Abstract
The article is a collection of ideas and experimental results that show that existing ideas about metallic alloys should go away to history of Metal Science. Here we present to the reader the results of experiments obtained as an example on four binary and two ternary alloys. Briefly, the main conclusions of the experiments are as follows: Interatomic chemical interactions exist in all metal alloys at temperature of both solid and liquid states. Chemical bonds in alloys have an amazing property to change their signs at a certain temperature (there is a phase transition “ordering – separation”). The reason for this transition is the electronic transition “ionic bond ↔ covalent bond”. The process of formation of new phases in binary alloys begins with the formation of solute clusters, in ternary alloys – with the formation of diffusion micro-pairs during the melting of the alloy. Such cardinal differences between the existing now notions about the nature of alloys and those revealed in this work should definitely lead to changes in the technology of heat treatment of alloys, to changes in the binary phase diagrams and to a change in the principles of creating new alloys.
Keywords
Introduction alloys, Chemical bonds, Transmission electron microscopy, Microstructure, Phase transition “ordering – separation”, Electron transition “ionic bond ↔ covalent bond”, Diffusion micro-pairs, Chemical bonds, Transmission electron microscopy, Microstructure; Phase transition “ordering – separation”, Electron transition “ionic bond ↔ covalent bond”
Introduction
Development of human civilization is inconceivable without progress in the advancement of its material base, in which metals and metal alloys play one of the leading roles. In recent years, we have observed significant changes for the better in the elaboration of new alloys. However, unfortunately, they achieved in a long, empirical way. This is because our ideas about the nature of alloys are still at the level of almost 100 years ago. This circumstance hinders progress in the development of new alloys and does not allow researching alloys in a necessary direction. The source of the existing now theory of alloys dates back to when researchers, studying the solubility of a salt in water with decreasing temperature, found that in time, salt crystals precipitate from the solution. They suggested whether the process of nucleation of particles of a new phase during the tempering of quenched metal alloys does not occur in the same way as during the isolation of salt crystals from a supersaturated aqueous solution. The idea turned out to be tempting, and since then, metal science has been living with the illusions of the past: a solid solution disordered after quenching becomes to supersaturated with a decrease in the heat treatment temperature, and particles of the excess phase precipitate. Only then, chemical bonds arise between the atoms of this phase. This means that not chemical interatomic bonds cause the formation of chemical compounds, but chemical compounds cause the occurrence of chemical interatomic bonds (!). Obviously, the comparison of the process of formation of a new phase during the tempering of a metal alloy and the process of precipitation of salt crystals with a decrease in the temperature of the water-salt solution is highly incorrect. However, this idea is so ingrained in our heads that even the discussion on this topic, held by the journal Acta Metallurgica in 1960-80 [1,2], ending in favor of existing ideas. This is not surprising, since in all universities of the world for many decades now, all professors have been presenting these ideas as an axiom that does not require proof.
The author of this article, starting his experimental work on the above topics, chose the direct method for studying the microstructure – TEM, and studied the microstructure of each alloy at all heat treatment temperatures (with an interval of about 200-300°C and, including in the liquid state). We hope that the reader already knows some of our papers or monography [3], where it is described what the “ordering-separation” phase transition is in binary alloys. This is a phase transition that occurs at a well-defined temperature, when, in the AxBy alloy, atoms A and atoms B, instead of mutual attraction experience mutual repulsing. This is not surprising if we consider alloys from the point of view of the structure of their electronic structure. As you know, in a pure metal between its atoms, takes place a 100% metallic bond. However, when atoms of another metal are added to one metal (i.e., when creating a binary alloy), a certain part of the metal bonds in the alloy are replaced by chemical ones, namely, ionic and/or covalent ones [4]. The ionic component of the chemical bond appears when, with a change in the alloy’s temperature, the B atoms become the nearest neighbors of the А atoms. Because of this instantaneous proximity, their valence orbitals localize the chemical compound AxBy form. The covalent component of a chemical bond forms when two atoms of the dissolved component B become the nearest neighbors. Because of this instant proximity, their valence orbitals hybridize, i.e. a cluster forms comprising these two atoms B. Thus, we understand how an ionic bond forms and how a covalent bond forms. However, it does not understand why, at the temperature characteristic of each alloy, these bonds pass one into the other, i.e., why an electronic transition “ionic bond ↔ covalent bond” occurs.
Materials and Methods
In this article, we will consider some binary alloys, for example, Ni75Mo25, Fe50Cr50 and Ni88Al12, as well as two ternary Ni65Mo20Cr15 and Ni53Mo35Al12 alloy, as examples. We smelted alloys from pure components in an induction furnace. We cut out specimens from them for research at different temperatures. After holding at any temperature, the specimens immediately cooled in water to fix the microstructure at this temperature. One of the specimens melted down and the contents of the crucible poured into water to study the microstructure of the liquid state. Foils cut out from all the blanks for study on a transmission electron microscope EM-125.
Results
Alloy Ni75Mo25. For those who had to deal with the problem of pouring liquid Ni-Mo alloys, it seemed surprising that its very low fluidity of that alloy even when it was overheated by 200°C above the solidus line. Now it becomes clear what we connect this with the phenomenon. The Figure 1a shows dark particles of molybdenum, which formed at the melting of the charge. This means that even at temperatures of the liquid state, the alloy under study has the tendency to phase separation. Indeed, we observe on the electron diffraction pattern a system of additional reflections {1 1½ 0} as four symmetrical pairs (Figure 1b). These reflections formed near each of the fundamental reflections {110} and {200}. Quenching of the same alloy from 1300̊°C, i.e. temperature, at which “a disordered solid solution” forms gives the same picture.
