Abstract
Large amounts of byproducts are generated by the food processing industries in the 21st century including bioactive compounds such as polysaccharides, polyphenols, carotenoids, and dietary fiber. Due to a lack of sustainable extraction techniques, these processing wastes are regarded as having negligible value in comparison to processed fruit or vegetables. Conventional extraction has limitations in terms of time, energy, and solvent usage. In comparison with conventional methods of extraction, ultrasonic-assisted extraction can extract bioactive components in a short period, at low temperatures, and with less energy and solvent, as well as maintaining the functionality of the components. However, UAE-related variables such as frequency, power, duty cycle, temperature, time, solvent type, and liquid-solid ratio must be understood and optimized for each byproduct. This article provides the mechanism, concept, and factors that influence bioactive compound extraction, with a focus on tomatoes.
Keywords
Bioactive, Phytochemical, Nutraceutical
Introduction
With growing demand, the production as well as consumption of fruits and vegetables has increased by huge manifolds as their losses in the form of waste (upto 60%) and by-products according to the Food and Agriculture Organization (FAO) which demands an increasing awareness among the growing population [1]. The bioactive components for the development of functional food products are lost due to the unavailability of extraction techniques [2]. The viewpoint towards fruit and vegetable by-products has changed due to the increasing demand for natural bioactive compounds namely pectin and antioxidants having huge potential in terms of nutritional and therapeutic value [3].
The development of nutraceutical products involves the extraction of bioactive components as a primary step. Depending on the type of compounds to be extracted several extraction methods are available such as solvent extraction, mechanical expelling, supercritical extraction, and microwave extraction. These methods’ limitations include the need for an aqueous phase in microwave-assisted extraction, high capital requirements for supercritical fluid extraction, low yields in mechanical expelling, and the need for additional solvent in solvent extraction [4]. UAE offers advantages over these techniques, including lower energy and time requirements, low-temperature extraction, and preservation of extract quality.
In UAE by increasing mass transport, the ultrasonic waves disrupt the plant tissue through physical forces created during acoustic cavitations and facilitate the rapid release of extractable components from the solvent [1].
With the use of ultrasound technology, it is possible to successfully extract various plant matrices, including whole plants and their byproducts, of aromatic compounds, polysaccharides, carotenoids, and polyphenols. The variables linked to the UAE, including power, frequency, duty cycle, temperature, time, type of solvent, and liquid-solid ratio, must be accurately managed to get the best extraction. Many studies have looked at how these factors affect the extraction of bioactive chemicals from fruit and vegetable by-products, both separately and in combination [5-7].
UAE is the method of extracting target compounds from various plant matrices using ultrasonic energy and solve [8]. The human hearing range is 20 Hz to 20 kHz, however, the mechanical waves known as ultrasounds have frequencies (>20 kHz) that are higher. These waves, which can move through a solid, liquid, or gaseous media, are composed of a series of cycles of compression and rarefaction that cause molecules to move and get dislodged from their original places.
When a sound wave is strong enough to cause rarefaction, the molecules are pulled apart by the strong attraction between them, resulting in cavitation bubbles. These bubbles form during coalescence, burst during the compression phase, and produce an intense local environment and hot area. Up to 5000 K of temperature and 1000 atmospheres of pressure rise are possible. These hot spots quicken the nearby metabolic reactions [7,9-11].
The primary sources of serotonin (5-hydroxytryptamine), an indoleamine monoamine neurotransmitter, are animal blood platelets, serotonergic neurons in the central nervous system (CNS), and enterochromaffin cells in the gastrointestinal tract (GI tract). The pineal gland of the brain produces melatonin (5-methoxy-N-acetyltryptamine), also known as the “hormone of darkness” [12]. It is an indole hormone that regulates several bodily processes. Melatonin secretion starts three months after birth; before that, it is mostly obtained from the mother’s or cow’s milk. In addition to being a potent antioxidant, it also strengthens immunity, increases resistance to illness and infection, inhibits certain types of cancer, and improves neurological conditions. Tryptophan is an important amino acid that is the precursor of both melatonin and serotonin in the mammalian brain. First, 5-hydroxytryptophan (5-HTP), the direct nutritional precursor of the neurotransmitter serotonin 5-hydroxytryptamine (5-HT), is produced from L-tryptophan in the pineal gland. Melatonin is produced in the pineal gland by methylating and acetylating serotonin [13].
