Abstract
The Isua Banded Iron Formation (BIF) formed just after Late Heavy Bombardment (3.8 Ga) and the first life produced through abiotic means appeared in a solution of essential elements on the early Earth. The volcanic layer in the Isua BIF contained no carbon, and the carbon present in the sedimentary layer contained more 12C than 13C. Iron-bearing lava was emitted intermittently into the primitive sea through volcanic eruptions. Bubbles were produced then Fe mixed with carbonated water, which may form through multiple chemical pathways. Hydrocarbon (CnH2n+2) was simultaneously produced via collisions between H+ from solar wind and early atmospheric CO2. Since long-chained molecule of CnH2n+2 is hydrophobic, they would have floated with membrane of Fe(HCO3)2 on the surface of the seawater. The carbonated water was neutralized by dissolved ions of Fe+2 and a floating solution of Fe(HCO3)2 developed in the presence of the BIF. Thus, the floating materials were gathered on the surface of the water. The intermolecular bonds in there floating materials exchanged neighboring atoms as the structure deformed, such that 12C was preferentially uptaken from carbon derived from the dissolution of CO2 molecules into the primitive sea at ~3.8 Ga. Given that Fe acts as a deoxidation agent, primitive photosynthesis was achieved in the CnH2n+2 and Fe(HCO3)2 via the chemical reaction of Fe(HCO3)2 in which the hydrophobic CnH2n+2 molecule was used as a scaffold for replication. Thus, the first life on Earth arose from abiotic processes due to metabolic intermolecular interactions.
Keywords
Isua Banded Iron Formation, Carbonated seawater, Origin of life, Iron(Ⅱ) hydrogen carbon, Metabolic intermolecular interaction, Primitive photosynthesis, Cyanobacteria
Introduction
In the 1970s, the world’s oldest stratum was explored via radiation dating. Although the oldest rock found on Earth to date is from the Acasta Gneiss Complex of northwest Canada [1], the oldest intact rock from the Isua sediment was of interest to many researchers as it showed signature of life. The Isua Banded Iron Formation (BIF) is classified as Algoma type that was formed 3.7~3.8 billion years ago, while BIF of Superior type first appeared around 3 billion years ago. A fundamental question remains about the oldest BIF that iron in BIF was oxidated by the oxygen that was absent throughout the early sea [2]. However, those previous studies focused on the ratio of 12C to 13C to verify the possibility that striped iron deposits were generated via photosynthesis by early organisms. Even though little is known about the earliest cyanobacteria, existing studies provide evidence that, unless some unknown abiotic process exists, which is able both to create such isotopically 12C rich and then selectively incorporate it into the grains, life on Earth emerged at least 3,8 billion years ago [3-5]. However, the isotope variations in nature cannot be uniquely ascribed to biology until nonbiological isotope effects are better understood [6]. A bubble has adaptability for external variation due to metabolic intermolecular interactions. Since the first life was produced abiotically, the definition of first life should be different from that used for today’s living creature. The strata in the Isua BIF included layers of lava and sediment layers that had piled up between the multiple lava layers [3]. Lava released from submarine volcanoes produced floating materials and dissolved Fe2+. The floating materials gather on the surface of water, and the energy from Sun’s irradiation triggers inorganic chemical reactions which produce iron dioxide from the floating materials. These materials ultimately form the Algoma-type striped iron deposit. Karasawa reported that mixing of iron powder with carbonated water creates bubbles and produces complex floating materials [7]. Metabolic intermolecular interactions within such bubbles gave rise to the earliest life. Thus, the environment in the early sea provided the conditions for abiotic process of the formation of the earliest BIF and that first life.
