DOI: 10.31038/CST.2023822
Abstract
Gastric cancer (GC) is one of the three most deadly among cancers. Although several new drugs have been introduced for metastatic disease, the median overall survival (OS) remains 11-14 months. Perioperative chemotherapy (CT) is the current standard of care for resectable cT2-4 and/or N+ GC, and FLOT (5-fluorouracil, oxaliplatin, docetaxel) is the treatment of choice. To date, no predictive factor of response has been identified. Considering their synergism in DNA repair, ataxia telangiectasia mutated (ATM) and sirtuin-1 (SIRT1) merit investigation for prognostic stratification.
We evaluated by immunohistochemistry the expression levels of ATM and SIRT1 in the surgical specimens from 42 patients with a resectable GC or gastroesophageal junction adenocarcinoma treated with neoadjuvant CT and surgery. In the entire population, median DFS was 22.2 months (95%CI 14.9 – NR) and median OS was not reached (95%CI 26.9 – NR). DFS was significantly longer in patients achieving tumor regression grade (TRG) 2-3 compared with those achieving TRG 4-5 (median DFS not reached vs. 14.9 months; HR=0,36 CI=0,14-0,97; p=0.034), and a trend toward a better OS was also observed across the two subgroups (p=0.068). The proportion of patients who obtained a major/medium pathological regression was higher in the ATM-absent group than in the ATM-expressed group (69% vs. 50%; X-squared=6.05; p=0.1). In the overall population, OS and DFS did not have a significantly different distribution according to ATM and SIRT1 expressions. In contrast, in the TRG 4-5 subgroup, the ATM expression seems to be associated with inferior DFS (7.7 months vs. 32.1 months; p=0.055), particularly when combined with absence of SIRT1. In conclusion, TRG has been confirmed a surrogate of survival, ATM expression correlates with TRG and TRG-high/ATM-expressed/SIRT1-absent profile should be studied as a prognostic marker in prospective trials.
Keywords
Gastric cancer, Prognostic biomarker, Predictive biomarker, Epigenetic, DNA damage repair, ATM, SIRT1, TRG, DNA double-strand break
Key Points
- No prognostic markers are available in gastric cancer patients after neoadjuvant chemotherapy.
- TRG is a surrogate of survival outcomes.
- TRG-high/ATM-expressed/SIRT1-absent profile should be prospectively investigated as a marker for prognostic stratification.
Introduction
Gastric cancer (GC) is one of the three most deadly among cancers, and the fifth most commonly diagnosed tumor worldwide, with about 950,000 new cases per year [1]. Although several new drugs have been introduced for metastatic disease, the median overall survival (OS) remains 11-14 months [2,3]. In recent years, perioperative chemotherapy (CT) has been considered the standard of care for the vast majority of patients with resectable GC, and FLOT (5-fluorouracil, oxaliplatin, docetaxel) is the treatment of choice in patients fit for intensive CT. Doublets containing fluoropyrimidine and platinum are considered feasible for frail, comorbid or elderly patients [4-6]. To date, no reliable predictive factor of benefit from perioperative treatments has been identified and a tailored strategy is yet to be applied [7]. An appropriate patient selection for perioperative therapy is challenging, since only a few features have been associated with tumor regression and survival outcome after FLOT treatment. In the FLOT4-AIO trial, diffuse histotype has been associated with a reduced rate of pathological complete response (pCR) compared to the intestinal one (3% vs. 23%, respectively). Among the tumor-centered endpoints, pCR is currently considered of great interest as it could be a surrogate for survival outcomes [8]. Unfortunately, in resectable disease, only limited evidence is available regarding a molecular biomarker-based patient selection [7]. Although several molecular subgroups have been identified as potentially associated with a prognostic or predictive effect, stratification in prospective trials is still needed. Among the putative biomarkers of interest, microsatellite instability (MSI) seems to be the best candidate to drive treatment choice in the near future [7,9,10]. Consistent with previous evidence, a recent international meta-analysis conducted by Pietrantonio et al. confirmed MSI-high (MSI-H) status as a favorable prognostic factor in patients with resected GC. The 5-year disease-free survival (DFS) and overall survival (OS) rates were higher in patients with MSI-H GC than in those with microsatellite stable (MSS) tumor (5-year DFS: 71.8% vs. 52.3%, respectively; 5-year OS: 77.5% vs. 59.3%) [9]. In addition, the benefit from neoadjuvant CT in resectable MSI-H GC seems to be limited, and that ongoing studies are evaluating immune checkpoint inhibitors (ICIs) as neoadjuvant therapy or potentially curative strategy in patients achieving a complete clinical-pathological-molecular response [10]. Although evidence is limited, ataxia telangiectasia mutated (ATM) protein and sirtuin-1 (SIRT1) have demonstrated a deep synergism of action and could be considered for prognostic stratification, since they are involved in the repair of DNA double-strand breaks (DSB) and epigenetic regulation [11-13].
