Abstract
Etodolac (ETD), a member of non-steroidal anti-inflammatory drugs (NSAIDs), has a poor aqueous solubility. Long term administration of ETD causes severe gastrointestinal disturbances such as peptic ulcer and bleeding. The enhancement of its solubility and dissolution profile is expected to improve its bioavailability and reduce its side effects. In the present study, we tried to enhance the aqueous solubility and dissolution rate of ETD by two co-evaporation techniques. The first one is the formation of solid dispersion with different hydrophilic carriers, including polyethelene glycol (PEG 4000), polyvinyl pyrrolidones (PVP K25 and PVP K90) and urea. The second method is the formation of solid adsorbates using inert carriers such as avecil PH 101, bentonite and aerosil 200 as adsorbents. Co-evaporates were prepared at (1:1 w/w), (1:3 w/w) and (1:5 w/w) ETD to carrier ratios and the corresponding physical mixtures were also prepared. The solubility and dissolution studies of all formulations were measured. Moreover, the physicochemical properties of the modified co-evaporates were characterized using different techniques including, differential scanning calorimetry (DSC), infrared spectroscopy and X-ray diffractometry (XRD) analysis. The results showed that the co-evaporates exhibited higher dissolution rate than the corresponding physical mixtures and both showed higher dissolution rate than the unmodified drug. Increase polymer concentration led to increase in the dissolution rate of drug. Plus, the dissolution rate was enhanced by increasing the temperature of the dissolution medium. Avecil PH 101 exhibited the highest dissolution rate over all other polymers. Infrared studies showed no interaction between the drug and the investigated carrier. The DSC and XRD studies indicated the conversion of ETD to an amorphous state. The enhancement of the drug solubility may be attributed to the increase of drug surface area, the wettability, formation of hydrogen bonds and the conversion to amorphous state.
Keywords
Etodolac, Co-evaporate, Solubility, Dissolution rate, Solid dispersion, Adsorbate, Hydrophilic carriers
Introduction
Rheumatoid arthritis is a chronic and systemic inflammatory disorder that primarily affects joints [1]. Such disease could be revealed by using one of the non-steroidal anti-inflammatory drugs (NSAIDs) which are considered as a classical treatment for such rheumatic disorders. Etodolac is a non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic and antipyretic properties. The mechanism of action of such drug based on its ability to inhibit prostaglandin biosynthesis. It is indicated for the relief of signs and symptoms of rheumatoid arthritis and osteoarthritis [2]. Etodolac appears to be associated with a higher incidence of adverse effects, mainly irritation to the stomach, compared to other NSAIDs. Therefore, this issue limits its use for the treatment of patients for whom other NSAIDs have been ineffective [3]. According to the Biopharmaceutical Classification System (BCS), ETD belongs to class II drugs which are characterized by low solubility (69 mg/L) and high permeability. Therefore, there is an urgent need for the enhancement of ETD solubility and dissolution profile, which ultimately lead to a significant reduction of gastric residence time after oral administration. Consequently, this will be very useful for both the reduction of drug side effects to the stomach and improvement of its bioavailability [4,5]. There are several techniques utilized for improving the aqueous solubility and dissolution rate of poorly water soluble drugs such as inclusion complexation [6], micronization [7], recrystallization [8], co-melting [9], co-grinding [10], lyophilization [11] and co-evaporation techniques including solid dispersion [12] and surface adsorption [13]. Especially, the co-evaporation technique using different hydrophilic polymers (solid dispersion) or inert adsorbents (adsorbates) has attracted a considerable interest as an efficient mean for improving the dissolution rate and hence the bioavailability of wide range of poorly water soluble drugs without changing the parent drug. Once the solid co-evaporate was exposed to aqueous media, the drug was released as very fine colloidal particles [14]. The solubility enhancement of drug was related to different reasons, including the improvement of drug surface area, wettability and porosity as well as the reduction of its crystallinity [15,16]. Regarding solid dispersion, there are several types of hydrophilic carriers that could be used in the preparation of solid dispersion systems such as polyethylene glycols (PEGs) [17], polyvinyl pyrollidones (PVPs) [18], cellulose derivatives [19], urea [20], sugars (lactose, mannitol and sorbitol) [21] and organic acids such as citric acid [22-24]. Alternatively, several insoluble inert carriershave been used for drugs deposition (surface adsorption), including disintegerants [25], microcrystalline cellulose (Avicel) [26], colloidal silicon dioxide (Aerosil 200) [27], porous calcium silicates (Florite R) [28], magnesium aluminum silicate (kaolin) [29] and colloidal hydrated aluminum silicate (Bentonite) [30]. Solid co-evaporates of ETD with different excipients can be characterized by different physicochemical methods such as powder x-ray diffraction analysis (XRD), IR spectroscopy, ultraviolet spectrophotometry [15] and thermal analysis such as the differential scanning calorimetry (DSC), thermo mechanical analysis (TMA), hot stage microscopy (HSM) and thermogravimetry (TG). PEGs and PVPs are the most commonly used carriers due to their excellent water solubility and a wide range of molecular weights, ranging from 200 Da to 300,000 Da in case of PEGs and from 2,500 Da to 3,000,000 Da in case of PVPs [31]. The main purpose of the present work was to increase the aqueous solubility of ETD using solid co-evaporation technique. Two different methods were utilized or preparation of drug co-evaporates. The first one is solid dispersion method using PEG 4000, PVP k25, PVP k90 and urea as hydrophilic carriers. On the other hand, the second technique based on the preparation of ETD co-adsorbates with Avicel PH 101, Aerosil 200 and Bentonite as inert carriers. Also, the effect of drug/polymer ratio was studied by preparing different drug to polymer ratios. The enhancement of the dissolution rate was evaluated using in vitro dissolution studies. Moreover, the effects of these excipients on the physicochemical properties of drug were studied by different analytical methods such as IR, DSC and XRD patterns which used to investigate drug/carrier interactions and their effect on the dissolution rate of the drug.
