Abstract
Shale, a significant rock formation with potential as a source and reservoir rock, presents challenges in drilling due to its unique chemical composition and mechanical properties. To address the instability caused by shale mineralogy, various shale inhibitors are used as drilling fluid additives, interacting with clay minerals to stabilize the clay by neutralizing surface charges. While numerous research groups have extensively studied shale stabilization, clay chemistry, and novel inhibitors, there remains a gap in comprehensive studies that correlate evidence of shale instability from recent literature with clay mineralogy and Zeta potential. This review article fills this gap by presenting pioneering work and recent evidence from literature to provide a clear rationale behind the role of clay mineralogy in shale instability. Notably, a novel correlation has been developed that predicts clay swelling based on Zeta potential for sodium bentonite clay with a smectite percentage ranging from 70-90%. This review work serves as a foundation for future researchers in selecting appropriate shale samples for their shale stabilization studies and estimating clay swelling based on Zeta potential. Ultimately, it presents a more nuanced understanding of the mechanism of shale swelling inhibition, contributing to the advancement of knowledge in this field with a cerebral approach.
Keywords
Clay mineralogy; Shale instability; Shale swelling; Shale hydration
Introduction
Shale is the most abundant clastic sedimentary rock roughly accounting for 70 % of the sedimentary rock type in the Earth’s crust [1]. Shale is a laminated and fissile rock comprising of clay and silt size particles ranging from 0.02 – 0.05 mm in diameter [2,3]. Shales are often located with the layers of limestone or sandstone and typically generate in the environment where silts, muds and other sediments were deposited by the mellow currents followed by the compaction e.g., basins of shallow oceans, payas, river flood plans and floor of deep-oceans [4,5]. The permeability and porosity of shale rocks depend upon the size of the constituent grain particles while their colour is mainly attributed by their composition e.g., the higher the organic content of the clay, the darker will be its colour; purple and reddish colour is due to the existence of hematite and limonite, blackish, brown, and blue hues correspond to the presence of iron ores while calcareous shale are yellowish or light grey [6,7]. Organic shales containing kerogens (a complicated mixture of hydrocarbons generated from animals and plants remains) in substantial quantity, produce oil when subjected to pyrolysis, are called oil shales [8-10]. The term shale oil is also used for the crude oil which is produced from oil bearing shale formations with low permeability and to avoid the confusion with the generic crude oil, the term ‘tight oil’ can be used for shale-oil [11]. The shale oil reservoirs are usually classified by the (Vitrinite reflectance) Ro = 0.6%-1.2%, TOC (Total organic content) > 2% and having complex mineral composition with ultra low permeability (0.001-0.0001) mD and low porosity (<5%) [12,13]. Though, shale is an important reservoir and source rock but drilling through shale formation is a havoc due to its brittle nature. Roughly 70% of the wellbore instability issues are associated with the shale dispersion, swelling and sloughing [14,15]. Wellbore instability issues may arise due to bit balling, pipe stucking, caving and lost circulation which predominantly happens due to shale swelling [16,17]. Shales can undergo through instability mainly due to its chemical composition or mechanical failure [18]. However, in this detailed review, shale instability has been related with the clay chemistry. The pieces of evidence have been collected from the recent literature which directly correlates the clay mineral content with shale instability. Moreover, this review paper also sheds lights on the pioneer work carried out which basically laid the foundation of the role of mineralogy in shale hydration. More importantly, this paper also relates the shale types with its mineralogy and ultimately with instability, paving way for the researchers to choose the suitable candidate for their shale stabilization studies.
Structure of Paper
To begin with, a concise introduction to shale has been presented, followed by an in-depth discussion of clay chemistry and its pivotal role in shale instability, drawing on pioneering research. Next, the impact of clay mineralogy on different types of shales and their resultant instability has been extensively examined. Furthermore, recent literature has been cited to demonstrate the effects of clay chemistry on shale instability. A new correlation, known as the M.H. correlation, has been developed, which establishes a link between clay instability and zeta potential. Finally, the study concludes with a summary of the key findings in the conclusion section.
Clay Chemistry
Understand the Term ‘Shale Instability’
Since, in this section clay chemistry will be discussed in context of ‘shale instability’ thus it is imperative to understand this term before moving forward. The term ‘instability’ is subjective; therefore, it is important to understand this term to make a clear narrative. In context of shale, the shale instability can be depicted by two phenomena: (1) dispersion (2) sloughing. Sloughing or swelling mainly takes place in swelling shale containing expandable clays such as smectite. Generally, cations with high valences are strongly adsorbed to the clay and thus are less prone to swelling as compared to the clay containing low valence exchangeable cations [19]. Unlike swelling, dispersion is mainly the continual disintegration of the shale which is induced when the bonding between clay layers is weakened when it hydrates [20,21]. This leads to the strength reduction of the shale formation which may collapse the wellbore [22].
Clay Mineralogy
Shale is comprised of many mineral grains which are predominantly clay-sized granules. IBorysenko (2009) presented the mineral composition for Pierre shale as: quartz (29%); kaolinite and chlorite (8%); illite, muscovite and smectite (28%); dolomite, albite, orthoclase (13%); and mica (24%) [23]. All these clay minerals can be classified based upon the configuration of alumino-phyllosilicate sheets as 1:1 or 2:1 [24,25]. A 1:1 clay consists of one octahedral and one tetrahedral sheet which are uncharged (neutral) and are bonded by hydrogen bonding e.g., kaolin group (kaolinite, nacrite, halloysite, dickite etc.) [26]. However, 2:1 configure d clay minerals are comprised of an octahedral sheet which is sandwiched between two tetrahedral sheets bonded with cations such as K+ (in illite) or Ca+/Na+ (in smectite) and overall having a negatively charged surface e.g., smectite, illite and chlorite as shown in Figure 1 [27]. Swelling clays have exchangeable cations and layers are always lacking in positive charge due to cationic substitution and thus cations in interlayer are deemed to counter-balance the negative charge in clay layers [28].
