primary forming this pegmatite type is impossible in situ. We need high water and energy to develop a mass of pegmatite bodies shown there (see also Johannes and Holtz, 1996) [8]. In a couple of publications, Thomas [9-14] and Thomas et al. [15,16] have shown that by the finding of typical mantle minerals in granites and pegmatites, the supplier of the necessary water (energy and other components) can be supercritical fluids coming directly from the mantle region. From the unfinished HDAC experiment (see above), we have learned that during heating and cooling, many phase changes happen, primarily by liquid-liquid immiscibility (see exemplary Figures 2 and 3).
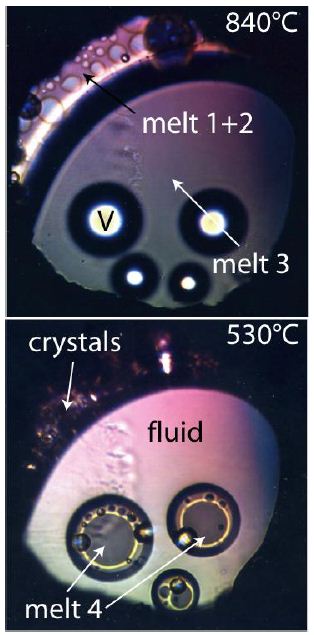
Figure 2: Look through the microscope at the sample chamber formed by an Ir-gasket with a hole of 300 µm between two diamonds of the HDAC at two different temperatures. Conspicuous are the different phases formed by liquid-liquid immiscibility.
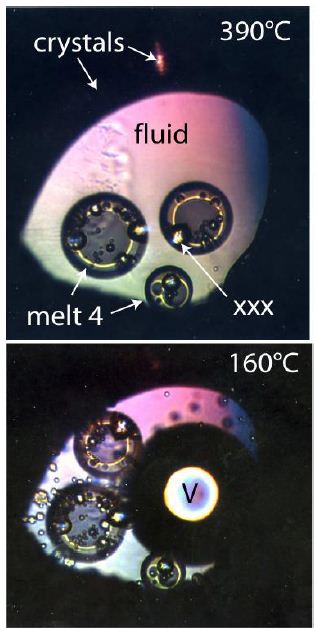
Figure 3: The same HDAC experiment at lower temperatures (390 and 160°C). V: Vapor, XXX: late-formed crystal. The “crystals” marked area stands for the melt 1+2, now wholly solidified.
Sample Material
The sample material (Qu8) comes from a miarolitic pegmatite body in the Sauberg tin mine near Ehrenfriederesdorf, Germany. A description of the locality is in Webster et al., 1997 [4]. The quartz sample was as big as your fist. Many 500 µm thick on both sides polished slices are produced from this sample. The vapor phase of some melt inclusions in this quartz contains high hydrogen, methane, and CO2 concentrations: XH2=0.58, XCH4=0.26, XCO2=0.16 (Thomas and Webster, 2000) [17]. In a quartz crystal (Qu8-45) from the same pegmatite, here not studied, we have found large aggregates of graphite and nanodiamond.
Methodology
The general used methods are described in Webster et al. 1997 [4] and Thomas [13] and references in there). For the microprobe study (main and trace elements), we mainly used the CAMECA SX50 microprobe. We used Raman spectroscopy (see Thomas 2023e and references) [13] to determine the water as the basis component for constructing the pseudo-binary solvus curves (see also Thomas and Davidson, 2016) [18].
Results
This contribution is only restricted to cesium (Cs) results. Cesium, with a Clarke value of about 5.0 ppm in granitic rocks (Rösler and Lange, 1975) [19], is enriched to extremely high values of about 160000 ppm. That corresponds to an enrichment of 32000 fold, an incredible value. Further, we see relationships between Cs and H2O, Cs and B2O3, and B2O3 with H2O. Figure 4a demonstrates the enrichment of Cs (as Cs2O) with water. The Cs shows here a good Lorentzian distribution. The data come from typical melt inclusion in the pegmatite quartz from the Sauberg mine. The same distribution type results for Cs and B in Figure 4b. This figure shows strong enrichment of Cs at a more or less constant B2O3 concentration of about 2.3% B2O3. According to He et al. (2020) [20], B2O3 reduces pollucite’s [(Cs, Na)(AlSi2)O6 • nH2O] crystallization temperature (maybe under 700-600°C) and improves immobilization through an encapsulation effect here by Al-silicates. The melt inclusions are relatively water-poor (~5%) and represent the heavy residue. Figure 4c shows the simplified solvus curve for the system melt-H2O versus B2O3 (Figure 1a). Figure 4d displays the extreme Cs enrichment near the solvus crest of the Melt-H2O – B2O3 system as Cs-pentaborate (Ramanite-(Cs). At first, this Cs-pentaborate was found in pegmatite material from the Isle of Elba (see Thomas et al., 2008) [21] – later also in Malkhan (Thomas et al., 2012) [22] and Ehrenfriedersdorf (Sauberg mine). The Lorentzian distribution of Cs vs. B is untypical, and Cs vs. H2O is typical for some elements (Be, Sn, and others [5,6]. Table 1 gives the fit data for the three Cs distributions.