Materials and Methods
Materials
Polyethylene glycol 4000 (PEG 4000) and polyvinyl pyrolidones (PVP K25 and PVP k90) were purchased from Fluka Bio Chemika (Switzerland). Urea was obtained from Chemajet Co. (Alexandria, Egypt). Microcrystalline cellulose (Avicel PH 101), Colloidal silicon dioxide (Aerosil 200) were obtained from Sigma Aldrich (Degussa Frankfurt, Germany). Bentonite was purchased from Nile Co. for Pharmaceutical and Chemical Industry (Cairo, Egypt). Etodolac was obtained by Pharco Pharmaceutical Co. (Alexandria, Egypt). Methanol and ethanol were purchased from El-Nasr Pharm. Chem. Co., (Cairo Egypt).
Phase Solubility Studies
The solubility of ETD was examined in distilled water, at different polymer concentrations (2.5-10% w/v)according to the method previously reported by Higuchi and Connors [32]. The selected hydrophilic polymers were PEG 4000, PVP K25, PVP K90 and urea. An excess amount of ETD (20 mg) was added to 20 ml stoppered glass tubes containing 10 ml of carrier solutions. The tubes were sonicated for 1 hr then transferred to a water bath previously adjusted at required temperatures 25°C and 37°C ± 1. Aliquots were withdrawn after 48 hours (equilibrium time), then filtered through a 0.45 µm membrane filter, and the filtrate was assayed spectrophotometrically at λ max 280 nm. The results are the mean values of three determinations ± standard deviation.
Preparation of Etodolac Co-evaporates
Co-evaporate of ETD with the selected hydrophilic carriers (PEG 4000, PVP K25, PVP K90 and urea) or solid inert carriers (Avicel PH 101, Aerosil 200 and Bentonite)were prepared at (1:1), (1:3) and (1:5) drug: carrier ratios using solvent evaporation technique. The calculated amount of each carrier was added to the ethanolic solution of ETD to give the desired drug/carrier ratios and the mixture was stirred for 30 minutes. The solvent was allowed to evaporate at room temperature and the residue was kept for 24 h in a desiccator containing anhydrous calcium chloride at room temperature. The resultant solid co-evaporate was scraped out, crushed and passed through sieve No. 60 (250 μm pore size) before packing in a tightly closed container [33]. The fraction of particle size range (125-250) µm was collected and used in the experimental studies.
Preparation of Physical Mixtures
Physical mixtures of ETD and the investigated carriers corresponding to co-evaporate were prepared by simple mixing using mortar and pestle.
Characterization of the Prepared Etodolac Co-evaporate Systems
Measurement of Drug Content. Known amounts of the prepared mixtures were dissolved in ethanol and then the drug concentration was evaluated spectrophotometrically at 280 nm. The drug content was calculated for each sample by using the following formula [34]:
% 𝒐𝒇 𝒅𝒓𝒖𝒈 𝒄𝒐𝒏𝒕𝒆𝒏𝒕 = 𝑨𝒄𝒕𝒖𝒂𝒍 𝒂𝒎𝒐𝒖𝒏𝒕 𝒐𝒇 𝒕𝒉𝒆 𝒅𝒓𝒖𝒈 𝒊𝒏 𝒕𝒉𝒆 𝒇𝒐𝒓𝒎𝒖𝒍𝒂/𝑻𝒉𝒆𝒓𝒐𝒕𝒊𝒄𝒂𝒍 𝒂𝒎𝒐𝒖𝒏𝒕 𝒐𝒇 𝒕𝒉𝒆 𝒅𝒓𝒖𝒈 𝒊𝒏 𝒕𝒉𝒆 𝒇𝒐𝒓𝒎𝒖𝒍𝒂 𝑿 𝟏𝟎𝟎
In vitro Dissolution Studies
Dissolution studies were carried out in triplicate using 6 paddles Hanson dissolution tester (Hanson Research Co., USA) for co-evaporates, physical mixtures and unmodified ETD. The dissolution test was performed at 37±0.5°C using the paddle method at 50 rpm. Experiments were run with certain USP modifications, whereby samples equivalent to 20 mg of ETD were placed in the dissolution medium (500 ml distilled water). The dissolution profiles were constructed from samples of 5 ml withdrawn after different time intervals and immediately followed by addition of an equal volume of fresh dissolution medium maintained at same temperature, to keep the volume of dissolution media constant [35]. The withdrawn samples were filtered through a membrane filter (0.45 µm), and the corresponding concentrations of ETD were analyzed spectrophotometrically at 280 nm. All results are the average of three measurements ± SD.
Differential Ultraviolet Absorption Study
This study was carried out in order to indicate the presence of any interference which may be raised from the investigated carriers on the maximum absorbance of the drug in the used dilution range [36]. So, A 1% solution of each polymer in distilled water was scanned in the presence and absence of the drug using distilled water as a blank.
Infrared Spectroscopy (IR)
The IR spectra of pure drug, carriers, physical mixtures and different prepared system with different carriers were measured using Shimadzu IR-476 spectrophotometer (Japan) at a range of 4000-400 cm-1 using KBr disk method. The samples were mixed with KBr and compressed into discs using IR compression machine [37].
Differential Scanning Calorimetry (DSC)
DSC analysis was performed using Shimadzu-Thermal analyzer DSC-T50 (Japan) calibrated with indium. The temperature range for the thermogram was 30 to 200°C, and the samples were heated at rate of 10°C/min [38]. Thermal analysis was carried out using TA 50 PC system with Shimadzu software program.
X-ray Diffraction (XRD) Studies
The powder X-Ray diffraction measurement was carried out using Philips PW1710 X-Ray diffractometer, USA. Typically, the investigated samples were irradiated by mono-chromatised Cu-Kα radiation with copper X-ray source (λ = 1.5406 Å) at 40 mA and 40 KV. Then, the obtained XRD patterns were collected over the 2θ range of 4-60° at a scan rate of 0.06°/sec [38].