Material Formation Process Revealed by the Environment
Origin of Water on Earth
It is generally accepted that the Earth was formed by meteorite collisions. However, meteorites will be broken in pieces by a collision that cannot bind into one solid substance without external forces. Moreover, meteorites are produced by large celestial bodies. The Sun accounts for 99.87% of the total mass of the solar system, and the planets and other celestial objects accounts for the remaining 0.13%. The Sun as the center of gravity holds most of materials in the solar system except for the material orbiting the Sun. According to Kepler’s third law, the relationship between the orbit and the period of a celestial body orbiting the Sun does not depend on the mass of the orbiting material, i.e., the orbiting speed of every celestial material in the orbit with the same radius of orbit is the same. Thus, materials on the orbit stays around the Sun for a long period. Although the gravitational force of cosmic dust is very weak, their van der Waals force have a glue-like effect that hold together all the material in the celestial body. The celestial bodies aggregated by adsorbing fine cosmic dust particles. Although the traditional formation theory of the solar system explains that the formation of planets occurred after the nuclear fusion of the Sun [8], it is challenging for a small celestial body to grow under the environment of the solar wind. The Earth formed due to accumulation of celestial materials containing with ice (H2O) and dry ice (CO2). For example, about 80% of the comet’s core contains ice (H2O), and the remaining 20% contains dry ice (CO2) and dust like SiO2 grains. The surface of the Earth did not feature a high temperature during the growing period in the cold accretion phase and it is considered that the initial stage of planet is like to that of the Sun. Measurements using radioactive isotopes have shown that most of the meteorites were formed by 4.6~4.5 billion years ago. The phenomenon that able to emit meteorites cannot be considered except nuclear fusion. There are a lot of meteorites in asteroid belt. There is a possibility that asteroid belt had formed at the period of Late Heavy Bombardment (3.8 Ga) by a nuclear fusion of once existed a planet. (cf. https://www.youtube.com/watch?v=QY8C7XK6k7I). A considered that a part of the material collected in the Sun was released as the form of meteorites when nuclear fusion began in the Sun. The emitted material was collected again in the Sun and the process was repeated. Some of the materials released from the Sun reached to Earth’s gravitational sphere and fell to the Earth’s ground. Those materials increase the mass of the primitive Earth. The energy released during the collision transformed to thermal energy, which heated up the surface of the Earth. Due to the increase of temperature of the ground, a magma ocean formed on surface of the Earth at the end stage of the growing period. Thus, H2O and CO2 were degassed from inside of the Earth at the end stage of the growing. As the meteorite impacts subsided, the Earth’s surface gradually cooled down. Consequently, the degassed CO2 and H2O formed the primitive atmosphere. When the surface of the Earth cooled, H2O present in the form of water vapor in the atmosphere condensed and the rain fall on the surface of the Earth; thus, the early sea was formed.
Formation of Heat Source at the Center of the Earth
When the primitive Earth was growing by accumulation of powder-like materials, the planet was rather homogeneous. The pressure on the central area of celestial body increased with its size. Energy state is lowered by condensed state due to the high pressure. The change of state releases energy to surroundings. Additionally, radioactive elements emit heat radiation contributing to the temperature increase. As increasing the temperature of inner Earth, heavy metal such as iron were accumulated at the center of the Earth, and oxides such as SiO2 formed the outer layer of the Earth. The energy due to the gravity can be considered as the main factor driving the rise of temperatures at the Earth’s core, to level of 3,500 to 5,000°C. Half of the gravitational potential is converted into kinetic energy according to the Virial theorem (it is utilized as the equilibrium condition in the field of mechanics, statistical mechanics, astronomy, and atomic physics). Gravitational energy (W) stored in spherical uniformly dense objects with universal gravitational constants G, mass M, and radius R can be calculated using Eq.(1) [9] and the value obtained for Earth is shown using Eq.(2).
W= (3/5) (GM2/R) (1)
(1/2) W=2.68 x 1031 [cal] = Δ4,500°C (cp =1, for Earth) (2)
Assuming the specific heat of the Earth is Cp =1, the amount of heat is evaluated as increase of temperature Δ4,500℃. The heat source contributed to formation of the Isua BIF in carbonated sea water.