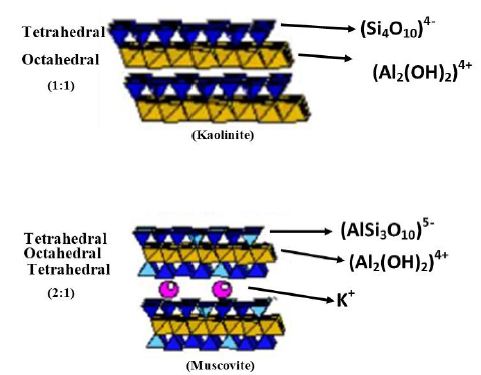
Figure 1: 1:1 and 2:1 configuration of clay mineral layers
Shale is composed of various clay minerals [29]. Among these minerals, smectite minerals and smectite/illite mixed layer are deemed to be the main reason behind clay swelling mainly due to their 2:1 configuration and cationic exchange capacity. O’Brien and Chenevert (1973) were one of the pioneers for their significant contribution in qualitatively relating shale swelling and its dispersion behaviour with its mineralogical composition. According to their qualitative study, shales rich in illite/smectite (S/I mixed layer) are more prone to dispersion and swelling. However, illite won’t alone result into sloughing, although it may result into the dispersion of the shale. They also found out that shales rich in S/I (mixed) layers are more prone to dispersion and sloughing. This is because at higher burial depth and certain conditions of temperature, illitization of smectite starts. Firstly, smectite converts to S/I (mixed) layer than to fully illite [30]. Therefore, matured shales hardly contain any smectite content. Table 1 adopted after O’Brien and Chenevert (1973) shows the relation between shale mineralogy and instability issues.
Table 1 shows that shales rich in mixed layer S/I (Smectite/illite) are more prone to dispersion and sloughing. However, shale containing no smectite originally will not undergo through illitization thus no mixed layer will be formed and eventually no prominent shale instability issues will rise [31].
Table 1: Role of clay mineralogy in shale instability
|
# |
Mineralogy |
Appearance |
Dispersion |
Sloughing |
| 1 | High smectite with little illite | Soft | High | Not observed |
| 2 | High illite with high smectite | Soft | High | Not observed |
| 3 | High S/I + illite + chlorite | Medium hard | Moderate | High |
| 4 | Moderate chlorite and illite | Hard | Little | Moderate |
| 5 | High illite + moderate chlorite | Very hard | Not observed | Not observed |
Before discussing the significant shale plays and their mineralogical composition, Table 2 summarizes the properties of various clay minerals which are usually found in the shale followed by a detailed discussion pertaining to all minerals. As a scope of this review, the discussion will be limited to Smectite, Illite, Chlorite and Kaolinite.
Table 2: Surface and chemical properties of problem causing slay minerals
|
# |
Mineral |
Layer Confg. |
Surface Charge mC/m2 [57] |
Cationic exchange capacity (meq/100g) [58] |
Basal spacing Ao [59,60] |
Chemical Formula |
Specific surface area m2/gm [61] |
||
|
Octahedral layer |
Tetrahedral Layer |
Coordination s |
|||||||
| 1 | Montmorillonite |
2:1 |
−6.03 ± 1.5 |
80-120 |
12.34 |
[(Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O] |
40-800 |
||
|
Al1.7Mg0.3 |
Si3.9Al0.1 |
O10(OH)2 |
|||||||
| 2 | Illite |
2:1 |
– |
20-40 |
10 |
(K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2·(H2O)], |
10-100 |
||
|
Al2 |
Si3.2Al0.8 |
O10(OH)2 |
|||||||
| 3 | Kaolinite |
1:1 |
−3.5 ± 1.5 |
1-10 |
10.6 |
Al2O3·2SiO2·2H2O |
5-40 |
||
|
Al2 |
Si2 |
O5(OH)4 |
|||||||
| 4 | Chlorite |
2:2 |
– |
20-40 |
14 |
(Mg,Fe)3(Si,Al)4O10(OH)2·(Mg,Fe)3(OH)6 |
10-55 |
||
|
Mg2.6Fe0.4 |
Si2.5Al |
O10(OH)2 |
|||||||
Montmorillonite is a significant member of the smectite mineral comprising silica tetrahedron and aluminum octahedral depicted in Figure 2A. The smectite group has wide spaces between base units and feeble bonding which makes it prone to swelling by intercalation of water cations into the lattice. The swelling can be reduced by substituting the counter-cations such as Ca+ and Na+. Smectite is mainly found in shallow intervals and at deep intervals it converts to illite which can also show swelling tendency.
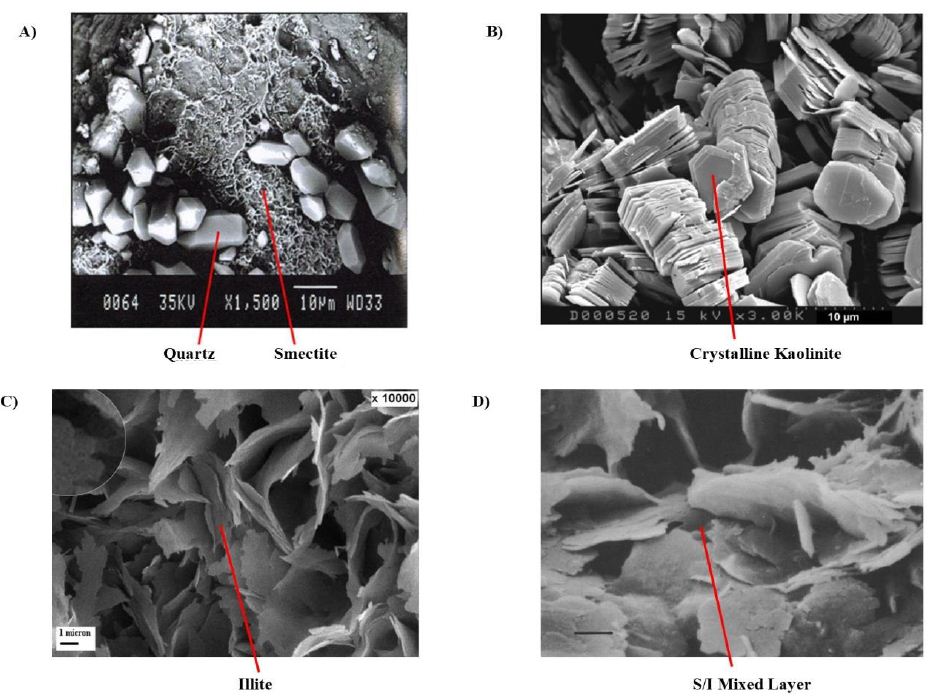
Figure 2: Scanning Electron Microscopy results of A) Smectite B) Kaolinite C) Illite [32] D) S/I mixed layer [33].