Results and Discussion
Solubility Studies
With an aqueous solubility of 69 mg/L (at 25°C), ETD is clearly considered as a poor water soluble drug. The results showed that the apparent solubility of ETD increased with the increaseof either temperature or carrier concentration. At the highest concentration (10% w/v), PVP K25 exhibited the highest value of solubilized drug [397.4 mg/l (5.8-fold)]. While as, urea showed a 3-fold increase in the solubility among the other investigated carriers at 37°C. The increase in ETD solubility by the investigated carriers was ranked in the following descending order: PVP K25 > PVP K90 > PEG 4000 > Urea (Figure 1).
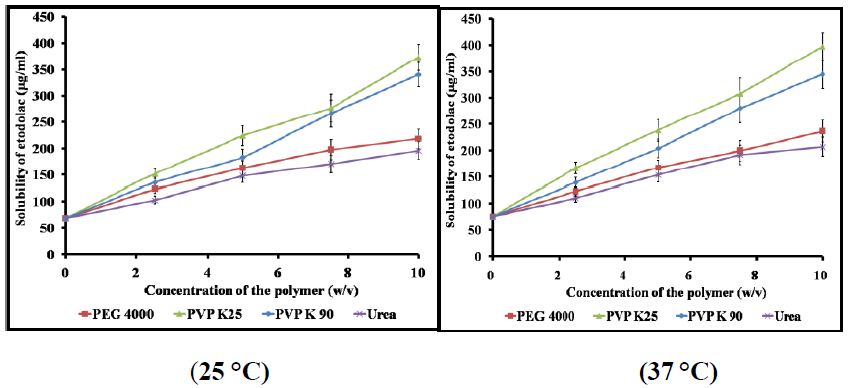
Figure 1: Aqueous solubility of ETD in the presence of different concentrations of the selected hydrophilic carriers at 25°C and 37°C.
Characterization of the Prepared ETD Solid Co-evaporate Systems
Differential Ultraviolet Absorption Study. It was found that the carriers used in this study showed no absorbance at the specified λ max of the drug (280 nm). By other words, the scanning of the drug in the presence of different carriers revealed that there is no change in neither the peak positions nor absorbance values of the drug, indication that there is no interference in measurement upon using such polymers.
Drug Content Measurement
The drug content for all the prepared systems was estimated using the following equation [39]; Conc. = Abs. x P.C x d.f (Where P.C is the procedural constant and d.f is the dilution factor). The obtained results showed that all the formulations were found to have drug contents in the range of 95.1-104.8% which are considered for further experiments.
In vitro Dissolution Studies
Generally, the rate of dissolution of pure ETD is very low when compared with that of the prepared systems and the rate of dissolution of ETD varied with the nature of the carrier used. More specifically, the results, illustrated in Figure 2, showed that co-evaporate of ETD/polymer exhibited a higher dissolution rate than the corresponding physical mixture at the same drug polymer ratio. Collectively, it could be concluded that the dissolution rate of ETD from either its co-evaporates or physical mixtures was arranged in a descending order as follows: (PVP K90 < PEG 4000 < PVP K25 < urea). The observed increase in the dissolution of ETD from different solid co-evaporates, compared to unmodified drug, could be attributed to improved wettability, dispersability, local solubilization, drug particles size reduction or formation of high energy amorphous phase [40]. Similarly, the adsorbates showed higher dissolution rate than the corresponding physical mixtures and the unmodified drug as illustrated in Figure 3.
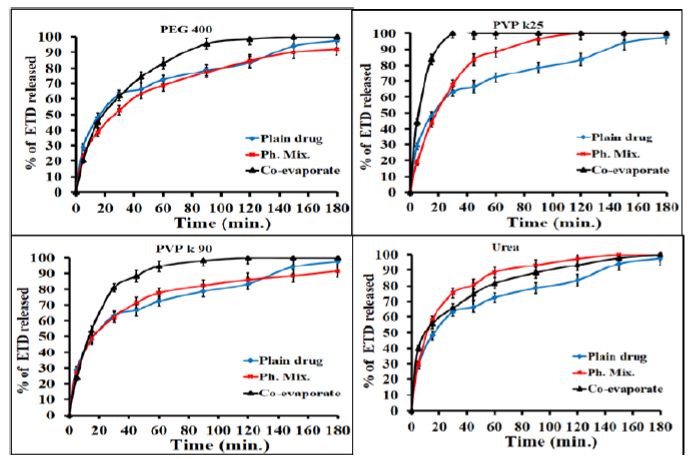
Figure 2: Dissolution profiles of ETD co-evaporates and physical mixture with PEG 4000, PVP K25, PVP K90 and urea at (1:1) w/v drug/polymer ratio.
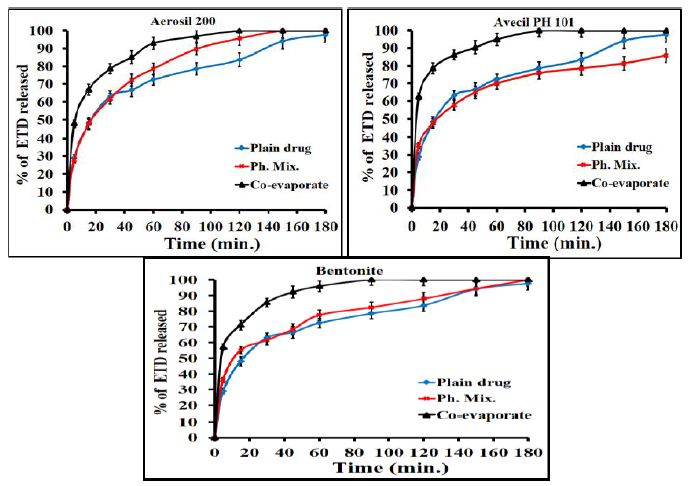
Figure 3: Dissolution profiles of ETD adsorbate (co-evaporates) and physical mixture with Avecil PH101 and aerosil 200 and bentonite at (1:1) w/v drug/carrier ratio.