“a disordered solid solution” forms gives the same picture.
Figure 1a shows dark particles of molybdenum, which formed at the melting of the charge. This means that even at temperatures of the liquid state, the alloy under study has the tendency to phase separation. Indeed, we observe on the electron diffraction pattern a system of additional reflections {1 1½ 0} as four symmetrical pairs. These reflections formed near each of the fundamental reflections {110} and {200}. Quenching of the same alloy from 1300̊ C, i.e. temperature, at which “a disordered solid solution” forms gives the same picture. Exactly the same picture you can watch after quenching this alloy from 1300°C. This means that the type of microstructure of the alloy is determined not by its state of aggregation at such temperatures, but by completely different parameters. A further decrease in the alloy’s temperature by only 100°C dramatically changes the type of microstructure: instead of the structure formed because of a tendency to separation, a chemical compound Ni3Mo forms as long square-section laths (Figure 2a and 2b). This shows that in the temperature range of 1200-1300°C, the “ordering-separation” phase transition occurs in the alloy under study.
Comparing the microstructures shown in Figures 1 and 2, we see that there are two diametrically opposed types of interaction between dissimilar atoms: separation at high temperatures (liquid state), ordering at a temperature of 1000°C. It is even more interesting to observe the phase transition “Ordering – separation” in alloy Fe50Cr50, where it occurs twice with a change in the temperature of the alloy.
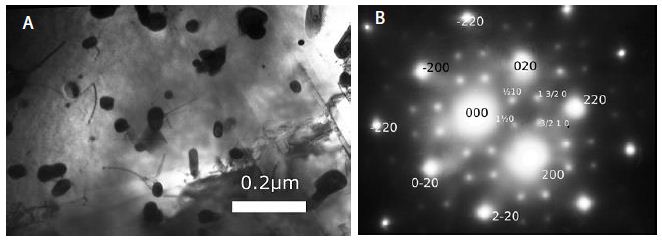
Figure 1: Alloy Ni75Mo25.Water quenching from liquid state: (a) microstructure (Mo-particles); (b) electron diffraction pattern.
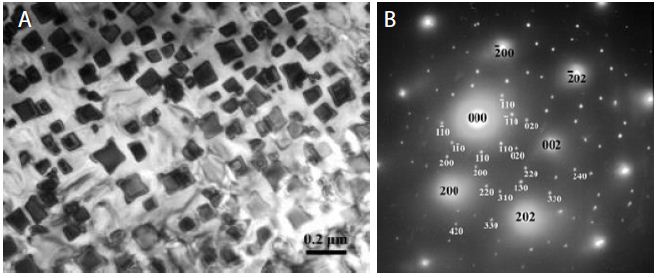
Figure 2: Ni75Mo25 alloy. Quenching from 1000°С. Bright-field image of the microstructure (a); electron diffraction pattern (b).
Fe50Cr50 alloy (Figure 3). The author [3], investigating the microstructure of alloys of the iron-chromium system by TEM, discovered a surprising phenomenon, which consists in the fact that at a quite definite heating temperature of these alloys, the sign of the chemical interaction between iron and chromium atoms changes. At some temperatures (above 1150°C), repulsion is observed between these atoms (particles of atoms of pure chromium with the Al2 lattice are formed – Figure 3a), at other temperatures (600-830°C) – attraction (a ϭ-phase is formed – Figure 3b). At the boundary between these two regions, when the sign of the chemical interaction passes through zero, a solid solution was found in the alloys of the iron-chromium system, and electronic domains were found during two-stage heat treatment (Figure 3c). Such domains, arising during the “ordering-phase separation” transition, best observed when defocusing the electron microscopic image of the specimens. We consider electronic domains as some regions of the alloy, inside which the sign of the ordering energy has already changed, in comparison with the surrounding matrix, where it remains the same. Since the chemical interaction energies have opposite signs on both sides of the domain wall, the electron beam passing through the foil is deflected in opposite directions, which, upon defocusing, leads to a deficiency (light lines) or an excess (dark lines) of electrons. Thus, electronic domains, as well as magnetic and ferroelectric domains, are a reflection on a conventional photographic plate of a phase transition occurring at the level of changes in the electronic structure of the alloy.
In contrast to, where the formation of electronic domains was considered as a temporary, unstable state of the alloy (a state of transition from a tendency to phase separation to a tendency to ordering), it was shown in Ref. [3] at domains in Fe-Cr alloys under certain conditions can represent a stable state of the alloy. The existence in the alloy’s bulk of such a state (incomplete phase transition ordering-separation) prevents the formation of the σ-phase in the entire volume of the alloy; the σ-phase forms only in a thin surface layer, where, apparently, the surface plays the role of a catalyst for the “ordering-separation” transition process. A further decrease in temperature to 500°С again leads to the formation of particles (or, possibly, highly concentrated clusters) of chromium atoms, Figure 3e. At the boundary between the regions of the ϭ-phase and low-temperature separation, we again found electronic domains (Figure 3f). The formation of electronic domains on the line of the “ordering-phase separation” phase transition (Figure 3c and 3f) tells us it is the changes in the electronic structure that underlie the microstructural phase transition “ordering-phase separation”. Low-temperature domains occupy the entire volume of the alloy, and therefore it can expected that such a microstructure of the alloy will have certain specific properties, such as magnetic or ferroelectric.