Illite has lesser tendency to swell in water even though it contains the same base unit as montmorillonite mainly because of strong electrostatic force of attraction and bonding between layers as shown in Figure 2C. Ion exchange may take place at the surface, but the volume expansion caused by this hydration is insignificant as compared to volume expansion in montmorillonite. They are formed by the weathering of muscovite and feldspar and its layers are bonded by poorly hydrated K+ ions which keeps it stable against hydration. However, the other type of illite which forms from smectite at HTHP conditions in deep interval has more tendency to swell as compared to the original version.
Chlorite and Kaolinite, unlike the other clay minerals, do not possess the hydration ability, although kaolinite shows a little dispersive behaviour. Kaolinite clays have lower swelling tendency and show poor ionic exchange capability. Shales rich in kaolinite are brittle in nature and are mainly the subject of mechanical failure in the formation. Not all shales contain every mineral which is responsible for shale instability.
But do shales really swell? It is also very important to mention here that shale doesn’t really swell in in-situ drilling conditions unless there are micro-fractures, exposed internal surface due to overburden or they are already drilled (in the form of cuttings). This above statement has always been a very important topic to debate among various research groups till now. However, the wellbore instability issues, and Non-productive time (NPT) caused by shale dispersion and its sloughing are something that everyone agrees on.
Since, from the pioneer work of O’Brien, it is established that chlorite doesn’t have much to do with the shale instability but infact these are mixed layers (S/I) and Illite which result into shale instability. Now, it is imperative to note if these types of minerals are found in major shale plays around the world. Table 3 presents a few of the major shale plays in North and Central America which depicts illite is usually the common mineral in most of major shale plays. Vermilion and Anahuac shale since is at lower burial depth thus it contains smectite while Atoka and Midway owing to the high burial depth, exhibit the process of illitization in the form of S/I (mixed layer).
Table 3: Mineralogical clay composition of major shale plays in North and Central America [34-36]
|
# |
Shale |
Smectite % |
Illite % |
S/I mixed layer % |
Chlorite % |
Kaolinite % |
| 1 | Vermilion |
25.4 |
5.5 |
– |
6.7 |
– |
| 2 | Anahuac |
40.4 |
5.5 |
– |
– |
– |
| 3 | Atoka |
– |
38.8 |
18.2 |
13 |
12 |
| 4 | Midway |
– |
35 |
15.0 |
15 |
15 |
| 5 | Wolfcamp |
– |
14.8 |
– |
3.2 |
19 |
| 6 | Canadian Hard |
– |
48.3 |
– |
8.3 |
10 |
| 7 | Barnett |
1-5% |
27 |
– |
8 |
– |
Significance of Diffused Double Layer (DDL)
Smectite contains montmorillonite which has the tendency to swell as shown in Table 1, due to weak bonding and 2:1 layer configuration which renders a negative charge on clay surface [37,38]. This negative charge is responsible for attracting water and other cations and thus leading to the swelling of the shale [39]. To understand the swelling caused by smectite mineral in shale, it is crucial to understand how the 2:1 configured negatively charged clay minerals formulate diffused double layer (DDL) or electrical double layer in first place. Clays are predominantly alumino-silicates in which silicon and aluminum ions are continuously being substituted by other cations leaving a net negative charge [21,40]. When clay particles are hydrated (or dispersed in a solution), they are surrounded by thin layer of cations (hydrosphere) from water [22]. Now, an electrical double layer will be formed which includes (i) negatively charged surface, (ii) surrounding cations (Stern layer) and (iii) a thin film of dispersing medium which contains high concentration of counter-ions as shown in Figure 3. Various shale inhibitors alter the DDL and neutralize the charge on clay surface to stabilize the clay against hydration [41,42].
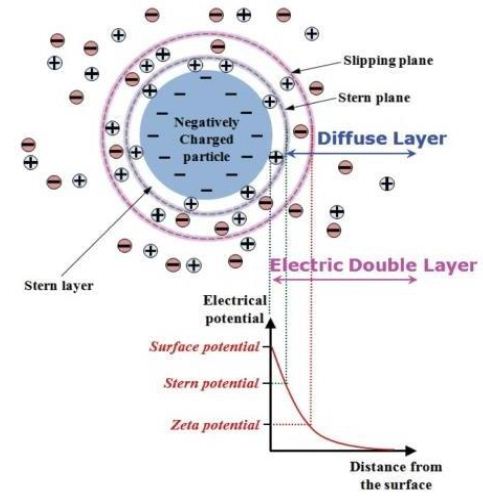
Figure 3: Components of Electrical Double Layer [43,44]
Shale Types and Clay Chemistry
Based upon shale appearance and its response to hydration it can be categorized into various categories to rationalize the swelling mechanism in a more comprehensible way [45].
Brittle Shales
Although they are quite cemented rocks, brittle shales crumble into tiny pieces when exposed to water. However, in the water, these parts don’t swell or get softer [46]. Brittle failure is brought on by: due to hydration of bedding planes and microfracture surfaces, shales first become weak, and clay then fails when surrounded by a matrix of non-swelling minerals like quartz and feldspar [47]. Brittle shales often have a high concentration of kaolinite, illite, and chlorite, all of which are unstable in high-pH surroundings [48]. The shape of cutting and bore hole instability might be severe depending upon the drilling direction (attack angle) with respect to drilling plane and the degree of rock anisotropy [49,50]. A brittle shale may act as a potential source rock for hydrocarbons, particularly gas deposits. Though, the age and brittleness of shales, which are determined by their elastic properties and mineral dominance, are the key factors that determine the success of production under these conditions. Generally, the success of hydraulic fracturing increases with increasing brittleness index [51]. The process includes creating fracture networks in the shale matrix, which would lead to the improvement in recovery factor of these extremely tight formations [52].