The effect of polymeric ratio on drug dissolution was studied and illustrated in figure. The results showed that the increase of polymer concentration led to an increase in the dissolution rate of drug since the effect is very clear in the case of co-evaporates compared to the physical mixtures. For example PVP K90 at (1:1), (1:3) and (1:5) w/w ratios exhibited faster dissolution of about 53.8%, 95.8% and 100%, respectively after 15 minutes as compared with 12.6% of pure drug (Figure 4). Regarding the adsorbate, illustrated in Figure 5, Avecil PH 101 showed dissolution rate of about 72.7, 78.5 and 100% after 15 min. corresponding to the ratios (1:1), (1:3) and (1:5) w/w ratios. In addition, the results, illustrated in Figure 6, showed that the dissolution rate of drug adsorbate with avecil PH 101 at (1:1) ratio was higher than that of drug PVP K90 solid dispersion at the same ratio.
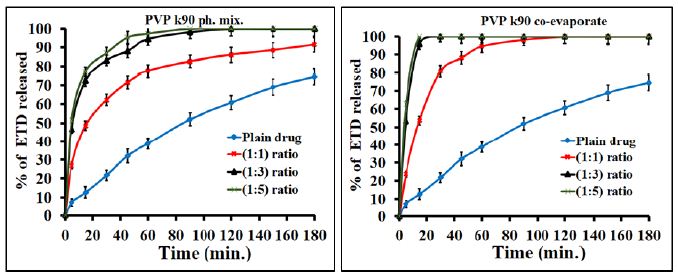
Figure 4: Dissolution profiles of ETD co-evaporates and physical mixture with PEG PVP K90 as a function of polymer concentration.
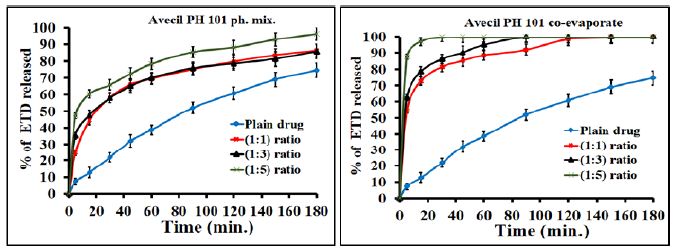
Figure 5: Dissolution profiles of ETD adsorbate (co-evaporates) and physical mixture with Avecil 101 as a function of polymer concentration.
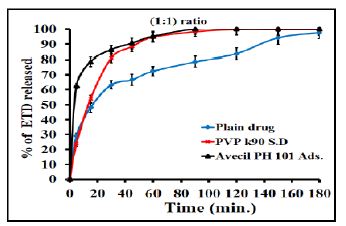
Figure 6: Dissolution profiles of ETD co-evaporates with PVP k90 (solid dispersion) and Avecil 101 (adsorbate) at (1:1) w/v drug/carrier ratio.
Regarding the effect of temperature on the drug solubility, the results indicated that the increase of temperature led to increase of the drug solubility (Table 1). The obtained results may be attributed to that the increase of temperature led to decrease of the intermolecular forces.
Table 1: Solubility of etodolac in the presence of different concentrations of the selected hydrophilic carriers at 25°C and 37°C.
|
Carrier conc. (%w/v) |
Solubility of etodolac (µg/ml) |
|||||||
|
PEG 4000 |
PVP K25 | PVP K90 |
Urea |
|||||
| 25°C | 37°C | 25°C | 37°C | 25°C | 37°C | 25°C |
37°C |
|
|
0 |
68.97 ± 4.3 | 75.96 ± 2.5 | 68.97 ± 4.3 | 75.96 ± 2.5 | 68.97 ± 4.3 | 75.96 ± 2.5 | 68.97 ± 4.3 | 75.96 ± 2.5 |
| 2.5 | 123.58 ± 3.7 | 122.75 ± 3.4 | 151.52 ± 3.7 | 166.22 ± 5.5 | 135.79 ± 3.7 | 138.9 ± 3.9 | 103.29 ± 4.3 | 110.12 ± 2.6 |
|
5 |
162.5 ± 4.6 | 166.64 ± 3.9 | 223.97 ± 3.8 | 238.46 ± 5.6 | 182.16 ± 4.5 | 203.27 ± 3.2 | 147.38 ± 5.4 | 153.8 ± 4.3 |
| 7.5 | 197.48 ± 6.3 | 199.13 ± 4.8 | 276.55 ± 4.1 | 308.02 ± 6.4 | 266.2 ± 4.7 | 280.28 ± 4.1 | 170.15 ± 3.9 |
191.27 ± 4.5 |
|
10 |
218.18 ± 5.8 | 236.39 ± 4.9 | 373.01 ± 5.3 | 397.44 ± 7.4 | 340.31 ± 5.2 | 344.66 ± 4.8 | 195.82 ± 6.2 |
207 ± 4.9 |
IR Spectroscopy
The IR spectra were recorded to illustrate the possible interaction between the drug and the selected carriers in the solid state. The IR spectrum of the drug, illustrated in Figures 7 and 8, band A, showed characteristic bands at wave number 1746 cm-1 which is corresponding to (C=O) stretching vibration of the carboxylic group, 3344 cm-1 due to single -NH stretching vibration of amine group and 2971 cm-1 for C-H stretching. Moreover, ETD which is present as ether form showed other characteristic bands corresponding to the C–O stretching vibration at 1034 cm-1. These data are in a good accordance with those reported previously [41-43]. Especially the two bands of (C=O) and (-NH) stretching will be highlighted in the present study in order to determine the possibility of interaction of ETD with the selected carriers. Also, the results showed no significant difference in the positions of the absorption bands indicating no marked chemical interaction between ETD and the tested polymer in the solid co-evaporate at the selected drug to polymer ratios. However, the spectra of PVP K25 and PVP K90 solid co-evaporates showed marked shifting of C-H stretching band from 2971 cm–1 for plain drug to 2954 cm–1. Furthermore, an intensive broad band was observed at 3417 cm-1 and 3428 cm–1 for PVP K25 and PVP K90 solid co-evaporates, respectively, which may be attributed to the presence of moisture in the PVP molecule [44] (Figure 7, band B). The most important finding, regarding IR spectra of PVPs co-evaporate, is the complete disappearance of carbonyl group (C=O) absorption band. This result was attributed to the existence of higher polymer concentration (1:3 for PVP K25 and 1:5 for PVP K90) which leads to overlapping of a broad peak of the polymer on the carbonyl group of ETD which present at the same region (Figure 7, band D).