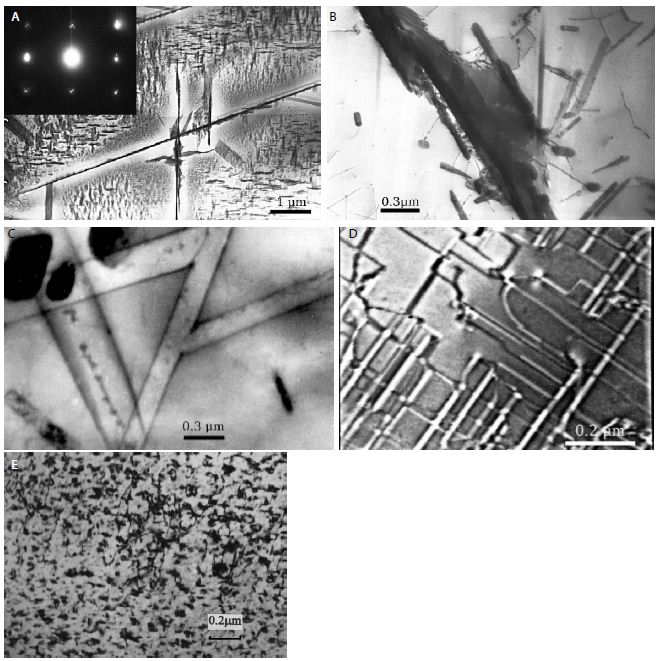
Figure 3: Fe50Cr50 alloy. Microstructure. Water quenching from 1400°C. Inset: electron diffraction pattern (a); σ-phase taken from subsurface layer (b). High-temperature (c) and low-temperature (d) electron domains. Microstructure of low-temperature phase separation (e).
Ni88Al12 Alloy
Figure 4a shows the electron diffraction pattern (a) and the dark-field image of the microstructure (b) got from a satellite near the (020) reflection [5,6]. Such formation of clusters usually shows the ordering of a binary alloy at 1300°C into particles comprising one component each, i.e. about the positive deviation of the investigated alloy from Raoult’s law. With a further decrease in temperature (1200, 1000 and 700°C), the sign of the ordering energy changes to the opposite and the chemical compound Ni3Mo (L12) forms in the alloy (Figure 4). Thus, we can conclude that in the Ni88Al12 alloy the “ordering-separation” phase transition occurs in the temperature range of 1200-1300°C.
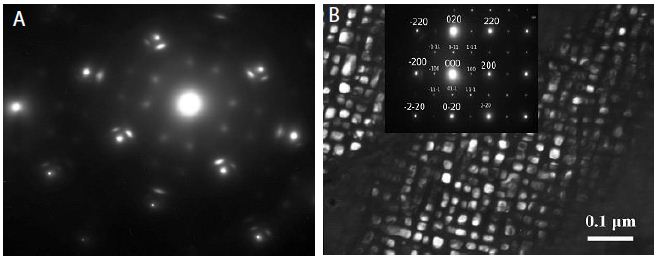
Figure 4: Ni88Al12 alloy. Quenching from a liquid state (tendency to separation) (a). Quenching from 1000°С: chemical compound Ni3Mo (L12). The electron diffraction pattern is in the Inset (b).
Alloys of Ni-Cr system
The phase transition “ordering-phase separation” in most of the binary systems studied by us occurs at a not quite definite heating temperature of the alloys (transition temperature). However, in alloys of the nickel-chromium system, a unique picture observed [7]. For example, in alloys with a chromium content of 32 wt.% and below at any heating temperature, there is only a tendency to phase separation since in the entire temperature range from 500 to 1500°C, clusters of chromium atoms are noticed in the microstructure of this alloy (Figure 5a and 5b). In alloys with a chromium content of 60 wt.% Cr and above at all temperatures is observed only a tendency toward ordering, since particles of the Cr2Ni compound are noticed in the microstructure (Figure 5c and 5d). In alloy Ni46Cr54, the composition of which is between concentrations from 32 to 60% Cr, with a change in the heating temperature of the alloy, the usual transition “ordering – phase separation” is observed at 1200°C (Figure 5e and 5f).