Swelling Shales
Although hydration (swelling) and dispersion are linked phenomena, however, the quantity and kind of clays present in the shale’s structure play a significant role in determining when they occur. Osmotic and surface (crystalline) hydrations induce swelling to occur [53,54]. Surface hydration which merely results in a small expansion brought on by the addition of a few water molecules on the surfaces of the clays, is not frequently thought to be of major relevance [55]. In this type of hydration, layers of water molecules form a quasi-crystalline structure between unit layers, increasing the c-spacing, and hydrogen bonds hold the water molecules to the oxygen atoms [56]. On the other hand, osmotic hydration is the main issue that results in the considerable expansion of clays and closure of the borehole [57]. It occurs when cation concentration between unit layers in a clay mineral is higher than those in the surrounding water. Consequently, water intercalates between the unit layers and causes the rise in c-spacing osmotically [38]. Osmotic swellings causes more swelling than caused by surface hydration, but only expandable clays like Montmorillonite can swell on exposure to hydration. Generally, most cations with high valences than those with low valences are more firmly adsorbed. Consequently, low-valence clays without exchangeable cations tend to swell less than clays with exchangeable cations possess high valences [53,58].
High Pressured Shales
Shales can produce abnormally pressured areas when: i) the pore pressure of the surrounding sandstones is slowly transferred over geological time to the shales [59]. When thick shales are compacted, the fluid cannot escape, leading to a considerable increase in pore pressure in deep intervals. ii) Any sandstone formations interbedded with or connected to the shale might produce a pressured zone if it is fully isolated [60]. When drilling through shale at abnormal pressures, shale shakers often show chipped drill cuttings. These small, thin, sharp cuttings are formed when the hydrostatic pressure of the drilling fluid is less than the pore pressure in the shale [61]. Under these circumstances, pore pressure fractures the shale downhole into a long, spalled, concave shape [59]. Hole enlargement from this type of failure would mean complete washout of the hole wall, and unlike hole failure in brittle shale, it does not only occur in certain directions [62]. In the case of stress anisotropy, more faulting could occur in certain directions, but the entire perimeter of the well will still be affected. As mentioned earlier, this failure is caused by incorrect mud weight selection and can be prevented by increasing the mud weight.
Tectonically Stressed Shales
This type of shale is often seen in regions where there is a large-scale deformation of Earth’s crust by natural processes [63]. Shales under these circumstances tend to have bed planes oriented in the direction of the applied stress. This stress, when released, will cause excessive downhole shear failure. The failure severity can increase if the cohesion of the bedding planes decreases due to adsorption of water. The type of cementation e.g. amorphous silica, aluminum or calcium silicate, or organic materials) can also play a critical role in stabilizing or disintegrating shale formations under stress [64]. Tectonically stressed shales usually have low mechanical strength, sub-compaction with a low degree of consolidation, strong tectonic stresses leading to ductile deformation and increasing pore pressure and structural pinnacle for dissolving water and hydrocarbons.
Significance of Clay Minerals in Various Shale Types for Shale Swelling
The above section briefly explains various types of shales usually encountered during exploration and drilling. Brittle shales are well cemented and consolidated, so they don’t tend to swell, however, they are prone to dispersion due to hydration because of the presence of illite and kaolinite which tend to disperse on hydration. Swelling shales usually contain illite, semectite/illite (mixed layer) formulated through illitiization, thus they are prone to swelling as well as disperse. However, in abnormally pressured and tectonically stressed shale; there may be instability due to clay mineralogy, but the main factor for shale instability in fore-mentioned shales is mechanical failure. For such type of shales, only adding shale inhibitors in drilling mud might not be the only solution but sealing the formation, controlling temperature and pressure might be other possible solutions to avoid shale from mechanical failures.
Pieces of Evidence on Clay Chemistry Linking to Shale Instability
From the recent literature, various evidences have been collected which helps in solidify the narrative which was created from the previous discussion. Though, many a research groups have studied shale stability instability but not all of them reported the clay mineralogy in their respective works thus the work summarized is not extensive, but it is enough to establish a clear narrative. Table 4 has been extracted from the available literature and thus has been used to correlate the clay minerals with the shale recovery (dispersion test) and linear swelling results.
Table 4: Pieces of evidence from literature relating shale instability and clay mineralogy
|
Author year |
Sample |
Clay Composition |
Shale recovery |
Shale swelling |
|||||
|
Smectite |
illite |
kaolinite |
Mixed layer |
Chlorite |
Mica |
||||
| [65] | 20% w/w bentonite powder |
ü |
ü |
ü |
x |
x |
x |
84.3% |
x |
| [66] | Tanuma shale |
x |
ü |
ü |
x |
x |
x |
76.4% |
5% |
| [67] | Agbada shale A |
ü?2.90% |
ü 14.90% |
ü 10.10% |
ü?19.30% |
x |
x |
38% |
35 |
| Agbada Shale b B |
x |
ü?17.10 |
ü?6.40 |
ü?20.10 |
x |
x |
39% |
42 |
|
| [68] | Taikang shale |
x |
ü?19.01 |
ü?14.15 |
ü?66.64 |
x |
x |
12% |
54% |
| [69] | shale |
x |
ü?12 |
ü?3 |
ü?82 |
x |
x |
50.4% |
55 |
| [70] | Shizhu shale |
x |
ü?8.47 |
x |
ü?1.14 |
x |
x |
x |
35 |
| Pengshui Shale |
x |
ü?14.63 |
x |
ü?7.44 |
x |
x |
x |
6.54 |
|
| [71] | Paraíba shale 1 |
x |
ü |
ü |
x |
ü |
ü |
35% |
x |
| Paraíba shale 2 |
x |
ü |
ü |
ü |
ü |
ü |
40 |
x |
|
| Paraíba shale 3 |
ü |
ü |
ü |
ü |
ü |
ü |
56 |
x |
|
| Paraíba shale 4 |
ü |
ü |
ü |
ü |
ü |
ü |
42 |
x |
|
| Paraíba shale 5 |
x |
ü |
ü |
ü |
ü |
ü |
51 |
x |
|
| Paraíba shale 6 |
ü |
x |
x |
x |
x |
x |
82 |
x |
|
From the above data, the relation between clay mineral and clay dispersion and swellings has been analyzed. Figure 4 shows the higher the content of illite is observed, the more shales are prone to less recovery (more dispersion) because illite tends to disperse. Similarly, the Figure 5 shows the higher the content of the Smectite/illite (S/1) mixed layer is, the higher trend in swelling is observed. The Figures 4 and 5 are in accordance with the discussion carried out above.