The ATM gene is located on chromosome 11 and encodes a serine/threonine protein kinase that contributes to maintaining genomic integrity transducing a DSB repair signal to effectors. ATM protein levels are decreased in GC compared to normal samples and low levels of phosphorylated ATM are associated with poor differentiation, lymph node metastasis and poor prognosis [14]. GC cells with defective ATM (expression or activity) determining homologous recombination deficiency are more sensitive to therapies that cause the accumulation of DNA DSBs [15]. In particular, in GC cell lines, ATM overexpression is associated with cisplatin-resistance and its inhibition, using the ATM inhibitor CP466722 or siRNA, induces the reversion of epithelial-to-mesenchymal transition [11]. Therefore, mediating platinum-resistance, we supposed that ATM expression could influence tumor regression in GC patients treated with platinum-based neoadjuvant CT.SIRT1 is a NAD+-dependent class-III histone deacetylase (HDAC) involved in several cell functions including DNA repair. Contributing to the identification of DNA damage sites and access of DNA repair proteins, SIRT1 has a crucial role in the epigenetic regulation of cell homeostasis by deacetylating both histone and non-histone proteins. SIRT1 acts both as tumor suppressor and tumor promoter, depending on location (nucleus vs. cytoplasm) and tissue type. DNA damage is a trigger for SIRT1 dissociation and re localization to DSB. In the SIRT1-DNA repair interplay, ATM preserves the efficient recruitment of SIRT1 to DSBs by signaling DNA damage. Simultaneously, SIRT1 stabilizes ATM at DSB sites and stimulates its autophosphorylation and activity [12,13].
The aim of this study was to investigate the prognostic role of ATM and SIRT1 expression in a cohort of patients who underwent neoadjuvant CT for resectable GC.
Materials and methods
Study Design and Population
This was a single-institution, observational, retrospective and prospective study, which enrolled patients with a resectable GC or gastroesophageal junction adenocarcinoma treated with neoadjuvant CT and surgery from September 2017 to April 2022.
Being an observational study, treatments were performed according to clinical practice and national and international guidelines, regardless of the inclusion in the study.
All patients had a histological diagnosis of GC or gastroesophageal junction adenocarcinoma and availability of tissue samples of primary tumor for translational analysis. Clinical stage was assessed in a multidisciplinary board according to the 7th edition of the International Union Against Cancer Tumour–Node–Metastasis classification [16]. A CT scan, FDG-PET scan, US-endoscopy and, wherever indicated, diagnostic laparoscopy, were routinely performed for staging. Patients with metastatic disease, squamous cell carcinoma, pure neuroendocrine carcinoma or esophageal cancer were excluded.
The primary objective was to describe the expression of ATM and SIRT1 in a cohort of patients with resectable GC who had received neoadjuvant CT. Secondary objectives included the description of clinical characteristics associated with specific patterns of expression and the identification of clinical and/or pathological characteristics that may be related to prognosis.
This study was approved by the Institutional review board of Azienda Ospedaliero-Universitaria Careggi (Comitato Etico Regionale for clinical experimentation of Toscana Region – Italy – Area Vasta Centro – 22070_BIO). Informed consent from each patient enrolled in the study was obtained.
Histopathological Evaluation and Immunohistochemical Staining
Immunohistochemistry (IHC) was performed on formalin-fixed paraffin-embedded (FFPE) tumor sections of GC, 3µm thick. Tissue sections were processed by fully automated detection and staining techniques through Discovery Ultra immunostainer (Ventana Medical Systems, AZ, USA). Slide sections were incubated with the following primary antibodies: anti-SIRT1 (#ab104833; mouse monoclonal, clone 1F3, 1:500, Abcam, Cambridge, UK) and anti-ATM (#ab32420; rabbit monoclonal, clone Y170, 1:100, Abcam, Cambridge, UK). Anti-SIRT1 signals were developed with UltraMap anti-mouse HRP, and anti-ATM with UltraMap anti-Rabbit HRP (Ventana Medical Systems, AZ, USA). The bound antibodies were visualized using Discovery ChromoMap DAB Kit (Ventana Medical Systems, AZ, USA). Finally, sections counterstaining was performed with Haematoxylin II ready-to-use; Ventana, AZ, USA). Healthy human colon for SIRT1 and testis for ATM were used as positive controls. Negative control was performed by replacing the primary antibodies with Mouse IgG1 and Rabbit IgG Isotype Control (Invitrogen). The negative and positive control sections were treated in parallel with the samples in the same run Immunohistochemical expression of SIRT1 was evaluated according to An et al. [17].