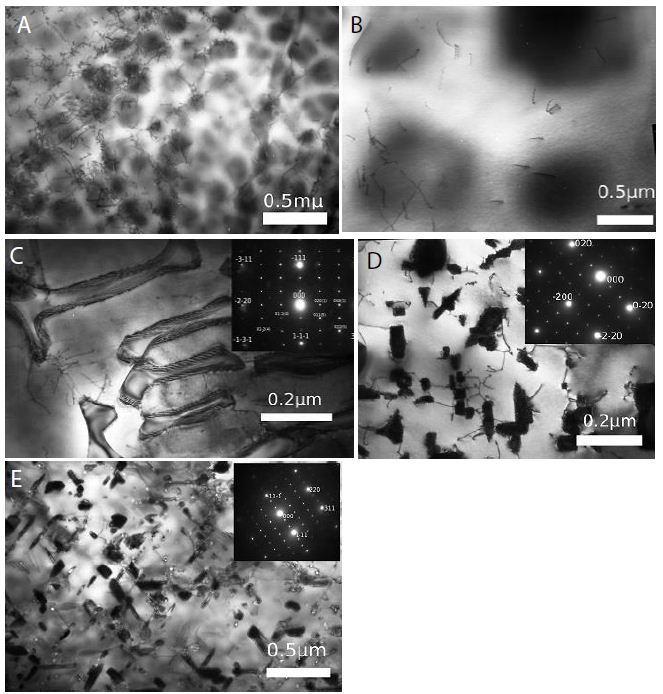
Figure 5: Alloys of the Ni-Cr system. (a) Ni68Cr32, water quenching from a liquid state. Absorption contrast. Cr-clusters; (b) Ni68Cr32, water quenching from 500°C; Absorption contrast. Cr-clusters; (c) Ni40Cr60 [9], water quenching from a liquid state. Ni2Cr particles. (d) Ni46Cr54, water quenching from a liquid state. Absorption contrast. Cr-clusters; (e) Ni46Cr54, water quenching from 1000°C. Ni2Cr particles [7].
As we see, we have investigated many other binary. It turned out that in almost every binary alloy there is a transition “ordering-phase separation”. In each alloy, this transition has its own characteristics, which manifested at the level of changes in the microstructure. In addition, in each alloy, we need to determine the temperature at which this transition occurs. The temperature can be any, but quite specific for each pair of atoms. For example, in the Co-30% Mo alloy, it is close to the solidus temperature. The regions of a so-called disordered solid solution located between the regions of phase separation and regions of ordering have not found in all systems where such a transition has occurred. In a number of alloys (for example, Ni-Co), the same image showed phase particles formed as a result of the appearance of a tendency to ordering in the alloy, and particles dissolving due to the disappearance of the tendency to phase separation in this alloy. We called such a transition the blurred “ordering -separation” one. As a result of this “mixing of phases”, a disordered solid solution was not found even on the line of the “ordering – separation” transition.
We have investigated many alloys. It turned out that in almost every binary alloy there is a transition “ordering-phase separation”. In each alloy, this transition has its own characteristics, which manifested at the level of changes in the microstructure. In each alloy, we need to determine the temperature at which this transition occurs. The temperature can be any. For example, in the Co-30% Mo alloy, it is close to the solidus temperature. The regions of a disordered solid solution located between the regions of phase separation and regions of ordering have not found in all systems where such a transition has occurred. In a number of alloys (for example, Ni-Co), the same image showed phase particles formed as a result of the appearance of a tendency to ordering in the alloy, and particles dissolving due to the disappearance of the tendency to phase separation in this alloy. We called such a transition a blurred “ordering – separation” transition. As a result of this “mixing of phases”, a disordered solid solution was not found even on the line of the “ordering – phase separation” transition.
Ternary Alloys
Ternary Ni65Mo20Cr15 alloy [7]. Electron microscopic analysis shows that in different sites of the same foil quenched from the liquid state (1450°C) accumulations of only two types of precipitates of the second phase found. In the Ni/Mo diffusion pair, observed single-component particles of molybdenum atoms, in the Ni/Cr diffusion pair – two-component clusters chromium atoms. Since molybdenum is a refractory metal, the formation of particles of Mo atoms in the alloy under study occurs at the maximum temperature that reached in the melting zone of the furnace. Whereas formation of the Cr-clusters (Figures 5-7), observe a fully formed microstructure up to the temperature of 1300°C. In the Ni/Mo diffusion pair, below 1250°C, the tendency to phase separation is substituted for the tendency to ordering (the “ordering-separation” transition), and the particles of Mo atoms dissolve.
After quenching the alloy from 1550°C, clusters of chromium atoms observed in diffusion pairs Ni/Cr as concentration waves of absorption contrast, which emanate from a certain light-colored center in a thin foil, forming apparently, in the moment of contact of the liquid alloy with water (Figure 6).
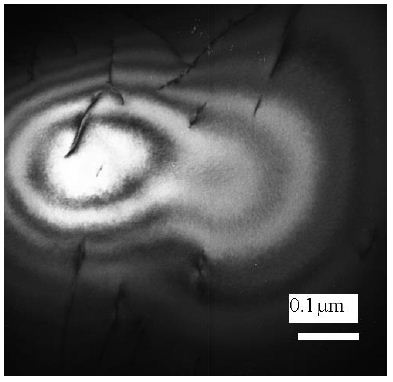
Figure 6: Ni65Mo20Cr15 alloy [8]. Nucleation of the Ni/Cr diffusion pair. Water quenching from 1550°C. Absorption contrast.
Comparing the microstructures in Figures 6-8, we can conclude that with a decrease in the temperature from which the alloy quenched the microstructure of the Cr-clusters forms over the entire volume of the alloy.
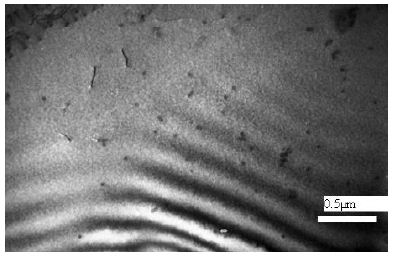
Figure 7: Ni65Mo20Cr15 alloy [9]. Ni/Cr diffusion pair. Water quenching from 1450°C. Absorption contrast.