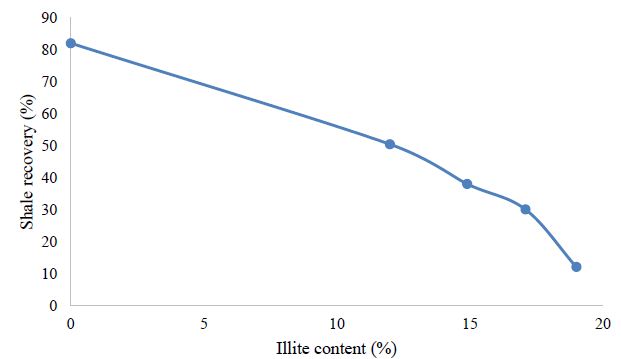
Figure 4: Effect of illite content in shale on shale recovery
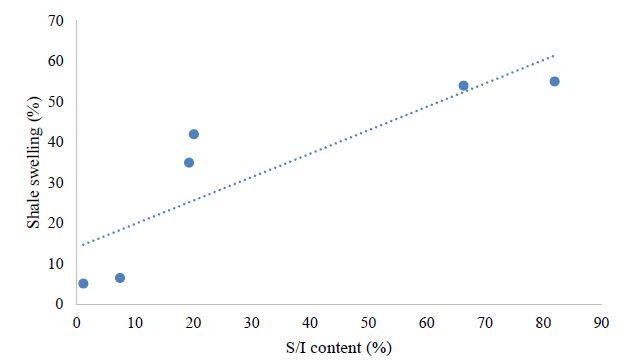
Figure 5: Effect of (S/1) mixed layer content on shale swelling
Shale Sampling for Stabilization Studies
The most tiresome task for shale stabilization studies is the choice of a proper shale sample especially when the role of any novel inhibitor is being studied. Because it will be useless to perform swelling experiments on shale outcrop which doesn’t have any smectite content or haven’t undergone through illitization [72-82]. Similarly, for shale samples which are low in illite and kaolinite, they shouldn’t be the suitable candidates for shale recovery test because illite and kaolinite rich shales tend to disperse more as compared to chlorite rich shales. Similarly, smectite (montmorillonite) or mixed layer shales tend to swell more as compared to only illite dominant shales. Shales usually have sandstone bedding between them, thus getting the virgin shale cores from subsurface might not be possible. More importantly, shale softens down thus getting consolidated shale cores isn’t really an easy task. Therefore, various researchers prefer bentonite wafers to mimic the effect of shale mainly during linear swelling tests because bentonite is rich in Montmorillonite thus making it a suitable candidate for shale swelling studies. However, for shale recovery test (to measure the extent of dispersion), shale samples which are rich in illite must be used.
M.H. Correlation for Predicting Clay Swelling based Upon Zeta Potential
Based upon the limited data available, a new correlation i.e. M.H. correlation has been developed which can help to estimate the clay swelling in Na-Bt hydrated slurry for the smectite (montmorillonite) content ranging from 70-90%. The data reported in Table 5 is carefully selected for only those research groups where simple Na-Bentonite slurry (with no additives) have been used for clay/shale swelling studies. Therefore, the reported data is limited, however, it helps in making a clear rationale in understanding the relationship between clay swelling and zeta potential. The relationship between zeta potential and clay swelling is complex and highly dependent on various factors such as the type of clay mineral, the properties of the surrounding solution, and the measurement techniques used to quantify zeta potential and swelling. In general, a higher absolute value of the zeta potential indicates a greater electrostatic repulsion between the clay particles, which can inhibit swelling as shown in Figure 6. This is because the repulsive forces between the clay particles prevent them from coming into close contact with each other, which limits the amount of water that can be absorbed into the interlayer space and reduces swelling. Conversely, a lower absolute value of the zeta potential, or a positive zeta potential, can promote swelling by reducing the electrostatic repulsion between the particles and allowing them to come into closer contact with each other. This can increase the amount of water absorbed into the interlayer space and promote swelling. However, the quantitative relationship between zeta potential and clay swelling is not straightforward and can vary depending on the specific conditions but an effort has been made to generalize the relation between clay swelling and Zeta potential as shown in Figure 6, Table 5 and eq. 1.
Shale swelling (%) = 3.25 (Z.P) + 194.3 (1)
(Where Z.P. is zeta potential in mV and the relation is only valid for Na-bentonite clay where smectite content is between 70-90%).
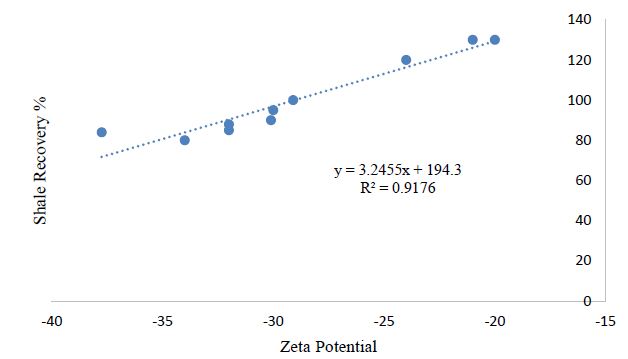
Figure 6: Relation between shale recovery and zeta potential
Table 5: Relation between Zeta potential and clay swelling
|
Research Group |
Zeta Potential (mv) |
Shale Swelling (%) |
| (Barati et al., 2017) [73] |
-40 |
80 |
| (Li et al., 2020) [74] |
-37.75 |
84 |
| (Zhong et al., 2015) [75] |
-34 |
80 |
| (Zhong et al., 2013) [76] |
-32 |
85 |
| (Rasool, Ahmad, & Abbas, 2022) [77] |
-32 |
88 |
| (An & Yu, 2018) [78] |
-30.1 |
90 |
| (Xuan et al., 2015) [79] |
-30 |
95 |
| (An & Yu, 2018) [78] |
-29.1 |
100 |
| (Murtaza et al., 2020) [80] |
-24 |
120 |
| (An et al., 2015) [81] |
-21 |
130 |
| (Xuan et al., 2013[82] |
-20 |
130 |
It is also worth mentioning here that the pH and ionic strength of the surrounding solution can greatly affect the zeta potential and swelling behavior of clay particles. At low pH values, the surface charge of clay particles may be positive, which can promote swelling. At high pH values, the surface charge may be negative, which can inhibit swelling. Additionally, changes in the ionic strength of the surrounding solution can affect the zeta potential and swelling behavior by altering the balance of attractive and repulsive forces between the clay particles. Furthermore, the relationship between zeta potential and clay swelling may be affected by the specific measurement techniques used to quantify these properties. Different techniques may yield different results due to variations in the assumptions, models, and experimental conditions used. Overall, while there is a general correlation between zeta potential and clay swelling, the quantitative relationship between these properties is complex and highly dependent on a variety of factors.