Characteristics of Sea Water under Atmosphere of CO2
When the surface of the Earth featured an ocean of magma heat convection became active at the surface layer of the Earth. Thus, there were mantle convections of upper layer and lower layers. Even though the heat released during meteorite collisions was large, temperature on the surface of Earth decreased as time progressed. However, mantle viscosity increases with a decrease in temperature, and consequently, the convection velocity slowed down. Thus, a layered structure comprising thin crust, thick silicate mantle, and iron core developed through prolonged convection. As discussed previously, water vapor fell to the surface of the Earth as rain when atmosphere cools down, However, when the surface of the Earth was still hot, the liquid water evaporate into the atmosphere. This repeated process and the thermal convections of water vapor contributed to the cooling down of the surface of the Earth. Gradually, an ocean was formed by the liquid water on the surface of the Earth, which comprised a SiO2 crust. However, the CO2 remained in the atmosphere until the temperature of the seawater cooled down to approximately 300℃; this is because CO2 does not dissolve in seawater at high temperatures. The CO2 in the sea water does not react with SiO2 on the seabed. The amount of the hydrogen ions in natural water can be determined by the reaction shown in Equation (3). Most of dissolved CO2 in the water stays as a molecule, while H+(aq) is produced as per the reactions outline in Equation (3) and (4).
CO2(aq) + H2O ⇔ H2CO3(aq) ; pKCO2 = 10-1.47 (3)
H2CO3(aq) ⇔ HCO3–(aq)+ H+(aq) ; pKa1 = 10-6.35 (4)
HCO3–(aq) ⇔ CO32-(aq) + H+(aq) ; pKa2 = 10-10.33 (5)
The equilibrium constants for the carbonate system are for fresh water at 25oC [10] p.221.
Floating Materials Formed by Intermolecular Bond of Fe(HCO3)2
Lava, released due to volcanic activity, supplied iron ions that neutralized carbonated water and produced HCO3- ions. The pH of water exposed to 100% CO2 atmosphere, soon decreases as pH< 7 [7]. However, when finely powdered iron is introduced to this carbonated water, the pH value slowly increases as pH>7. The speed with the pH changes depends on the surface state of iron powder [7] (Figure 1).
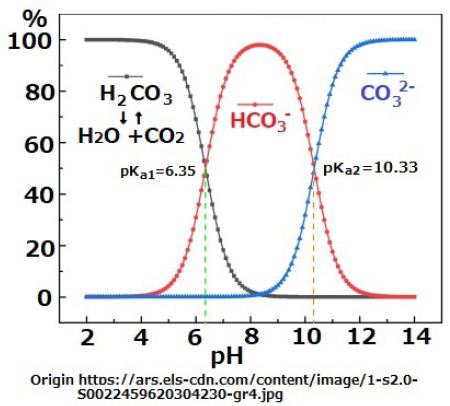
Figure 1: Variation of density of carbonate species as a function of pH
The proposed model suggests that the Isua BIF was formed due to the accumulation of materials released during volcanic eruptions that broke through silicate crust at the seabed. The iron ions released during the volcanic eruption mixed in carbonated water, which neutralized the water and released HCO3– ions.
As shown in Figure 2, the arrangement of Fe2+ surrounded by hydrogen-bonded HCO3– is the same as that of 2-dimensional planes of [Fe(OH)2+2CO2]. Since a flexible plane of Fe(HCO3)2 is yielded by possibility of plural electronic states on the same atomic arrangement, it forms robust membranes and bubble [7] (Figure 2).