We investigated many other binary alloys. It turned out that in almost every binary alloy there is a transition “ordering-phase separation”. In each alloy, this transition has its own characteristics, which manifested at the level of changes in the microstructure. In addition, in each alloy, we need to determine the temperature at which this transition occurs. The temperature can be any, but quite specific for each pair of atoms. For example, in the Co-30% Mo alloy, it is close to the solidus temperature. The regions of a so-called disordered solid solution located between the regions of phase separation and regions of ordering have not found in all systems where such a transition has occurred. In a number of alloys (for example, Ni-Co), the same image showed phase particles formed as a result of the appearance of a tendency to ordering in the alloy, and particles dissolving due to the disappearance of the tendency to phase separation in this alloy. We called such a transition the blurred “ordering -separation” one. As a result of this “mixing of phases”, a disordered solid solution was not found even on the line of the “ordering – separation” transition.
We have investigated many alloys. It turned out that in almost every binary alloy there is a transition “ordering-phase separation”. In each alloy, this transition has its own characteristics, which manifested at the level of changes in the microstructure. In each alloy, we need to determine the temperature at which this transition occurs. The temperature can be any. For example, in the Co-30% Mo alloy, it is close to the solidus temperature. The regions of a disordered solid solution located between the regions of phase separation and regions of ordering have not found in all systems where such a transition has occurred. In a number of alloys (for example, Ni-Co), the same image showed phase particles formed as a result of the appearance of a tendency to ordering in the alloy, and particles dissolving due to the disappearance of the tendency to phase separation in this alloy. We called such a transition a blurred “ordering – separation” transition. As a result of this “mixing of phases”, a disordered solid solution was not found even on the line of the “ordering – phase separation” transition. Ternary Ni53Mo35Al12 alloy [8]. As is known from the electronic theory, in ternary alloys, as in binary ones, the chemical interatomic interaction is pair-wise [8]. Therefore, ternary Laves phases cannot form in a ternary alloy. We have shown that the ABC ternary alloy, while still in the liquid state divided into diffusion micro-pairs A/B, A/C, and B/C. Each diffusion micro-pair occupies its own microscopic area. All phases formed in ternary alloys comprised atoms of no more than two components [8].
In the Ni53Mo35Al12 alloy, this process has its own characteristics, since the aluminum and molybdenum atoms form the Mo3Al chemical compound, the melting point of which is about 2600°C. This means that the chemical bond between the Al and Mo atoms is very strong. Therefore, in Figure 8 we see the microstructure of solid Mo3Al particles in the investigated liquid alloy. This compound forms instantly in the process of melting the charge. The same microstructure as in Figure 1 retained in the alloy under study after its quenching from 1300°C. As was shown above, at a temperature of about 1200°С, the “ordering–separation” transition occurs in binary alloys of the Ni–Mo system [5]. This means that the chemical orientation of the Ni and Mo atoms relative to each other changes sharply at separation – ordering phase transition.
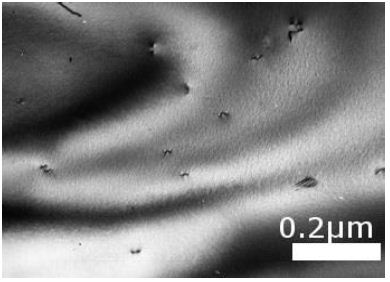
Figure 8: Ni65Mo20Cr15 alloy. Ni/Cr diffusion micro-pair. Water quenching from 1300°C. Absorption contrast.
Figure 10 shows the internal microstructure of a large solid Mo3Al particle in the liquid alloy under study. When the charge melted, this compound form instantly. As long as it exists, the Mo/Ni and Al/Ni diffusion pairs cannot form in the alloy. The alloy under study has the same microstructure. However, at a temperature of about 1200°C, when the “ordering-separation” phase transition should have occurred in diffusion Ni/Mo pairs, the microstructure of the studied alloy completely changes (Figure 9).
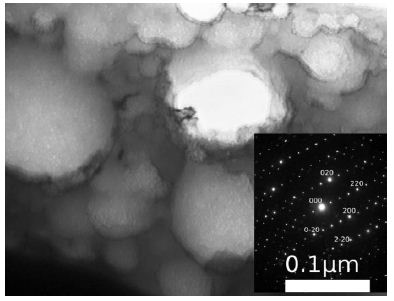
Figure 9: Ni53Mo35Al12 alloy [10]. Quenching from the liquid state. Light-field image. Accumulations of particles of the Mo3Al phase. Inset: Electron diffraction pattern (the zone axis is close to the direction <001>).
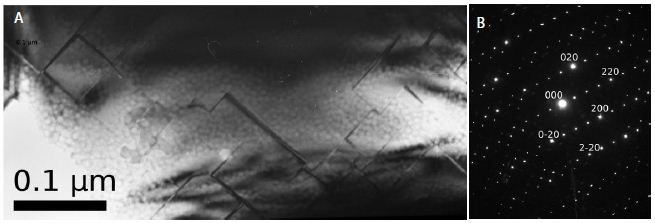
Figure 10: Ni53Mo35Al12 alloy. Quenching from the liquid state. Another bright-field image of the internal structure of a large Mo3Al particle (a). Electron diffraction pattern (b).