Conclusion
This review articles mainly focusses on presenting proofs from recent literature relating clay mineralogy with shale instability. The following conclusions can be drawn from the fore-mentioned discussion
- Shale instability either due to mechanical or chemical effect, is mainly its dispersion and sloughing (swelling).
- To foretell, either shale will fail mechanically or will show instability due to its mineralogy, shale types play a vital role. Brittle shales contain illite and are well consolidated, they don’t swell mainly but only disperse when water intercalates between layers and thus weakening the bonding. Swelling shales have S/1 mixed layers which, as the name indicates, makes them prone to swelling. However, abnormally pressured shales and tectonically stressed shales mainly undergo mechanical failures.
- Bentonite wafers can be used for shale swelling tests as finding a real time consolidated core from deep interval with high smectite content is hard. However, for shale recovery test, shale outcrop samples rich in illite can be used.
- The recent literature also exhibit the fact that the shales rich in S/I mixed layer will tend to swell more. It is also interesting to note here, that at high burial depth, smectite has already started converting to illite through illlitization, thus the illite formulate through this process also tend to swell.
- A M.H correlation for predicting clay recovery based upon zeta potential values can be used with limited application of bentonite clay with smectite content of 70-90%.
Recommendations
- Various shale types can be collected with their mineralogy known and they can undergo swelling and dispersion tests to empirically observe the effect of shale types and clay chemistry on shale instability.
- The effect of water content and its role on shale swelling can be studied in detail.
- The study on illitization and its role on dispersion and swelling can be studied.
- More work on categorizing the shale based upon mechanical failure and instability due to mineralogy can be done.
- Applications of Machine Learning can be used to categorize shale based upon mineralogy and thus predicting the shale instability behaviour.
- The combined effect of fracking and swelling can be studied on the shales with expandable clays.
- Case studies from the field can be collected reporting wellbore failure incidents due to shale instability and thus role of clay mineralogy can be analyzed in all case studies.
Acknowledgement
We would like to pay utmost gratitude to YUPT 015LC0-326 to provide financial assistance for this work.
Declaration Statement
The authors don’t have any conflict of interest to declare.
Authors’ Contributions
Muhammad Hammad Rasool: Writing original draft, conceptualizing, analysis; Maqsood Ahamad: Supervision, investigation.
References
- Chaturvedi KR, Singh AK, Sharma T (2019) Impact of shale on properties and oil recovery potential of sandstone formation for low‐salinity water flooding applications. Asia‐Pacific Journal of Chemical Engineering 14.
- Woo J, Lee HS, Ozyer C, Rhee CW (2021) Effect of lamination on shale reservoir properties: Case study of the Montney Formation, Canada. Geofluids 2021.
- Kang Z, Zhao Y, Yang D (2020) Review of oil shale in-situ conversion technology. Applied Energy 269.
- Li M, Wu S, Hu S, Zhu R, Meng S, et al. (2021) Lamination Texture and Its Effects on Reservoir and Geochemical Properties of the Palaeogene Kongdian Formation in the Cangdong Sag, Bohai Bay Basin, China. Minerals 11.
- Wan B, et al. (2020) A tale of three taphonomic modes: the Ediacaran fossil Flabellophyton preserved in limestone, black shale, and sandstone. Gondwana Research 84: 296-314.
- Yongsheng M, Xunyu C, Peirong Z (2018) China’s shale gas exploration and development: Understanding and practice. Petroleum Exploration and Development 45: 589-603.
- Dan MFM, Amaran SA, Madun A, Idris UA, Abu Talib MK, et al. (2020) The Impact of Mechanical Weathering on the Durability Strength Index of Ayer Hitam Shale Under Various Weathering Grades and Conditions. Journal of Computational and Theoretical Nanoscience 17: 1070-1078.
- Zou C, et al. (2019) Organic-matter-rich shales of China. Earth-Science Reviews189: 51-78.
- Hu T, et al. (2021) Movable oil content evaluation of lacustrine organic-rich shales: Methods and a novel quantitative evaluation model. Earth-Science Reviews. 214.
- Zhao Z, Tao L, Zhao Y, Halifu M, Chen C (2020) Mechanism of water imbibition in organic shale: an experimental study,” in Abu Dhabi International Petroleum Exhibition & Conference,: OnePetro.
- Song Z, et al. (2020) “A critical review of CO2 enhanced oil recovery in tight oil reservoirs of North America and China,” in SPE/IATMI Asia Pacific Oil & gas conference and exhibition,: OnePetro.
- Begum M, Yassin MR, Dehghanpour H, and U of Alberta (2019) Effect of kerogen maturity on organic shale wettability: A Duvernay case study. Marine and Petroleum Geology 110: 483-496.
- Wenzhi Z, Rukai Z, Suyun H, Lianhua H, Songtao W (2020) Accumulation contribution differences between lacustrine organic-rich shales and mudstones and their significance in shale oil evaluation. Petroleum Exploration and Development 47: 1160-1171.
- Rasool MH, et al. (2022) Rheological characterization of potassium carbonate deep eutectic solvent (DES) based drilling mud. Journal of Petroleum Exploration and Production Technology 12: 1785-1795.
- Rasool MH, Zamir A, Elraies KA, Ahmad M, Ayoub M, et al. (2021) Potassium carbonate based deep eutectic solvent (DES) as a potential drilling fluid additive in deep water drilling applications. Petroleum Science and Technology 39: 612-631.
- Abbas MA, et al. (2022) Characterization of nano based drilling fluid for shale swelling inhibition. Petroleum Science and Technology.
- Zamir A, et al. (2021) Influence of alkyl chain length in ionic liquid based drilling mud for rheology modification: a review. Journal of Petroleum Exploration and Production Technology.
- Guo Y, Huang L, Li X, Chen J, Sun J (2020) Experimental investigation on the effects of thermal treatment on the physical and mechanical properties of shale. Journal of Natural Gas Science and Engineering 82.
- Marsh A, Heath A, Patureau P, Evernden M, Walker P (2018) Alkali activation behaviour of un-calcined montmorillonite and illite clay minerals. Applied Clay Science 166: 250-261.