Some of the best sources of tryptophan are the seeds of pumpkins and squash (576 mg/100 g). Tryptophan-rich foods include brown rice, whole oats, wheat bran, and wheat germ. One of the best foods for high serotonin content is butternut (398 μg/g tissue). Plantains, apricots, cherries, peaches, and Chinese plums have also been found to have significant levels of serotonin [14-18]. Following its discovery in edible plants, melatonin has been detected in a wide variety of plants and plant parts, including the rind of tart cherries, tomato fruit grape skin, sunflower, mustard, and walnut seed roots. Its concentrations typically range from picograms to nanograms per gram of tissue. The first common tree nut about which melatonin has been researched from a nutritional standpoint is the walnut [13].
Additionally, serotonin plays a role in the hypothalamic regulation of pituitary secretion, specifically in the control of prolactin, growth hormone, and adrenocorticotropin (ACTH) secretion. Additionally, a direct synaptic connection has been shown between serotonergic terminals and neurons in the paraventricular nucleus of the hypothalamus that contain corticotropin-releasing hormone (CRH). To control development patterns, mating behavior, and specific motions like migration, metabolism, and other physiological processes, melatonin governs the circannual cycle. Light-induced melatonin production is regulated and considered an endogenous synchronizer of the circadian cycle. Because the peak generation of endogenous melatonin occurs simultaneously with the nightly drop in body temperature, it affects sleep in animals through thermoregulatory function [12]. Numerous calcium-dependent cellular processes are regulated by melatonin, which binds to Ca2+-calmodulin in cells. Recurrent depression during the short photoperiod is the hallmark of winter-type seasonal affective disorder (SAD), and photoperiodic variation is directly linked to the condition’s summertime remission.
Materials and Methods
Materials
The study involved selecting fresh tomatoes (Solanum lycopersicum) that were free of cuts and exterior deterioration from Jadavpur, Kolkata, West Bengal Supermarket. Specialty chemicals were obtained from M/s Sigma-Aldrich, Munich, Germany. These included acetonitrile (99.9% pure, HPLC grade), acetic acid (99.8% pure), and methanol (HPLC grade). AR-grade chemicals were all that were employed in the investigation.
Methods
Extraction of SER-MEL from Tomatoes Employing UAE
Little changes were made to the procedure described by Chakraborty and Bhattacharjee (2019) [18] for the extraction of SER, MEL, and the precursor molecule L-TRP from irradiation tomatoes. Figure 1 illustrates the whole process. As shown in Table 1, the initial trials were carried out by adjusting several parameters, including the solvent composition, sample solvent ratio, extraction time, extraction amplitude, and addition of anhydrous sodium sulfate (Na2SO4).
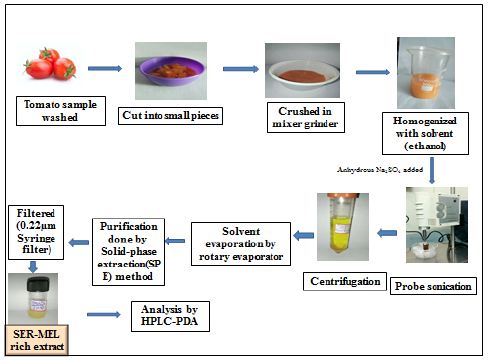
Figure 1: Extraction of L-TRP, SER and MEL from tomatoes by UAE
Table 1: Factors for extraction of phytochemicals
|
No. of runs |
Extraction time (mins) | % amplitude (nm) | Solvent composition | Sample solvent ratio | Na2SO4 addition | Yield of biomolecules from tomatoes (µg/g of dry weight) | ||
| L-TRP | SER |
MEL |
||||||
| 1 |
30 |
100 |
Ethanol |
1:1 |
Not added |
27.76a |
21.49b |
Not detected |
| 2 |
10 |
70 |
Ethanol |
1:1 |
Not added |
48.68a |
9.34b |
0.3b |
| 3 |
10 |
70 |
Ethanol and water |
1:3 |
Not added |
0.14a |
0.34b |
0.2b |
| 4 |
10 |
70 |
Ethanol |
1:3 |
Added post centrifugation |
0.64a |
1.67d |
0.19c |
| 5 |
10 |
20 |
Ethanol |
1:3 |
Added prior to sonication |
17.22a |
5.06b |
1.74c |
| 6 |
10 |
70 |
Ethanol |
1:3 |
Added prior to sonication |
5.63a |
3.70g |
1.03c |
| 7 |
10 |
100 |
Ethanol |
1:3 |
Added prior to sonication |
0.42a |
1.85b |
0.09c |
Using UAE, the phytochemicals L-TRP, SER, and MEL were extracted from tomatoes, and many parameters were adjusted, including batch size and the continuous way of establishing vibration length. 5 g of the material washomogenized after being crushed and ground (T 50 digital Ultra-turrax_, M/s Ika, and Staufen, Germany). Amber-colored beakers were used throughout the entire extraction process.