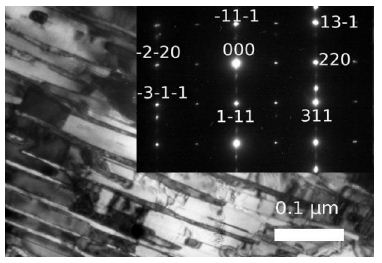
Figure 11: Alloy Ni53Mo35Al12. Quenching from 1000°С. Bright field images of the microstructure of diffusion micro-pairs Ni3Mo (a) and Ni3Al (L12).
The regions of a disordered solid solution located between the regions of phase separation and regions of ordering have not found in all systems where such a transition has occurred. In a number of alloys (for example, Ni-Co), the same image showed phase particles formed as a result of the appearance of a tendency to ordering in the alloy, and particles dissolving due to the disappearance of the tendency to phase separation in this alloy. We called such a transition a blurred “ordering – separation” transition. As a result of this “mixing of phases”, a disordered solid solution was not found even on the line of the “ordering – phase separation” transition.
When studying the Ni53Mo35Al12 alloy by TEM, we again encountered with the same fact that we discovered earlier in some other ternary alloys: in one region of the foil, we find one binary (or one-component) phase, in another region of the same foil, a completely different one. This immediately indicates that the alloy divided into certain sections that differ from each other in composition, i.e. into two different diffusion micro-pairs: Ni/Mo and Ni/Al.
In the Ni53Mo35Al12 alloy, this process has its own characteristics, since the aluminum and molybdenum atoms form the Mo3Al chemical compound, the melting point of which is about 2600°C. This means that the chemical bond between the Al and Mo atoms is very strong. Therefore, we see the microstructure of solid Mo3Al particles in the investigated liquid alloy. This compound forms instantly in the process of melting the charge. The same microstructure retained in the alloy under study after its quenching from 1300°C. As was shown above, at a temperature of about 1200°C, the “ordering–separation” transition occurs in binary alloys of the Ni–Mo system [5]. This means that the chemical orientation of the Ni and Mo atoms relative to each other changes sharply from separation to ordering.
Discussion
Our experimental detection of such transitions and their publication in the literature for during 20 years did not lead to any progress in the worldview of metallurgists, and, as a result, to the appearance of works by other authors on this topic. Although, thanks to our experiments, these changes simply suggested themselves, since their introduction would significantly improve the technological properties of all heat-treated parts and products made of metal alloys and more than halve the cost of their heat treatment [9]. It could happen in all the engineering plants in the world. Perhaps the complete lack of interest of metallurgists in these two transitions explained because, in this case, they would have to change their ideas about alloys and change your attitude towards binary phase diagrams [10]. However, it is difficult to make this step when the entire community of metallurgists thinks alternatively and university professors teach of students’ alternative.
Diffusion Micro-pairs
Usually, diffusion micro-pairs in multi-component alloys form during their smelting [11]. However, if a chemical compound’s melting point is higher than the alloy, we cannot fix these micro-pairs, and compounds will found first (for example, Mo3Al). If lower, then we will find the formation of chemical compounds takes place inside the diffusion micro-pairs.
Heat treatment of metal alloys “quenching + tempering (aging)” has known for a very long time and currently carried out at almost all machine-building plants in the world.
According to existing concepts, after quenching at high temperatures, a microstructure of a disordered solid solution forms in alloys. Subsequent tempering, carried out at a lower temperature, leads to the fact that the solid solution comprising quenching becomes “supersaturated” and “excess” phases are released from it (either by the spinodal mechanism or by the nucleation-growth mechanism).
In contrast to the existing theory of heat treatment, in alloys at all temperatures, there is a chemical interaction between similar (covalent bond) and dissimilar (ionic bond) atoms. Such an interaction exists both in the liquid and in the solid state of the alloy. This means that we can never get a disordered solid solution after quenching the alloy from high temperatures, even if we will quench from the liquid state. This also means that at each temperature forms own microstructure, which distinguished from any other microstructure by type or dispersity of precipitates. The final microstructure of an alloy is determined by the temperature of the final heat treatment, i.e. tempering. The heat treatment, such as quenching from region “disordered solid solutions”, does not affect the final microstructure of the alloy.
Because of this instantaneous proximity, their valence orbitals localized, i.e. the chemical compound AxBy forms. The covalent component of the chemical bond, which activated for the same reason, causes the two atoms of the dissolved component B to become the nearest neighbors. Because of this instantaneous proximity, their valence orbitals hybridize, i.e. a cluster forms comprising these two atoms B. However, our experimental detection of such transitions and their publication in the literature for over 20 years did not lead to any progress in the worldview of metallurgists, and, as a result, to the appearance of works by other authors on this topic. In addition, these changes simply suggested themselves, since their introduction would significantly improve the technological properties of all heat-treated parts and products made of metal alloys and more than halve the cost of their heat treatment. It could happen in all the engineering plants in the world. Perhaps the complete lack of interest of metallurgists in these two transitions explained because, in this case, they would have to change very many ideas about alloys. Moreover, it is difficult to take this step when the entire community of metallurgists thinks differently and university professors teach of students differently.