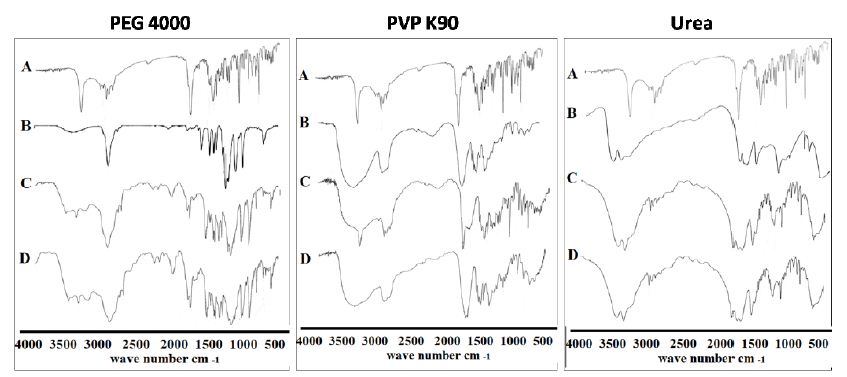
Figure 7: The IR absorption spectra of ETD co-evaporate and physical mixture with different hydrophilic carriers where: (A) Plain drug, (B) Hydrophilic carrier, (C) Physical mixture and (D) Co-evaporate.
Similarly, the IR spectra of ETD adsorbate systems with different adsorbents were illustrated in Figure 8. The IR apectra of Avicel PH 101 showed major broad peak at 3409 cm−1, corresponding to (-OH) stretching (Figure 8, trace B). The spectra of physical mixtures should, ideally, be equivalent to the addition spectrum of drug and adsorbent (Figure 8, trace C). In the case of ETD/Avicel PH 101 adsorbate spectra, the bands corresponding to (C=O) stretching and (-NH) stretching of the drug are disappeared (Figure 8, trace D). This may be due to hydrogen bonding between these functional groups of drug and the hydroxyl groups of Avicel PH 101 [45]. This finding, also, indicates the adsorption of the drug molecules on the surface of Avicel PH 101 which has high adsorption capacity due to its large surface area. With respect to the IR absorption spectra of ETD/Aerosil 200 systems in (1:1) ratio, the presence of a broad prominent peak at 1107 cm-1 corresponding to strong (Si-O) linkage is characteristic to Aerosil 200. The characteristic peaks of the drug in both adsorbate and physical mixture are still present at the same position as plain drug but with an apparent reduction in the intensity, confirming the absence of any suspected interaction between the drug and Aerosil 200. Regarding the IR absorption spectrum of ETD/Bentonite system which is characterized by complete absence of infrared absorption bands of bentonite due to its inorganic nature (aluminum phyllosilicate), the drug bands are still present at the same position but with low intensity.
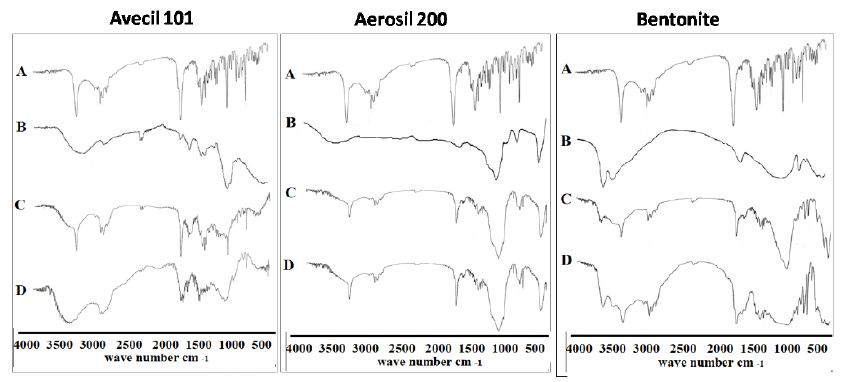
Figure 8: The IR absorption spectra of ETD adsorbate and physical mixture with different adsorbents where: (A) Plain drug, (B) Hydrophilic carrier, (C) Physical mixture and (D) Co-evaporate.
Differential Scanning Calorimetry (DSC)
The DSC thermograms of the drug before and after modification were illustrated in Figure 9. Regarding the untreated drug (Figure 9, band A), the results showed an endothermic peak at 152.3°C, corresponding to the melting point of the drug. The observed sharp melting endotherm confirms the crystallinity of the drug [43]. Also, PEG 4000 showed a single endothermic peak at 58.2°C, corresponding to its melting point (Figure 9, band B). On the other hand, physical mixture (Figure 9, band C) and solid co-evaporate (Figure 9, band D) of ETD with PEG 4000, showed complete disappearance of endothermic peak of the drug and the appearance of new endothermic peaks at both 58.9°C and 61.3°C for solid co-evaporate and physical mixture, respectively. The disappearance of ETD peak in case of physical mixture could be explained on the basis that the drug was dissolved in the molten polymer [46]. However, the complete disappearance of the endothermic peak of the ETD in its solid co-evaporate with PEG 4000 may be, also, attributed to the formation of the amorphous form of the drug [47]. Regarding to DSC thermogram of ETD/PVPs system, it was clear that PVP band showed a shallow, broad endothermic peak ranging from 80 to 120°C due to the presence of residual moisture contents in PVP which is in agreement with the previously obtained results [48]. Physical mixture of ETD/PVPs showed a marked reduction of the drug which is accompanied with a partial shift to lower melting points, 125.9°C in the case of PVP K25 and 102.9°C in the case of PVP K90. This result indicated that the drug still present in crystalline form, however the lower melting point may indicate a formation of different crystalline polymorph at higher temperature. On the other hand, solid co-evaporate of ETD binary systems with both PVP K25 and PVP K90 showed the complete disappearance of drug characteristic endothermic peak and, only, the characteristic peak of the polymer still present. This finding indicated the conversion of the drug to amorphous state. The obtained results and interpretations were in a good accordance with those obtained previously [44,48,49]. Also, Figure 9 showed the DSC thermograms of ETD solid co-evaporate and physical mixture with urea. The characteristic peak of ETD is still present but shifted to a lower temperature, 131.7°C and 132.5°C for solid co-evaporate and physical mixture, respectively. This finding may be attributed to the existence of crystalline nature of urea and its higher melting point 134°C which is in agreement with the previously obtained finding [50]. Regarding the DSC thermograms of ETD adsorbate systems with different adsorbents, Figure 9 illustrated the thermograms of drug/Avicel PH 101 in (1:1) ratio adsorbate, physical mixture and the individual components. The DSC of Avicel PH 101 exhibited a shallow broad endothermic peak at about 85 ℃, which might correspond to the volatilization of adsorbed water [45]. In case of ETD/Avicel PH 101 adsorbate, the melting endothermic peak of the drug was completely disappeared indicating transformation of the drug to amorphous state. On the other hand, thermogram of the corresponding physical mixture revealed the crystallinity of the drug. Regarding to DSC of pure Aerosil 200, it did not show any peaks in the thermogram proving that this adsorbent was almost in an amorphous state. Also, it was clear that the endothermic peak of the drug still presents in both physical mixture and loaded adsorbate. These results indicate a weak or no interaction between drug and adsorbent. Finally, DSC thermograms of ETD/Bentonite (1:1) ratio prepared systems and individual components were illustrated, also, in Figure 10. The endothermic peak of the drug still present in both physical mixture and adsorbate with the apparent shifting to the lower melting temperature in the case of physical mixture.