The expression score was assessed by combining staining intensity score and the positive percentages. The expression was scored as follows: <10%, 10-24%, 25-49%, 50-74%, and ≥75%. Immunohistochemical expression of ATM was evaluated according to Miller et al. [18]. The staining was evaluated based on nuclear DAB signal, and the intensity score was assessed as: 0 to 3, scaled in 0.25-point increments (0=totally negative; +/3=weak positive; ++/3=moderate positive; +++/3=strongly positive).
Statistical Analysis
Demographic and clinical data, molecular alterations, disease and treatment characteristics were analyzed using descriptive statistics. Statistical comparisons for categorical variables were performed using X2 test. Time-to-event endpoints were described by Kaplan-Mayer curves. Survival distributions for specific subgroups of patients were tested with the log-rank test. A p-value of 0.05 or lower was considered statistically significant. All the analyses were corrected for multiple testing when appropriate and challenged with comprehensive multivariate modeling.
Results
Between September 2017 and April 2022, a total of 42 patients were prospectively and retrospectively enrolled. Twenty-nine were men (69%) and 13 were women (31%) with a median age of 68 years (range 49-79). The primary tumor location was corpus-antrum in 79% of cases and gastroesophageal junction in 21%. A vast majority of the patients (90%) had an optimal performance status (ECOG PS0) at the time of the initial diagnosis. Among the most common presenting symptoms, weight loss more than or equal to 5 kg was reported in 19%. As neoadjuvant treatment, 79% (n=33) of patients had received a taxane-based triplet, while 21% (n=9) received a taxane-free doublet. Postoperative CT was administered in only 50% of cases, due to suboptimal recovery from surgery or postoperative complications, and 85% of them required a dose reduction due to toxicities. In the surgical specimens, lymph node involvement was reported in 50% (n=21) of patients and pT3-4 in 64% (n =27). Clinico-pathological characteristics are summarized in Table 1.
Table 1: Clinical and pathological characteristics of patients
| Patients N (%) | |
| Sex | |
|
Male |
29 (69%) |
|
Female |
13 (31%) |
| Age | |
|
Median |
68 (range 49-79) |
| Location | |
|
Corpus antrum |
33 (79%) |
|
gastroesophageal junction |
9 (21%) |
|
Baseline weight loss |
|
|
No |
24 (57%) |
|
< 5Kg |
10 (24%) |
|
> 5Kg |
8 (19%) |
| ECOG PS | |
|
0 |
38 (90%) |
|
1 |
3 (7%) |
|
2 |
1 (3%) |
| Type of Surgery | |
|
Ivor Lewis |
7 (17%) |
|
Partial Gastrectomy |
17 (40%) |
|
Total Gastrectomy |
18 (43%) |
| ypN | |
|
0 |
21 (50%) |
|
1 |
10 (24%) |
|
2 |
5 (12%) |
|
3 |
6 (14%) |
| ypT | |
|
0 |
0 (0%) |
|
1 |
9 (21%) |
|
2 |
6 (15%) |
|
3 |
24 (57%) |
|
4 |
3 (7%) |
| Intraoperative metastases | |
|
No |
39 (93%) |
|
Yes |
3 (7%) |
| Pre-operative Treatment | |
|
Platinum-based doublet |
9 (21%) |
|
Taxane-based triplets |
33 (79%) |
| Post-operative Treatment | |
|
Fluoropirimidine single agent |
3 (7%) |
|
Platinum-based doublet |
9 (21%) |
|
Taxane-based triplets |
21 (50%) |
|
No |
9 (21%) |
| Grading | |
|
G1 |
2 (5%) |
|
G2 |
23 (55%) |
|
G3 |
15 (35%) |
|
NA |
2 (5%) |
| TRG Mandard | |
|
2 |
10 (24%) |
|
3 |
14 (33%) |
|
4 |
8 (19%) |
|
5 |
8 (19%) |
|
NA |
2 (4%) |
| Recurrence | |
|
No |
25 (60%) |
|
Yes |
17 (40%) |
At the time of the analysis, 16 patients were deceased and 26 patients were still living. Disease recurrence occurred in 40% of patients (n=17). Median DFS was 22.2 months (95%CI 14.9 – NR) and median OS was not reached (95%CI 26.9 – NR). Among the other clinico-pathological factors, univariate analysis showed that weight loss at diagnosis (p=0.03), pathological nodal involvement (p=0.002) and number of neoadjuvant cycles (p=0.03) were significantly associated with DFS. In addition, ypT (p=0.05), ypN (p=0.007), number of neoadjuvant cycles (p=0.0018) and number of adjuvant cycles (p=0.008) were significantly associated with OS. The multivariate analysis confirmed the association between the number of adjuvant cycles and DFS (p=0.018) and OS (0.023) and between the number of neoadjuvant cycles and OS (p=0.042).