- Rana A, Arfaj MK, Saleh TA (2020) Graphene grafted with glucopyranose as a shale swelling inhibitor in water-based drilling mud. Applied Clay Science 199.
- Lei M, et al. (2020) Synthesis of carboxymethyl chitosan as an eco-friendly amphoteric shale inhibitor in water-based drilling fluid and an assessment of its inhibition mechanism. Applied Clay Science 193.
- Du J, Hu L, Meegoda JN, Zhang G (2018) Shale softening: Observations, phenomenological behavior, and mechanisms. Applied Clay Science 161: 290-300.
- Borysenko A, et al. (2009) Experimental investigations of the wettability of clays and shales. Journal of Geophysical Research: Solid Earth 114.
- Lutyński M, Waszczuk P, Słomski P, Szczepański J (2017) CO2 sorption of Pomeranian gas bearing shales–the effect of clay minerals. Energy Procedia 125: 457-466.
- Diaz-Perez A, Cortes-Monroy I, Roegiers J (2007) The role of water/clay interaction in the shale characterization. Journal of Petroleum Science and Engineering 58: 83-98.
- Schaef HT, et al. (2015) Competitive sorption of CO2 and H2O in 2: 1 layer phyllosilicates. Geochimica et Cosmochimica Acta 161: 248-257.
- Berthonneau J, Hoover CG, Grauby O, Baronnet A, Pellenq RJM, Ulm FJ (2017) Crystal-chemistry control of the mechanical properties of 2: 1 clay minerals. Applied Clay Science 143: 387-398.
- Wang G, et al. (2021) Technical development of characterization methods provides insights into clay mineral-water interactions: A comprehensive review. Applied Clay Science 206.
- Chen S, Han Y, Fu C, Zhu Y, Zuo Z (2016) Micro and nano-size pores of clay minerals in shale reservoirs: Implication for the accumulation of shale gas. Sedimentary geology 342: 180-190.
- Du J, Cai J, Chao Q, Song M, Wang X (2021) Variations and geological significance of solid acidity during smectite illitization. Applied Clay Science 204.
- Du J, Cai J, Chen Z, Lei T, Zhang S, et al. (2019) A contrastive study of effects of different organic matter on the smectite illitization in hydrothermal experiments. Applied Clay Science 168: 249-259.
- Gualtieri AF, et al. (2008) Structural characterization of the clay mineral illite-1M. Journal of Applied Crystallography 41: 402-415.
- Huggett JM (1995) Formation of authigenic illite in palaeocene mudrocks from the central North Sea: A study by high resolution electron microscopy. Clays and clay minerals 43: 682-692.
- O’Brien DE, Chenevert ME (1973) Stabilizing sensitive shales with inhibited, potassium-based drilling fluids. Journal of petroleum technology 25: 1089-1100.
- Chenevert ME (1970) Shale alteration by water adsorption. Journal of petroleum technology 22: 1141-1148.
- Wilson M, Wilson L (2014) Clay mineralogy and shale instability: an alternative conceptual analysis. Clay Minerals 49: 127-145.
- Stillinger FH Jr, Kirkwood JG (1960) Theory of the diffuse double layer. The Journal of Chemical Physics 33:1282-1290.
- Al-Bazali T (2021) Insight on the inhibitive property of potassium ion on the stability of shale: a diffuse double-layer thickness (κ−1) perspective. Journal of Petroleum Exploration and Production Technology 11: 2709-2723.
- AL-Bazali T (2022) Insight into Debye Hückel length (κ− 1): smart gravimetric and swelling techniques reveals discrepancy of diffuse double layer theory at high ionic concentrations. Journal of Petroleum Exploration and Production Technology 12: 461-471.
- Muhammed NS, Olayiwola T, Elkatatny S (2021) A review on clay chemistry, characterization and shale inhibitors for water-based drilling fluids. Journal of Petroleum Science and Engineering 206.
- Song H, Liu H, Bu H, Liu D, Li Y, et al. (2021) Effects of montmorillonite charge reduction on the high-temperature/high-pressure pyrolysis of organic matter. Applied Clay Science 213.
- Rasool MH, Zamir A. Elraies KA, Ahmad M, Ayoub M, et al. (2022) A Deep Eutectic Solvent based novel drilling mud with modified rheology for hydrates inhibition in deep water drilling. Journal of Petroleum Science and Engineering 211.
- Tawari SL, Koch DL, Cohen C (2001) Electrical double-layer effects on the Brownian diffusivity and aggregation rate of Laponite clay particles. Journal of Colloid and Interface Science 240: 54-66.
- Park SJ, Seo MK (2011) Interface science and composites. Academic Press.
- Gholami R, Elochukwu H, Fakhari N, Sarmadivaleh M (2018) A review on borehole instability in active shale formations: Interactions, mechanisms and inhibitors. Earth-Science Reviews 177: 2-13.
- Yongxue SBXBL, Jiang X (2012) CT imaging and mechanism analysis of crack development by hydration in hard-brittle shale formations. Acta Petrolei Sinica 33.
- Han S, Gao Q, Cheng Y, Yan C, Han Z, et al. (2020) Experimental study on brittle response of shale to cryogenic fluid nitrogen treatment. Journal of Petroleum Science and Engineering 194.
- Wang D, Wang X, Ge H, Sun D, Yu B (2020.) Insights into the effect of spontaneous fluid imbibition on the formation mechanism of fracture networks in brittle shale: An experimental investigation. ACS omega 5: 8847-8857.
- Zhang J, Yu Q, Li Y, Pan Z, Liu B (2022) Hydraulic Fracture Vertical Propagation Mechanism in Interlayered Brittle Shale Formations: An Experimental Investigation. Rock Mechanics and Rock Engineering.
- Gui J, Ma T, Chen P, Yuan H, Guo Z (2018) Anisotropic damage to hard brittle shale with stress and hydration coupling. Energies 11.
- Yan X, You L, Kang Y, Li X, Xu C, et al. (2018) Impact of drilling fluids on friction coefficient of brittle gas shale. International Journal of Rock Mechanics and Mining Sciences 106: 144-152.
- Liu T, Liu H, Meng Y, Han X, Cui S, Yu A (2020) Multi-coupling stress field and evaluation of borehole stability in deep brittle shale. Arabian Journal of Geosciences 13: 1-9.