A titanium probe with a 3 mm tip diameter, 80 mm length, and a sonication capacity of 5–200 ml sample was used to treat the homogenized sample to UAE using a probe sonicator (Labsonic M, M/s Sartorius, Melsungen, Germany). The probe had a maximum power of 100 W and a maximum frequency of 30 kHz. To allow real sound wave incursion without contacting the vial’s surface, the extraction procedure was carried out while keeping a distance of 1 cm from the probe’s tip. The extraction vials were placed in an ice bath with a temperature controlled between 4 and 8°C [18].
The extracted materials were centrifuged at 4°C for 15 minutes at 4000 rpm. The obtained supernatants were subjected to solvent evaporation at 50°C for 10 minutes and 25± 5°C under vacuum using a rotary vacuum evaporator. For the contents of SER, MEL, and L-TRP, the extracts were kept in screw-capped glass vials with an amber color at -20°C.
Quantification and Purification of L-TRP, SER, and MEL in the UAE by High Performance Liquid Chromatography (HPLC)
The extracts underwent purification using a 0.22 μm membrane filter, and the simultaneous quantification of L-TRP, SER, and MEL was performed using the high-performance liquid chromatography-photo diode array (HPLC-PDA) analytical technique.
According to the procedure, the phytochemicals were tracked using a PDA detector and a D2 light at 280 nm [19-23]. Based on the standard retention time of L-TRP, SER, and MEL Sigma standards, where 20 μl of the produced standard or extract (UAE extract) was injected and run, the peaks of these biomolecules were found. The HPLC method’s parameters, including the mobile phase composition, operating mode, and flow rate, were tested in multiple preliminary runs. The condition that produced the maximum yield of the standards for SER, MEL, and L-TRP was selected as the optimal one.
The HPLC was operated in gradient elution method with acetic acid and methanol (both HPLC grade) in mobile phase in a flow rate of 1 ml/min which showed distinct peaks of SER, MEL, and L-TRP at their respective elution time.
Statistical Analyses
The mean ± SD of three separate experimental runs has been used to express the yield of L-TRP, SER, and MEL. The mean ± SD of three values is also used to describe the results. One-way analysis of variance (ANOVA) was used to do the statistical analysis of the data. Duncan’s multiple-range test was used to identify significant changes in means. The tests were verified to be significant using a p-value of B 0.05. STATISTICA 8.0 software (Statsoft, Oklahoma, USA) was utilized in this study to test the outcomes of the experiments.
Results and Discussion
The three main component concentrations varied in the extracts examined by HPLC-PDA at various solvent ratios. The optimal conditions were selected based on which extracts with the highest yield of the three components were deemed to be the best. A few data from our experiment and a bar graph based on the outcomes of the preliminary trials are shown in Figure 2 below. The antioxidants in the preliminary trail exhibited their maximum yield when the amplitude was 20 nm. By employing ethanol as the solvent and adding Na2SO4 before sonication, the antioxidants, namely L-TRP, SER, and MEL, demonstrated a good synergistic co-existence, as evidenced by the SE value of 1.08, which is greater than unity. The refined extracts were kept at -20°C in the dark in screw-capped bottles with an amber color. The co-extraction of additional antioxidants from tomatoes resulted in the observation of multiple additional peaks in the HPLC profile.
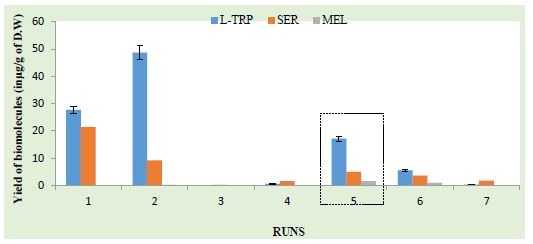
Figure 2: Highest concentration of antioxidants in the fifth run
Conclusion
Using ethanol as the solvent and adding Na2SO4 before sonication (5th run), the trials showed that the best yields of SER, MEL, and L-TRP were obtained at low ultrasonication amplitudes. The extract can be further used for production of nutraceutical food product such as tomato soup with enhanced contents of the three phytochemicals. The same can be used for production of medicinal supplements such as nasal sprays, ointments as they are naturally obtained antioxidants.