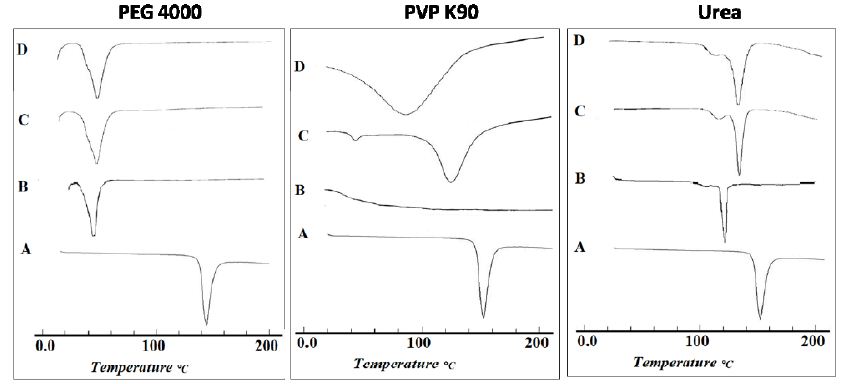
Figure 9: DSC thermograms of ETD co-evaporate with different hydrophilic carriers where: (A) Plain drug, (B) Hydrophilic carrier, (C) Physical mixture and (D) Co-evaporate.
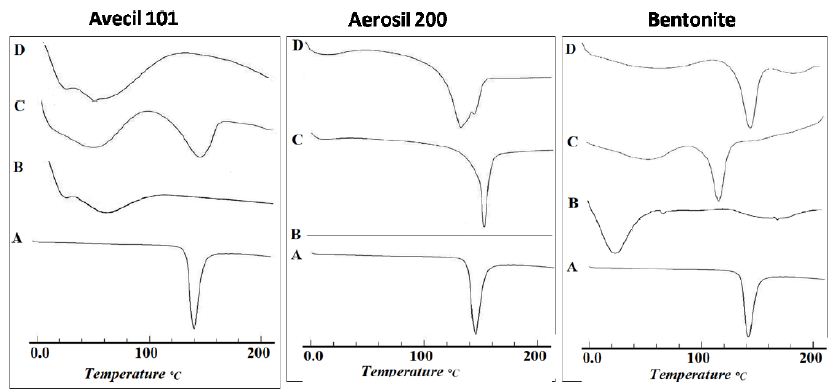
Figure 10: DSC thermograms of ETD adsorbate and physical mixture with different adsorbents where: (A) Plain drug, (B) Hydrophilic carrier, (C) Physical mixture and (D) Co-evaporate.
X-ray Diffraction Analysis (XRD)
In an attempt to get further evidence on the solid state changes, x-ray diffraction spectra were carried out on drug alone, carrier alone, ETD/carrier binary systems (co-evaporates and corresponding physical mixtures) which was illustrated in Figure 11. The results showed the presence of numerous distinct peaks in the x-ray diffraction spectrum of ETD at diffraction angles of 2θ at 9.16°, 13.6°, 14.38°, 18.58°, 22.9° and 27.28° with relative intensities of 44, 54, 100, 35, 65 and 30, respectively, indicating the crystalline nature of the drug (Figure 11, band A). In contrast, the X-ray diffraction spectrum of PVP K90 showed no diffraction peaks, indicating the existence of the polymer in an amorphous state. The XRD pattern of ETD/PVP K90 physical mixture showed the absence of some diffraction peaks of pure drug, indicating the partial crystallinity of ETD in physical mixture. This finding may be attributed to the dilution factor of high polymer ratio (Figure 11, and C). On the other hand, no diffraction peak was observed in the case of the corresponding solid co-evaporate indicating the complete conversion of ETD to amorphous form (Figure 11, and D). The obtained results and interpretations were in a good agreement with the previously reported data [44]. Similar results were obtained in the case of ETD Avicel-PH 101. The X-ray diffraction spectrum of Avicel showed no diffraction peaks, indicating the existence of the carrier in an amorphous state (band B). The characteristic peaks of ETD are clearly noticed in physical mixture of ETD and Avicel PH 101 indicating the crystallinity of the drug in the physical mixture (band C). The phase transformation of crystalline ETD to the amorphous form in the case of adsorbate system with Avicel PH 101 (band D) was explained by adsorption of the drug within the pores of the carrier matrix. Adsorption may take place via Vander Waals forces or through formation of hydrogen bonds between the (-NH) or (=CO) groups of the drug and (-OH) group of the carrier as confirmed by IR results [51].