- Ahmed HM, Kamal SM, Al-Harthi M (2019) Polymeric and low molecular weight shale inhibitors: A review. Fuel 251: 187-217.
- Li Z, et al. (2021) The influence of shale swelling on casing deformation during hydraulic fracturing. Journal of Petroleum Science and Engineering 205.
- Rasool MH, Ahmad M, Ayoub M, Zamir A, Abbas MA (2022) A review of the usage of deep eutectic solvents as shale inhibitors in drilling mud. Journal of Molecular Liquids .
- Abbas MA, Zamir A, Elraies KA, Mahmood SM, Rasool MH (2021) A critical parametric review of polymers as shale inhibitors in water-based drilling fluids. Journal of Petroleum Science and Engineering 204.
- Cao H, Zhang Z, Bao T, Sun P, Wang T, et al. (2019) “Experimental investigation of the effects of drilling fluid activity on the hydration behavior of shale reservoirs in Northwestern Hunan, China. Energies 12.
- Rezaei A, Shadizadeh SR (2021) State-of-the-art drilling fluid made of produced formation water for prevention of clay swelling: experimental investigation. Chemical Engineering Research and Design 170: 350-365.
- Li J, et al. (2021) Origin of abnormal pressure in the Upper Paleozoic shale of the Ordos Basin, China. Marine and Petroleum Geology 110:162-177.
- Mingshui S, Huimin L, Yong W, Yali L (2020) Enrichment rules and exploration practices of Paleogene shale oil in Jiyang Depression, Bohai Bay Basin, China. Petroleum Exploration and Development 47: 242-253.
- Jinhua F, Shixiang L, Xiaobing N, Xiuqin D, Xinping Z (2020) Geological characteristics and exploration of shale oil in Chang 7 member of Triassic Yanchang Formation, Ordos Basin, NW China. Petroleum Exploration and Development 47: 931-945.
- Ju W, et al. (2020) Predicting the present-day in situ stress distribution within the Yanchang Formation Chang 7 shale oil reservoir of Ordos Basin, central China. Petroleum Science 17: 912-924.
- Hu L (2022) A review of mechanical mechanism and prediction of natural fracture in shale. Arabian Journal of Geosciences 15: 1-16.
- Liu R, et al. (2020) Influence of tectonic exhumation on porosity of Wufeng–Longmaxi shale in the Fuling gas field of the eastern Sichuan Basin, China. AAPG Bulletin 104: 939-959.
- Beg M, Haider MB, Thakur NK, Husein M, Sharma S, et al. (2021) Clay-water interaction inhibition using amine and glycol-based deep eutectic solvents for efficient drilling of shale formations. Journal of Molecular Liquids 340.
- Mohammed K, Alwassiti AA, Al-Bidry MA (2019) Experimental Study on Tanuma Shale Stability Using Drilling Fluids with Different Additives, in IOP Conference Series: Materials Science and Engineering, , vol. 579, no. 1: IOP Publishing, p. 012002.
- Okoro EE, Dosunmu A (2014) Experimental analysis of shale for evaluating shale drilling fluid interaction in Agbada formation. British Journal of Applied Science & Technology 4.
- Jia H, et al. (2019) Investigation of inhibition mechanism of three deep eutectic solvents as potential shale inhibitors in water-based drilling fluids. Fuel 244: 403-411.
- Xu Jg, Qiu Z, Zhao X, Mou T, Zhong H, et al. (2018) A polymer microsphere emulsion as a high-performance shale stabilizer for water-based drilling fluids. RSC advances 8: 20852-20861.
- Shi X, Wang L, Guo J, Su Q, Zhuo X (2019) Effects of inhibitor KCl on shale expansibility and mechanical properties. Petroleum 5: 407-412.
- Leal C, Brunet M, Amorim L, Lira H, Nogueira F, et al. (2019) Influence of reactivity and mineralogical composition on instability due to the disintegration of shales from Paraíba and Ceará States, Brazil. Cerâmica 65: 400-406.
- Krinari G, Khramchenko M (2018) Interstratified illite-smectite phases: formation mechanisms and practical applications. Russian Geology and Geophysics 59: 1120-1128.
- Barati P, Shahbazi K, Kamari M, Aghajafari A (2017) Shale hydration inhibition characteristics and mechanism of a new amine-based additive in water-based drilling fluids. Petroleum 3: 476-482.
- Li X, Jiang G, Wang J, Ni X (2020) Inhibitive properties comparison of different polyamino acids in water-based drilling fluids. Journal of Natural Gas Science and Engineering 83.
- Zhong H, Qiu Z, Sun D, Zhang D, Huang W (2015) Inhibitive properties comparison of different polyetheramines in water-based drilling fluid. Journal of Natural Gas Science and Engineering 26: 99-107.
- Zhong H, Qiu Z, Huang W, Xie B, Wang W (2013) Bis (hexamethylene) triamine as potential shale inhibitor in water-based drilling fluid. The Open Petroleum Engineering Journal 6.
- Rasool MH, Ahmad M, Abbas MA (2022) A Double Action PD (Polymer-Deep Eutectic Solvent) Based Shale Inhibitor in Drilling Mud. Journal of Advanced Research in Fluid Mechanics and Thermal Sciences 99: 149-157.
- An Y, Yu P (2018) A strong inhibition of polyethyleneimine as shale inhibitor in drilling fluid. Journal of Petroleum Science and Engineering 161: 1-8.
- Xuan Y, Jiang G, Li Y, Yang L, Zhang X (2015) Biodegradable oligo (poly-l-lysine) as a high-performance hydration inhibitor for shale. RSC Advances 5: 84947-84958.
- Murtaza M, Kamal MS, Hussain SMS, Mahmoud M, Syed NA (2020) Quaternary ammonium gemini surfactants having different spacer length as clay swelling inhibitors: Mechanism and performance evaluation. Journal of Molecular Liquids 308.
- An Y, Jiang G, Ren Y, Zhang L, Qi Y, et al. (2015) An environmental friendly and biodegradable shale inhibitor based on chitosan quaternary ammonium salt. Journal of Petroleum Science and Engineering 135: 253-260.
- Xuan Y, Jiang G, Li Y, Wang J, Geng H (2013) Inhibiting effect of dopamine adsorption and polymerization on hydrated swelling of montmorillonite. Colloids and Surfaces A: Physicochemical and Engineering Aspects 422: 50-60.