A histopathological review was performed by dedicated pathologists focusing on the assessment of tumor regression grade (TRG) according to Mandard. As reported in Table 1, TRG 2 was reported in 24% of cases (n=10) and TGR 3 was reported in 33,3% (n=14), while both TGR 4 and TGR 5 were described in 19% of cases (n=8). Then, we assessed whether TRG was associated with survival outcomes. Consistent with the literature, DFS was significantly longer in patients achieving TRG 2-3 compared with those achieving TRG 4-5 (median DFS not reached vs. 14.9 months; HR=0,36 CI=0,14-0,97; p=0.034) and a trend toward a better OS was also observed across the two subgroups (median OS not reached vs. 26.9 months; HR=0,39; CI=0,14-1,11; p=0.068) (Figures 1 and 2).
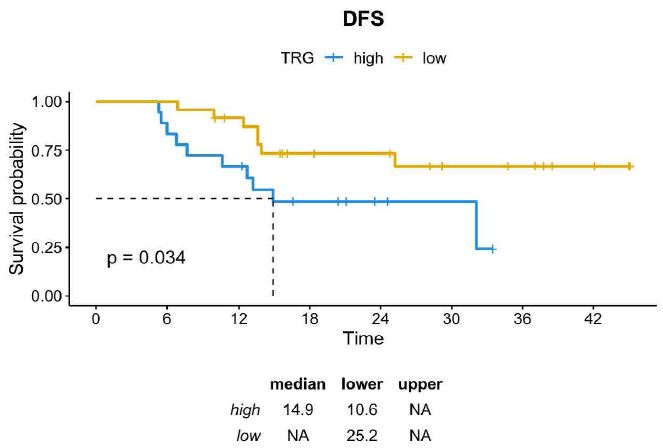
Figure 1: DFS in GC patients achieving TRG 2-3 vs TRG 4-5
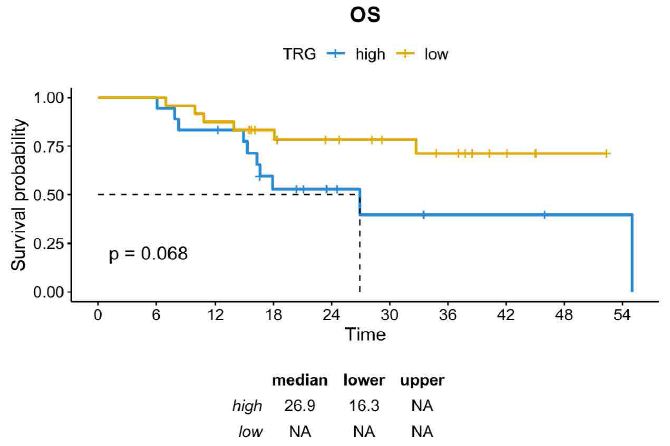
Figure 2: OS in GC patients achieving TRG 2-3 vs TRG 4-5
We evaluated the expression of ATM and SIRT1 in the surgical specimens by IHC. Although optimal ATM staining cutoffs were controversial, according to Kim et al. the criteria for negative cases were set as less than 10% of cells stained as weak positive (+/3) or higher intensity, that is, more than 90% of cells showing totally negative (0) or equivocal staining (±) [15]. For example, if more than 90% of tumor cells showed equivocal (±) or negative (0) staining and less than 10% showed any positive (+, ++ or +++/3) staining, a case was defined as negative (Figure 3).