References
- Kshitiz Kumar Shivmurti Srivastav, Vijay Singh Sharanagat (2020) Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review.
- Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG (2018) Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci Food Saf 17: 512-531. [crossref]
- Kosseva MR Academic Press (2013) Functional food and nutraceuticals derived from food industry wastes. Food Industry Wastes 103-120.
- Samaram S, Mirhosseini H, Tan CP, Ghazali HM, Bordbar S, Serjouie A (2015) Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: Oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem 172: 7-17. [crossref]
- Vilkhu K, Mawson R, Simons L, Bates D (2008) Applications and opportunities for ultrasound assisted extraction in the food industry – a review. Innovative Food Sci Emerg Technol 9: 161-169.
- Chemat F, Rombaut N, Meullemiestre A, Turk M, Perino S, et al. (2017) Review of green food processing techniques. Preservation, transformation, and extraction. Innov Food Sci Emerg Technol 41: 357-377.
- Roselló-Soto E, Galanakis CM, Brnčić M, Orlien V, Trujillo FJ, et al. (2015) Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci Technol 42: 134-149.
- Freitas de Oliveira C, Giordani D, Lutckemier R, Gurak PD, Cladera-Olivera F, Ferreira Marczak LD (2016) Extraction of pectin from passion fruit peel assisted by ultrasound. LWT – Food Sci Technol 71: 110-115.
- Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, et al. (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. UltrasonicsSonochem 34: 540-560.
- Šic Žlabur J, Voca S, Dobricevic N, Brncic M, Dujmic F, et al. (2015) Optimization of ultrasound assisted extraction of functional ingredients from Stevia rebaudiana Bertoni leaves. Int Agrophys 29: 231-237.
- Zinoviadou KG, Galanakis CM, Brnčić M, Grimi N, Boussetta N, et al. (2015) Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res Int 77: 743-752.
- Utiger RD (1992) Melatonin: The hormone of darkness. New England Journal of Medicine 327: 1377-1379. [crossref]
- Bhattacharjee P, Ghosh PK, Vernekar M, Singhal RS (2016) Role of Dietary Serotonin and Melatonin in Human Nutrition; In: Serotonin and Melatonin: Their Functional Role in Plants, Food, Phytomedicine, and Human Health, GA Ravishankar & A Ramakrishna (Eds.), CRC Press-Taylor & Francis Group, United States 317-333.
- Bhattacharjee P, Chakraborty S (2018) Neurotransmitters in Edible Plants Implications in Human Health.
- García-Moreno C, Rivas-Gonzalo JC, Pena-Egido MJ, Mariné-Font A (1983) Improved method for determination and identification of serotonin in foods. Journal of Association of Official Analytical Chemists 66: 115-117. [crossref]
- Garrido M, Paredes SD, Cubero J, et al. (2010) Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxy melatonin and total antioxidant capacity in the urine of middle-aged and elderlyhumans. Journals of Gerontology Series A: Biological Sciences and Medical Sciences 65: 909-914.
- Gónzalez-Flores D, Velardo B, Garrido M, et al. (2011) Ingestion of Japanese plums (Prunus salicina Lindl Crimson Globe) increases the urinary 6-sulfatoxy melatonin and total antioxidant capacity levels in young, middle-aged and elderly humans: Nutritional and functional characterization of their content. Journal of Food and Nutrition Research 50: 229-236.
- Chakraborty S, Bhattacharjee P (2020) Ultrasonication-assisted extraction of phytomelatonin-rich, erucic acid-lean nutraceutical supplement from mustard seeds: an antioxidant synergy in the extract by reductionism. J Food Sci Technol 57: 1278-1289. [crossref]
- Huang X, Mazza G (2011) Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. Journal of Chromatography A 1218: 3890-3899. [crossref]
- Eh A, Teoh SG (2012) Novel modified ultrasonication technique for extraction of lycopene from tomatoes. Journal of ultrasonics sonochemistry 19: 151-159. [crossref]
- Marti R, Brondo ML, Lahoz I, Campillo C, Cebolla-Cornejo J, et al. (2018) Polyphenol and L-ascorbic acid content in tomato as influenced by high lycopene genotypes and organic farming at different environments. Journal of food chemistry 239: 148-156. [crossref]
- Ramakrishna A, Giridhar P, Ravishankar GA (2011) Phytoserotonin: A review. Plant Signaling and Behavior 6: 800-809. [crossref]
- Ranganna S (2000) Handbook of analysis and quality control for fruit and vegetable products, 3rd edn. Tata and McGraw-Hill, New Delhi.