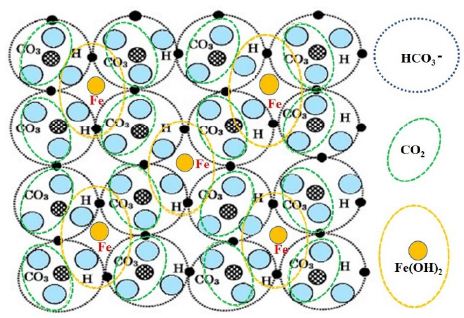
Figure 2: Floating plane formed by resonating structure of intermolecular bonds of Fe(HCO3)2 = Fe(OH)2+2CO2 [6]
Figure 2 shows resonating structure of intermolecular bonds of Fe(HCO3)2=Fe(OH)2+2CO2. The resonating structure of intermolecular bonds of Fe(HCO3)2 leads to a plane structure of floating materials. Those floating materials gather on the surface of the water. The structure also forms the membrane of a bubble. The floating materials are gradually deposited on the seabed as chemical compounds through inorganic process including irradiated by ultraviolet rays of the Sun.
Observations
Bubbles formed in Carbonated Water by Mixing of Iron Powder
Mixing of powdered iron in carbonated water forms bubbles and complex floating materials as shown in Figure 3.

Figure 3: Bubbled and floating materials generated after a mixture of powdered iron and carbonated water left undisturbed for several hours
The inorganic bubble responds to external changes due to internal metabolic intermolecular interactions. The bubble is always renewing and have adaptability for external changes. Even if a part of the bubble is cracked, it is repaired immediately through the formation process. (A video on inorganic bubble responds to external changes by a metabolism:
http://www.youtube.com/watch?v=7mLPULp-il8)
Deoxidation of Carbon Dioxide by Oxidation of Iron Atoms
The floating materials were oxidized taking many years of long time after mixing of powdered iron in carbonated water as shown in Figure 4.

Figure 4: Floating materials were oxidized at a) floating state, and b) precipitate state
The Pauling electronegativity values of Fe, H, and C are 1.80, 2.30, and 2.54, respectively; thus, it is considered that the carbon particles in sediment of Isua BIF were generated through deoxidation of CO2 as shown in Eq. (6).
4Fe(HCO3)2→2Fe2O3 + 4H2O + C +7CO2 (6)
The oxidation of ultrafine iron powder exposed to solid state of carbon dioxide has been demonstrated previously (A Video on Oxidation of ultra-fine iron particles by solid state of carbon dioxide, https://www.youtube.com/watch?v=eyq3qbxFahw).
Electronic Configurations of Fe2+ and Fe3+
The electronic configurations of Fe2+ and Fe3+ are shown in Figure 5. When one electron of the 3d orbit in Fe2+ is emitted, the 3d orbit becomes a semi-closed shell and stabilizes. Although Fe3+ exists as a relatively stable ion, oxidation of Fe2+ to Fe3+ takes a long time. The flexibility of Fe3+ compound on structure decreases compared with that on Fe2+ compounds.
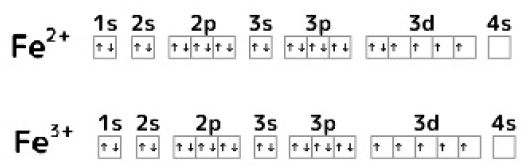
Figure 5: Electronic configuration of Fe2+ and Fe3+
Early Photosynthesis via Metabolic Intermolecular Interactions in Floating Material
The ultrafine powder of iron acts as a reducing agent for CO2. Hence, it is considered that the earliest instance of photosynthesis will be carried out via oxidation of iron atom, as described in Equation (7),
As for anoxygenic photosynthesis at acidity of pH<6.3
3mCO2 + 3nH2O +4mFe → 3Cm (H2O)n +2mFe2O3↓ (7)
In order to keep floating materials, there is a possible reaction as described Equation (8) occurred in the metabolic intermolecular interactions of Fe(HCO3)2 membrane with the hydrophobic CnH2n+2 molecule.
As for photosynthesis with producing oxygen in 6.3<pH<10.3,
Cn-1H2n + 4Fe(HCO3)2 → 2Fe2O3↓+ CnH2n+2 + 3H2O +7CO2 [O] (8)
Although the fossil evidence of photosynthesis can be tracked back to 3.5 billion years ago [11], It is estimated that the earliest life was produced soon after the development of oldest BIF.