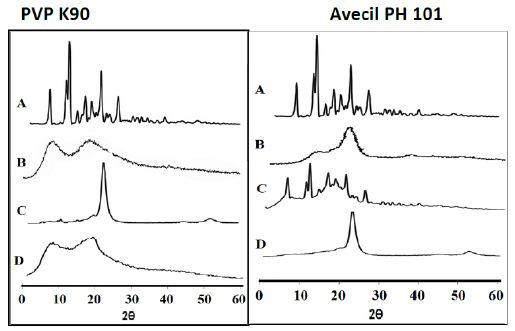
Figure 11: The x-ray powder diffraction patterns of ETD co-evaporate with PVP K90 and Avecil PH 101 where: (A) Plain drug, (B) Hydrophilic carrier, (C) Physical mixture and (D) Co-evaporate.
Conclusion
The solid dispersion and adsorbates of ETD were prepared by co-evaporation method and characterized by IR, DSC and X-ray. The solubility of ETD was enhanced markedly in the presence of different investigated polymers since PVPk90 and Avicel PH 101 exhibited the highest effect. Generally, all co-evaporates showed higher dissolution rate than the corresponding physical mixtures. However, both co-evaporate and physical mixtures exhibited higher dissolution rates than the unmodified ETD. The increase in polymer concentration led to an increase in the drug dissolution rate. The enhancement of drug solubility and dissolution rate was attributed to the reduction of the particle size, enhanced wettability and the conversion of drug from crystalline form to amorphous one as proved by XRD and DSC analysis.
Declaration of Interest
The authors report no conflicts of interest in this work.
References
- Guo Q, Qiang G, Yuxiang W, Dan Xu, Johannes N, Nathan J, et al., (2018) Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Research 6: 1-14.
- Pareek, A, Nitin Chandurkar,Tenpe SR, Yeole PG (2014) research article Evaluation of anti-inflammatory activity of etodolac and colchicine combination in experimental animals. International Journal of Clinical Pharmacology and Therapeutics.
- Harirforoosh S, W Asghar, F Jamali (2013) Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. Journal of Pharmacy & Pharmaceutical Sciences 16: 821-847.
- Khaleel NY, et al., (2011) Solubility and dissolution improvement of Ketoprofen by solid dispersion in polymer and surfactant using solvent evaporation method. International Journal of Pharmacy and Pharmaceutical Sciences 3: 431-435.
- Buckley ST, Kerstin JF, Gert F, Martin B, et al., (2013) Biopharmaceutical classification of poorly soluble drugs with respect to “enabling formulations”. European Journal of Pharmaceutical Sciences 50: 8-16. [crossref]
- Chaudhary V, Patel J (2013) Cyclodextrin inclusion complex to enhance solubility of poorly water soluble drugs: A review. International Journal of Pharmaceutical Sciences and Research 4.
- Rasenack N, Müller BW (2002) Dissolution rate enhancement by in situ micronization of poorly water-soluble drugs. Pharmaceutical Research 19: 1894-1900. [crossref]
- Chiou WL, Chen SJ, Athanikar N (1976) Enhancement of dissolution rates of poorly water‐soluble drugs by crystallization in aqueous surfactant solutions I: Sulfathiazole, prednisone, and chloramphenicol. Journal of Pharmaceutical Sciences 65: 1702-1704. [crossref]
- Otsuka M, Ofusa T, Matsuda Y (1998) Dissolution improvement of water-insoluble glybuzole by co-grinding and co-melting with surfactants and their physicochemical properties. Colloids and Surfaces B: Biointerfaces. 10: 217-226.
- Rascioni R (2016) Effect of particle size reduction and crystalline form on dissolution behaviour of nimesulide. Journal of Thermal Analysis and Calorimetry 123: 2213-2223.
- Dhore PW, Vivek SD, Suprit DS, Yamini SB, Connor Mack, et al. (2017) Enhancement of the aqueous solubility and permeability of a poorly water soluble drug ritonavir via lyophilized milk-based solid dispersions. Pharmaceutical Development and Technology. 22: 90-102. [crossref]
- Chokshi RJ, Hossein Z, Harpreet KS, Navnit HS, Waseem AM et al., (2007) Improving the dissolution rate of poorly water soluble drug by solid dispersion and solid solution—pros and cons. Drug Delivery 14: 33-45. [crossref]
- Friedrich H, Bernd F, Karl K, Roland B et al., (2006) Dissolution rate improvement of poorly water-soluble drugs obtained by adsorbing solutions of drugs in hydrophilic solvents onto high surface area carriers. European Journal of Pharmaceutics and Biopharmaceutics 62: 171-177. [crossref]
- Sharma D (2016) Solubility enhancement strategies for poorly water-soluble drugs in solid dispersions: A review. Asian Journal of Pharmaceutics (AJP): Free full text articles from Asian J Pharm 1.
- Das SK, Abha Doshi, Bhagyashri Joshi, Vandana Wankhede, Jesal Doshi, et al., (2012) Solid dispersions: an approach to enhance the bioavailability of poorly water-soluble drugs. International Journal of Pharmacology and Pharmaceutical Technology. 1: 37-46.
- Zayed G (2014) Dissolution Rate Enhancement of Ketoprofen by Surface Solid Dispersion with Colloidal Silicon Dioxide. Unique J. Pharm. and Bio. Sci 1: 33-38.
- Biswal S, J Sahoo, PN Murthy, RP Giradkar, JG Avari (2008) Enhancement of dissolution rate of gliclazide using solid dispersions with polyethylene glycol 6000. AAPS PharmSciTech 9: 563-570. [crossref]
- Balata G, M Mahdi, Rania AB (2011) Bakera, Improvement of solubility and dissolution properties of clotrimazole by solid dispersions and inclusion complexes. Indian Journal of Pharmaceutical Sciences. 73. [crossref]
- Xie T, L.S. Taylor (2016) Improved release of celecoxib from high drug loading amorphous solid dispersions formulated with polyacrylic acid and cellulose derivatives. Molecular Pharmaceutics 13: 873-884.
- Kuharski RA, Rossky PJ (1984) Solvation of hydrophobic species in aqueous urea solution: a molecular dynamics study. Journal of the American Chemical Society. 106: 5794-5800.