In the experimental part of the article, we presented the reader with the results of our studies of binary and ternary substitution alloys. The cardinal difference between our research (TEM) and those works (XRD) thanks to which the current theory of alloys built is immediately striking. Now that it has become clear to us that the X-ray method is not suitable for these purposes, and that those authors who used the TEM method in the discussion of 1960-80 were right, there is no doubt that our ideas about the existence of some disordered solid solution in alloys should go down in the history of alloy science. If this is really the case, then any quenching in water from any heating temperature of the alloy should lead to formation of the microstructure that formed in the alloy at this temperature. It has been known that almost every machine-building plant in the world uses “double” heat treatment of alloys – quenching from the region of a “disordered” solid solution + tempering at low temperatures. Select a few parts from the batch intended for quenching and simply do not make it.
Compare their microstructure and properties with those parts that have been as quenched. I should note that for the purity of the experiment, samples (or parts) that have quenched should cooled in water to fix the microstructure characteristic of your tempering temperature. Even if you are not strong in alloy theory, you will be able to deduce whether you need that quenching to get a “disordered solid solution” before tempering.
High-entropy Alloys
When a ternary alloy forms three diffusion micro-pairs at once, the uphill diffusion of atoms of each component will be on coming. As a result, the rate of formation of one or another diffusion pair will slow down significantly, and perhaps even drop to zero. Most likely, these streams separated either in time or in temperature. However, when many number of streams of different directions, they completely suppressed to each other. Therefore, the more components an alloy contains, the slower one or other diffusion micro-pairs will form in it. That is why in high-entropy alloys (HEA), which contain five or more components, diffusion micro-pairs do not form. By performing a straightforward calculation, it becomes apparent that formation 10 diffusion micro-pairs in 5-component alloy is possible only on paper. In reality, it is impossible. This is why long-distance diffusion is completely absent in HEA and diffusion micro-pairs do not form. This meant that chemical bonds between the atoms of the components cannot manifest themselves in these alloys and no chemical compounds can form in them. The question arises: whom needs such alloys that do not have the properties of alloys and microstructure of which represents randomly arranged different atoms (only sometimes -small solid solution regions).
In their properties and structure, they are more similar to metal ore. It turns out interesting with HEA. A person has invested considerable effort to receive pure metals from natural metal ore, only to produce a new synthetic metal ore from these metals later. Typically, diffusion micro-pairs in multicomponent alloys form during their smelting, when the crystal lattice of the alloy has not yet formed. In the solid state of the alloy, uphill diffusion of atoms of the dissolved component also occurs, but much more slowly. I am not maintaining, but it is hardly possible to expect that in the solid state in a ternary alloy, uphill diffusion of atoms of two components at once is possible. Most likely, either on time or on temperature separate these flows. However, with a large number of flows in different directions, they are completely suppress each other. Consequently, the more components an alloy contains, the lower the likelihood of the formation of certain diffusion micro-pairs and, consequently, particles of new phases of one or other type.
It should be noted that to obtain true alloys, one should avoid not only adding a large number of alloying components (usually more than two), but also adding a small amount of any component (usually 3% or less). With such alloying, one must proceed from the principle that the amount of the alloying component would been enough to form diffusion micro-pairs in the alloy, i.e. so that particles containing this component formed. Otherwise, such doping can considered as simple clogging of the crystal lattice with foreign atoms. Our understanding of this or that physical phenomenon always changes with time and usually corresponds to the level of experimental technique in the period under consideration. Let us recall the once fashionable classical theory of the “nucleation and growth” of a new phase, the theory of the “in situ” nucleation of special carbides in alloyed steels, and so on. Where are they now? They forgotten because their description of processes occurring in nature differs from reality. At the same time, there are theories and ideas with a different fate, which came to us from the past century, but are so deeply rooted in our minds that even now, when the experiment does not confirm them, we believe that they are an indisputable truth. For example, we cannot imagine equilibrium phase diagrams without solid solution regions at high temperatures, although a solid solution, from the point of view of thermodynamics, is not an equilibrium phase at any temperature. We cannot imagine the probability of decomposition of a quenched solid solution without its “supersaturation” in solutes, which occurs at a decrease of heat treatment temperature. We cannot imagine the heat treatment carried out to obtain a highly dispersed two-phase structure, which would not include the preliminary high-temperature quenching from the solid solution region, and so on.
The discovery of the ‘ordering-phase separation’ transition in alloys puts an end to these ideas. It becomes clear that the ideas about the nature of alloys that we acquired in our universities turn out to be largely outdated, as they based on experimental data obtained as far back as the mid-twentieth century and without the use of the method of TEM. From the above discussion, it follows that introduction of such a concept as the ‘ordering-phase separation’ transition into common use changes our previous understanding of the driving forces of the process of new phase formation. In addition, it becomes apparent that in order to change this situation a great number of experimental studies is to upgrade existing phase diagrams. The ‘ordering-phase separation’ transition, regarded as changes in the chemical interaction sign, is such a transition, in the process of which the ionic component of the chemical bond between the atoms, due to the electron-phonon interaction, replaced by the covalent component, or vice versa. All processes occurring in alloys during heat treatment and leading to a change in their properties considered from the point of view that the disordered solid solution is the initial phase in these processes, and the elastic forces arising between dissimilar atoms are the driving force. The experimental results presented in this monograph debunk this point of view and show that it is necessary to take the liquid state of the alloy as the initial phase, and interatomic chemical interactions as the driving force of all diffusion processes occurring in the condensed state of an alloy.