Non-biological Origin of Organic Materials on Primitive Earth
Volcanic Origin of Carbon on Primitive Earth
The carbon particles of the Isua BIF are present as size of 2~5μm in thick layers that are sandwiched between thin layers formed by submarine volcanoes, and the biological origin carbon, of which 13C is about 2% less than non-biological origin, is included in these carbon particles. These carbon particles are included the max of 0.5% the clay layers, each of clay layers is several centimeters, the clay layers are sandwiched between thin volcanic layers of approximately several millimeters. The volcanic layers do not contain carbon [12] pp.123. In case of the pH value around volcanic rocks is much higher than 7, it I considered as FeCO3 precipitate without free carbons. Thus, the thin layers of iron oxide can be attributed to volcanic explosions, and it can be considered that carbon contained in the clay layer once floating materials and gradually deposited on the seabed with clay.
Atmospheric Origin of the Organic Carbon at Early Earth
Solar winds containing H+ influence the atmosphere on the Earth [13] (Figure 6).
H+ at approximately 500 km/s impacts atmospheric CO2. Hydrocarbons were synthesized in the collisions. Thus, floating materials contain atmospheric organic carbon of non-biological origin as shown in Figure 6.
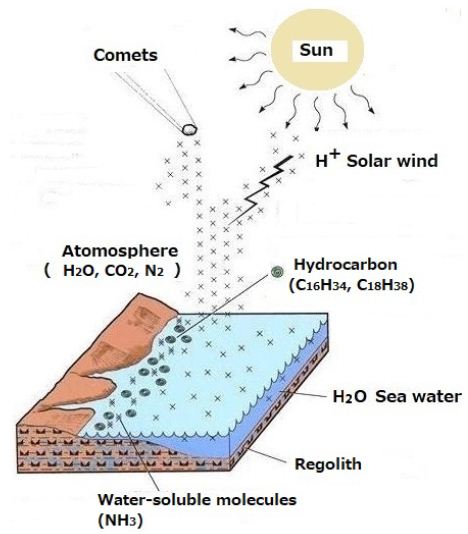
Figure 6: Proposed model to demonstrate the generation of atmospheric organic carbon of non-biological origin
Small hydrocarbon molecules such as CH4 and those involved in repetitive chemical reactions remained as gas molecules in the upper sky. However, the remaining carbon atoms bounded with hydrogen atoms to form long chain hydrocarbons without branches.
These hydrocarbon molecules formed floating materials that gathered on the surface of the early ocean.
Table 1 shows the melting and boiling points of various hydrophobic long chain hydrocarbons. In particular, the fatty acids possessing 16 or 18 pieces of carbon are of great importance as they are the main components of cell membranes.
Table 1: Temperature characteristics of hydrophobic long chain hydrocarbon molecules
|
Molecule |
Melting point [℃] | Boiling point [℃] |
Specific gravity [20℃] |
| Tetradecane; C14H30 |
4~6 |
253~257 |
0.76 |
| Hexadecane; C16H34 |
18 |
287 |
0.773~0.776 |
| Octadecane; C18H38 |
28~30 |
317 |
0.777 |
| Eicosane; C20H42 |
36~38 |
343 |
0.7886 |
Individual Chickens are Formed from Individual Egg
These molecules shown in Table 1 remained in a liquid state for a prolonged period, which was made possible evolution of the molecules due to intermolecular bond. However, the same membrane must be reproduced without the mold, because the membrane that is adhered to peptide-bounded amino acids cannot be replicated using mirror symmetrical mold. Because the mold of the left-hand protein molecule cannot be copied from the right-hand protein molecule. The mechanism that able to reproduce protein cannot be considered except mechanism of the reproduction. Thus, the self-replication of primitive life is achieved by the memorization of the order of self-production. It will be possible via photosynthesis in the floating substance of hydrocarbon molecules containing metabolic intermolecular interactions of Fe(HCO3)2. Although it is reported that oldest fossils evidencing life were formed at 3.5 billion years ago, the developed floating materials may have initiated the processes necessary for early life to develop, shortly after the formation of the sea water on the surface of the Earth. However, many steps of evolutions are necessary for the development of a systematic cell system of replication such as use of RNA.