- Saha R, et al., (2002) Solubility enhancement of nimesulide and ibuprofen by solid dispersion technique. Indian Journal of Pharmaceutical Sciences 64.
- Lima,ÁA, et al. (2011) The use of solid dispersion systems in hydrophilic carriers to increase benznidazole solubility. Journal of Pharmaceutical Sciences 100: 2443-2451.
- Thenmozhi K, Yoo YJ (2017) Enhanced solubility of piperine using hydrophilic carrier-based potent solid dispersion systems. Drug Development and Industrial Pharmacy 43: 1501-1509. [crossref]
- Waghmare A, Pore Y, Kuchekar B (2008), Development and characterization of zaleplon solid dispersion systems: a technical note. Aaps Pharmscitech 9: 536-543.
- Law S, Chiang C (1990) Improving Dissolution Rates of Griseofulivin by Deposition on Disintegrants. Drug Development and Industrial Pharmacy. 16: 137-147.
- Ismail A, K Saleh, S Khalaf (2004) Preparation and evaluation of naproxen adsorbate as an attempt to improve drug dissolution characteristics. Drugs 9.
- Chella N, N Shastri, RR Tadikonda (2012) Use of the liquisolid compact technique for improvement of the dissolution rate of valsartan. Acta Pharmaceutica Sinica B 2: 502-508.
- Qian KK, RH Bogner (2012) Application of mesoporous silicon dioxide and silicate in oral amorphous drug delivery systems. Journal of Pharmaceutical Sciences 101: 444-463.
- Srikanth M et al., (2013) Dissolution rate enhancement of bicalutamide by adsorption process. African Journal of Pharmacy and Pharmacology 7: 1357-1362.
- Putra, E.K., Ramon P, Jaka S, Nani I, Suryadi I et al., (2009) Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Research 43: 2419-2430.
- Leuner C, Dressman J (2000) Improving drug solubility for oral delivery using solid dispersions. European Journal of Pharmaceutics and Biopharmaceutics 50: 47-60. [crossref]
- Higuchi T, Connors K (1965) Phase solubility diagram. Adv. Anal. Chem. Instrum,. 4: p. 117-212.
- S Singh , RS Baghel, L Yadav, (2011) A review on solid dispersion. International Journal of Pharmacy & Life Sciences 2.
- Sharma A, Jain C (2010) Preparation and characterization of solid dispersions of carvedilol with PVP K30. Research in Pharmaceutical Sciences 5.
- Kumar SGV, Mishra DN (2006) Preparation, characterization and in vitro dissolution studies of solid dispersion of Meloxicam with PEG 60001. Yakugaku Zasshi 126: 657-664.
- Everaerts M, Van den Mooter G (2019) Complex amorphous solid dispersions based on poly (2-hydroxyethyl methacrylate): Study of drug release from a hydrophilic insoluble polymeric carrier in the presence and absence of a porosity increasing agent. International Journal of Pharmaceutics 566: 77-88.
- Ibrahim M, et al., (2010) Formulation and evaluation of ketorolac tromethamine-Eudragit solid dispersions of potential sustained-release properties. STP Pharma Pratiques 20.
- Ismail , El-Mahdy M, Al-Kubati S (2009) Enhancement of solubility and dissolution of nimesulide using solubilization, solid dispersion and complexation techniques. Bulletin of Pharmaceutical Sciences. Assiut 32: 321-338.
- Swinehart D (1962) The beer-lambert law. Journal of Chemical Education 39.
- Bobe K, Cr Subrahmanya, Sarasija Suresh, Dinanath Gaikwad, et al., (2011) Formulation and evaluation of solid dispersion of Atorvatstatin with various carriers. Int J Comp Pharm 1: 1-6.
- Dwivedi A, Misra N (2010) Quantum chemical study of Etodolac (Lodine). Der Pharma Chemica,. 2: 58-65.
- Al-Shammary F, Mian N, Mian M (1992) Analytical Profiles of Drug Substances and Excipients. Ed. Brittain HG Academic Press Inc. USA,.
- Abdelbary A, et al., (2013) Influence of Various Polymers on the Improvement of Etodolac Solubility and Dissolution Rate via. Solid Dispersion Technique. Inventi Impact: Pharm Tech.
- Ismail S, El-Mahdy M, Al-Kubati S (2009) Enhancement of solubility and dissolution of nimesulide using solubilization, solid dispersion and complexation techniques. Pharm. Sci 32: 321-338.
- Ambike AA, Mahadik K, Paradkar A (2005) Spray-dried amorphous solid dispersions of simvastatin, a low T g drug: in vitro and in vivo evaluations. Pharmaceutical Research 22: 990-998.
- Moseson DE, Andrew SP, Christopher JG, Andrew AS, Stephen PB et al., (2019) Dissolution of indomethacin crystals into a polymer melt: Role of diffusion and fragmentation. Crystal Growth & Design 19: 3315-3328.
- Patil MP, Gaikwad NJ (2009) Preparation and characterization of gliclazide-polyethylene glycol 4000 solid dispersions. Acta Pharmaceutica 59: 57-65.
- Wu K, Jing L, Wayne W, Denita AW (2009) Formation and characterization of solid dispersions of piroxicam and polyvinylpyrrolidone using spray drying and precipitation with compressed antisolvent. Journal of Pharmaceutical Sciences 98: 2422-2431. [crossref]
- Yoshioka M, Hancock BC, Zografi G (1995) Inhibition of indomethacin crystallization in poly (vinylpyrrolidone) coprecipitates. Journal of Pharmaceutical Sciences 84: 983-986. [crossref]
- Chiou WL, Niazi S (1971) Phase diagram and dissolution‐rate studies on sulfathiazole‐urea solid dispersions. Journal of Pharmaceutical Sciences 60: 1333-1338. [crossref]
- Li Y, Huishi P, Zhefei G, Ling L, Yixuan D, et al., (2014) Interactions between drugs and polymers influencing hot melt extrusion. Journal of Pharmacy and Pharmacology 66: 148-166. [crossref]