Table 1: Lorentzian fit data
|
Distribution |
Area |
Center (%) |
Width (%) |
Offset (%) |
Height (%) |
R2 |
| Cs2O-B2O3 |
5.226 |
2.264 |
0.553 |
1.621 |
6.012 |
0.90983 |
| Cs2O-H2O |
26.592 |
25.991 |
5.631 |
0.134 |
3.006 |
0.96752 |
| Ramanite-(Cs) |
13.309 |
29.992 |
0.900 |
5.676 |
9.419 |
0.99436 |
In comparison to Figure 4a, the distribution of Cs2O vs. H2O shown in Figure 4d is a little bit shifted to higher water concentrations (maybe the real critical point of the system) or by the crystal water in the formula of Ramanite-(Cs) [CsB5O8 • 4H2O] – see Thomas et al. 2008) [21].
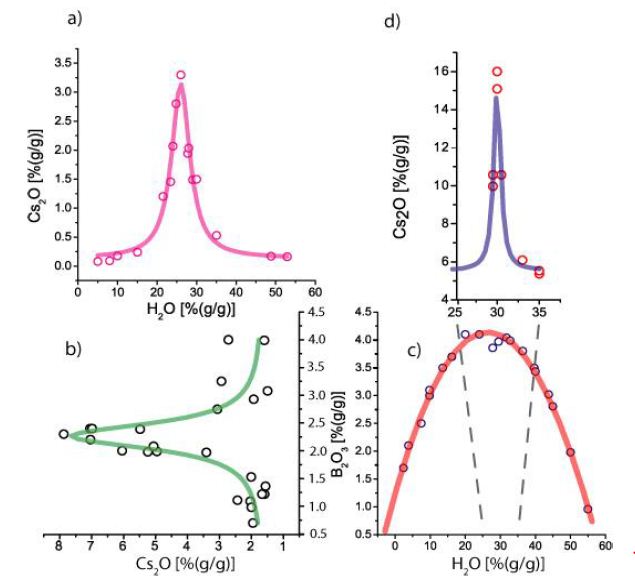
Figure 4: (a) Distribution of Cs with H2O, (b) Cs with B, (c) H2O and B, (d) Cs as Ramanit-(Cs) with H2O. The microprobe data for (b) are from Thomas et al. 2019 [5].
Discussion
The here-shown enrichment of Cs during the crystallization of a pegmatite-forming melt related to the Variscan tin deposit Ehrenfriedersdorf is unexpectedly high. The origin of such distributions is, at the moment, not clear. More research is indispensable. Are such Lorentzian-type distributions of elements with water a characteristic feature of the participation of supercritical fluids at the tin mineralization here? Some elements (Be) are Gaussian distributed (see Thomas 2023d) [12]. What are the reasons for the different distribution types? Which physicochemical processes are the determining steps? Figure 4 shows that the behavior of Cs in water-rich high-temperature melt-water systems is very complicated. Therefore, is storing the radioactive 137Cs as boron-stabilized pollucite doubtful (see Yokomori et al., 2014) [23,24]. Because each element in the supercritical state can enriched to high values, we assume, in principle, that universal cooperative interactions between the particles under these conditions will work. The opposite is, up to now, not proved.
Acknowledgment
The first author thanked I-Ming Chou and William (Bill) A. Bassett for their professional introduction to the HDAC technique during my visit to the Argonne National Laboratory in the summer of 2001. Iliya Veksler and Christion Schmidt are thanked for the joint HDAC experiments on synthetic pegmatite melts.
References
- Thomas R, Förster HJ, Heinrich W (2003) Behaviour of Boron in a peraluminous granite-pegmatite system and associated hydrothermal solutions; a melt and fluid-inclusion study. Contribution to Mineralogy and Petrology 144: 457-472.
- Veksler IV, Thomas R (2002) An experimental study of B-, P- and F.rich synthetic granite pegmatite at 0.1 and 0.2 GPa. Contribution to Mineralogy and Petrology 143: 673-683.