Conclusion
This paper describes the processes involved in the formation of the oldest BIF and earliest life on Earth based on evidence. The concept of the first life is different from that considered at the standard for today’s living creature, because the first life was produced abiotic process. The BIF in the Isua region was formed through abiogenic processes including intermittent volcanic explosions in a sea of carbonated water. Volcanic activity led to the formation of membranes or bubbles at the surface of the sea water; these membranes or bubbles are attributed to the 2-dimensional structure of Fe(HCO3)2, where Fe2+ is surrounded by four HCO3– ions linked to each other by hydrogen bonds. Such inorganic bubble responds to external changes via metabolic intermolecular interactions. The floating materials gather on the surface of water, following which, the neighboring atoms or ions in the materials were able to interchange positions via thermal motions. During this stage, the material became isotopically enriched in 12C. Since Fe atom acts as a reducing agent for CO2, the floating material that had participated in the reduction reaction dropped down to the seabed where it mixed with SiO2. Thus, the intermittent volcanic explosions and abiotic processes contributed to the layered structure of Isua BIF and the earliest life from an abiogenic perspective. The earliest instance of photosynthesis may have occurred in the membrane of Fe(HCO3)2 using hydrophobic CnH2n+2 molecule in floating materials as the catalyst and irradiation of the Sun as the energy. However, the development of the systematic cell replication system observed in living creature today require several steps of evolution and elucidating the metabolic intermolecular interactions of the floating materials is of great significance in improving our understanding of the life on the early Earth.
Acknowledgement
I would like to thank Editage (www.editage.com) for English language editing
References
- Bowring SA, Williams IS, Compston W (1989) 3.96 Ga gneisses from the Slave province, Northwest Territories, Canada. Geology 17: 971-975.
- Demoulin CF, Yannick JL, Luc C, Camille F, Denis B, et al. (2019) Cyanobacteria evolution: Insight from the fossil record. Free Radic Biol Med. 140: 206-223.
- Rosing MT (1999) 13C-Depleted Carbon Microparticles in >3700-Ma Sea-Floor Sedimentary Rocks from West Greenland. Science 283: 674-676. [crossref]
- Schidlowski M, Peter WUA, Rudolf E, Christian EJ, et al. (1979) Carbon isotope geochemistry of the 3.7×109-yr-old Isua sediments, West Greenland: implications for the Archaean carbon and oxygen cycles. Geochimica et Cosmochimica Acta 43: 189-199.
- Mojzsis SJ, Arrhenius G, McKeegan KD, Harrison TM, Nutman AP, et al. (1996) Evidence for life on Earth before 3,800 million years ago. Nature 384: 55-59.
- Roe JE, Anbar AD, Barling J (2003) Nonbiological fractionation of Fe isotopes: Evidence of an equilibrium isotope effect. Chemical Geology 195: 69-85.
- Karasawa S (2014) Prebiotic reactions in the bubble that was formed in carbonated water by iron atoms. Viva Origino 42: 12-17.
- Hayashi C, Nakazawa K, Nakagawa Y (1985) Formation of the solar system, Protostars & planets Ⅱ, Arizona Press pg: 1100-1153.
- Maeder A (2009) Physics, Formation and Evolution of Rotating Stars, (Astronomy and Astrophysics Library) S1.2, The Potential Energy, Springer.
- White WM (2013) Geochemistry Chap 6, pg: 217-267, John Wiley & Sons, Ltd.
- Schopf JW (2011) The palaeobiological record of photosynthesis. Photosynth Res. 107: 87-101. [crossref]
- Rosing MT (2004) Search of the Earth’s oldest life, NHK special-Global Evolution-1, pg: 123, (written in Japanese).
- Karasawa S (2022) Effects of Solar Wind on Earth’s Climate. Geol Earth Mar Sci 4: 1-5.