Cardinal Changes in Heat Treatment of Alloys
The detection of the ‘ordering-phase separation’ transition allowed for the conclusion that, at a certain temperature, the sign of the chemical interaction between dissimilar atoms can change. This means that it is only at the temperature of such a transition, when the energy of the interatomic chemical interaction is close to zero, which a disordered solid solution can form in the alloys. However, such a clearly pronounced change in the microstructure not observed in the alloys of all systems. In the alloys of some other systems, at a transition temperature one can see a mixture of microstructures of phase separation and ordering. It should also note that the microstructure of the solid solution could observed in alloys in which the energy of the interatomic chemical interaction is low at all temperatures. When any composition of the alloy is in a molten state, it subdivided into microscopic areas, each of which enriched with one alloying component or another (in other words, one or several types of diffusion pairs formed in it). Binary chemical compounds (tendency to ordering) or clusters (tendency to phase separation) can form inside these diffusion pairs. When the heat treatment temperature decreases, the average chemical composition of such diffusion pairs is always preserved, and at the same time the distribution of components inside the diffusion pairs themselves becomes more heterogeneous: chemical compounds (if their melting point is higher than the melting temperature of the alloy itself) or clusters similar to the corresponding binary alloys are formed. The driving force of such a partitioning process is the tendency of the similar atoms to form clusters in the solvent lattice or the tendency of dissimilar atoms to form a chemical compound.
The ‘ordering-phase separation’ transition, which at the microstructure level manifests itself as changing of the sign of energy of the chemical interaction between the atoms A and B, at the electronic level manifests itself in exactly the same way. It is a transition from the state when all pairs of valence electrons localized on atoms A and B and forming ionic bonds between atoms come out from this state. At the same time, some pairs of valence electrons, due to the electron-phonon interaction, are involved in the formation of hybridized orbitals between two B atoms (which leads to the formation of B clusters, i.e. phase separation). This means that the ‘ordering-phase separation’ transition in an alloy is a consequence of the electronic ‘covalent bond ↔ ionic bond’ transition. This article shows metallurgists should come to terms with the existence in nature of such a type of microstructure as diffusion micro-pairs and say goodbye to such a type of microstructure as a disordered solid solution. And the sooner they do this, the less effort and money will be spent all over the world on heat treatment of alloys (water-quenching “to get a disordered solid solution” will disappear), on the design of new and improvement of existing alloys. The phase diagrams of binary alloys will become more informative and truthful (because of the regions of non-existent disordered solid solutions will disappear). We will understand many of the mysteries of nature regarding metallic alloys that nature has hidden from us for many years. In the case, all of us, metallurgical scientists, will need to contribute to this work.
Conclusion
This article shows metallurgists should come to terms with the existence in nature of such a type of microstructure as diffusion micro-pairs and say goodbye to such a type of microstructure as a disordered solid solution. And the sooner they do this, the less effort and money will be spent all over the world on heat treatment of alloys (water-quenching “to get a disordered solid solution” will disappear), on the design of new and improvement of existing alloys. The phase diagrams of binary alloys will become more informative and truthful (because of the regions of non-existent disordered solid solutions will disappear). We will understand many of the mysteries of nature regarding metallic alloys that nature has hidden from us for many years. However, for this, all of us, alloy specialists, will need to contribute to this work.
References
- Nesbit LA (1981) Laughlin DE. Solid-state morphological instability of Ni4Mo precipitates. J Crystal Growth 51: 273-278.
- Van Tendeloo G, Amelinkx S (1985) De Fontaine D. On the nature of the “short-range order” in 1 ½ 0 alloys. Acta Crystallography.B41: 281-292.
- Ustinovshikov Yuri (2019) The ‘Ordering-Phase Separation’ Transition in Alloys. Cambridge Scholars Publishing.
- Ustinovshikov Y (2017) Changes in electronic structure of metallic alloys at the transition ‘ordering-phase separation’. J Alloys & Compounds 714: 476-483.
- Ustinovshikov Y, Shabanova I (2011) Phase transitions in alloys of the Ni-Mo system. Mat Chemic Phys 129: 975-980.
- Ustinovshikov Y (2012) Phase transition ‘ordering-phase separation’ in the Ni-12 at. % Al alloy. J Alloys & Compounds 528: 141-145.
- Ustinovshikov Y (2018) Multi-component alloys: Regularities in the formation of microstructures. J Alloys & Compounds. 735: 2298-2302.
- Ustinovshikov Y (2016) Formation of microstructures responsible for remarkable properties of the Ni- and Co-based super-alloys. Acta Mater 111: 66-74.
- Ustinovshikov Y (2014) A new paradigm for heat treatment of alloys. J Alloys & Compounds 614: 258-263
- Ustinovshikov Y (2017) Mapping of the transition ‘ordering-phase separation’ into phase diagrams. J Alloys & Compounds. 691: 713-720.
- Ustinovshikov Y (2014) Phase and structural transformation in the Ni65Mo20Cr15 alloy at changing the temperature of heat treatment. Journal of Alloys and Compounds 588: 470-473.