- Thomas R, Schmidt C, Veksler I, Davidson P, Beurlen H (2006) The formation of peralkaline pegmatitic melt fractions: evidence from melt and fluid inclusion studies. Extended abstract. XLIII. Congresso Brasileiro de Geologia, 2006, published 2008, 757-761.
- Webster JD, Thomas R, Rhede D, Förster HJ, Seltmann R (1997) Melt inclusion in quartz from an evolved peraluminous pegmatite: Geochemical evidence for strong tin enrichment in fluorine-rich and phosphorus-rich residual liquids. Geochimica et Cosmochimica Acta 61: 2589-2604.
- Thomas R, Davidson P, Appel K (2019) The enhanced element enrichment in the supercritical states of granite-pegmatite systems. Acta Geochim 38: 335-349.
- Thomas R, Davidson P, Rericha A, Voznyak DK (2022) Water-rich melt inclusions as “frozen” samples of the supercritical state in granites and pegmatites reveal extreme element enrichment resulting under non-equilibrium conditions. Mineralogical Journal (Ukraine) 44: 3-15.
- Schröcke H (1954) Zur Paragenese erzgebirgischer Zinnlagerstätten. Neues Jahrbuch Mineral. 87: 33-109.
- Johannes W, Holtz F (1996) Petrogenesis and experimental petrology of granitic rocks. Springer.
- Thomas R (2023a) Unusual cassiterite mineralization, related to the Variscan tin-mineralization of the Ehrenfriedersdorf deposit, Germany. Aspects in Mining & Mineral Sciences 11: 1233-1236.
- Thomas R (2023b) Ultrahigh-pressure and temperature mineral inclusions in more crustal mineralizations: The role of supercritical fluids. Geology, Earth and Marine Sciences 5: 1-2.
- Thomas R (2023c) A new fluid inclusion type in hydrothermal-grown beryl. Geology, Earth and Marine Sciences 5: 1-3.
- Thomas R (2023d) To the geochemistry of beryllium: The other side of the coin. Geology, Earth and Marine Sciences 5: 1-4.
- Thomas R (2023e) Raman spectroscopic determination of water in glasses and melt inclusions: 25 years after the beginning. Geology, Earth and Marine Sciences 5: 1-2.
- Thomas R (2023f) The Königshain granite: Diamond inclusions in Zircon. Geology, Earth and Marine Sciences 5: 1-4.
- Thomas R, Davidson P, Rericha A, Recknagel U (2023a) Ultrahigh-pressure mineral inclusions in a crustal granite: Evidence for a novel transcrustal transport mechanism. Geosciences 13: 94: 1-13.
- Thomas R, Recknagel U, Rericha A (2023b) A moissanite-diamond-graphite paragenesis in a small beryl-quartz vein related to the Variscan tin-mineralization of the Ehrenfriedersdirf deposit, Germany. Aspects in Mining & Mineral Sciences 11: 1310-1319.
- Thomas R, Webster JD (2000) Strong tin enrichment in a pegmatite-forming melt. Mineralium Deposita 35: 570-582.
- Thomas R, Davidson P (2016) Revisiting complete miscibility between silicate melts and hydrous fluids, and the extreme enrichment of some elements in the supercritical state – Consequences for the formation of pegmatites and ore deposits. Ore Geology Reviews 72: 1088-1101.
- Rösler HJ, Lange H (1975) Geochemische Tabellen. VEB Deutscher Verlag für Grundstoffundustrie Leipzig. 675 p.
- He P, Fu S, Wang M, Duan X, Wang O, et al. (2020) B2O3-assisted low-temperature crystallization of pollucite structures and their potential applications in Cs+ immobilization. Journal of Nuclear Materials 540.
- Thomas R, Davidson P, Hahn A (2008) Ramanite-(Cs) and ramanite-(Rb): New cesium and rubidium pentaborate tetrahydrate minerals identified with Raman spectroscopy. American Mineralogist 93: 1034-1042.
- Thomas R, Davidson P, Badanina E (2012) Water- and boron-rich melt inclusions in quartz from the Malkhan pegmatite, Transbaikalia, Russia. Minerals 2: 435-458.
- Thomas R, Davidson P (2007) The formation of granitic pegmatites from the viewpoint of melt and fluid inclusions and new experimental work. Granitic Pegmatites: the state of the art – International Symposium, Porto, Portugal, 13-16.
- Yokomori Y, Asazuki K, Kamiya N, Yano Y, Akamatsu K, et al. (2014) Final storage of radioactive cesium by pollucite hydrothermal synthesis, Scientific Reports 4, 4195: 1-4.