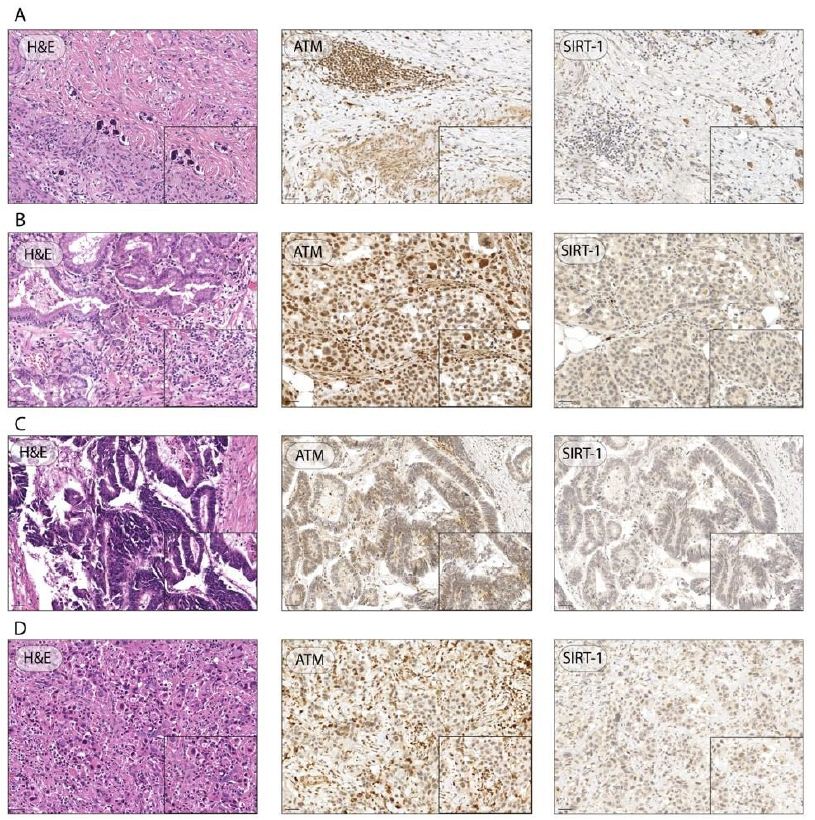
Figure 3: Representative H&E images and IHC of ATM and SIRT-1 expression in GC specimens. Representative images of GC patient with 0 ATM score, 3 SIRT1 score, and TRG of 2 (A); representative images of GC with 3+ ATM score, 0 SIRT1 score, and TRG of 5 (B); representative images of GC with ATM and SIRT1 score 0, and TRG of 5 (C); representative images of GC with 0 ATM score, 1 SIRT1 score, and TRG of 4 (D); (Magnification ×20, inset ×40; scale bar 100 μm, 50 μm, respectively).
Accordingly, as reported in Table 2, ATM score was 0 in 16/42 cases (38%), 1+ in 16/42 (38%), 2+ in 7/42 (17%) and 3+ in 3/42 (7%).
Table 2: ATM and SIRT1 expression
|
Patients N (%) |
|
| ATM | |
|
0 |
16 (38%) |
|
+/3 |
16 (38%) |
|
++/3 |
7 (17%) |
|
+++/3 |
3 (7%) |
| SIRT1 | |
|
<10% |
31 (74%) |
|
10-24% |
3 (7%) |
|
25-49% |
4 (10%) |
|
50-74% |
3 (7%) |
|
≥75% |
1 (2%) |
SIRT1 expression was <10% in 74% of cases, 10-24% in 7%, 25-49% in 10%, 50-74% in 7% and ≥75% in 2% [12]. We then we evaluated the correlations between the expression of ATM and SIRT1 and TRG. Of note, the proportion of patients who obtained a major/medium pathological regression (TRG 2-3) was higher in the ATM-absent subgroup than in the ATM-expressed subgroup (69% vs. 50%; X-squared=6.05; p=0.1) (Figure 4).
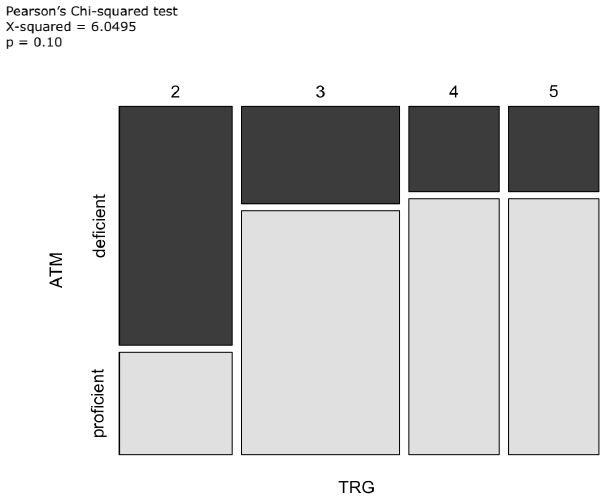
Figure 4: Mosaic plot including TRG and ATM as variables
In the overall population, OS and DFS did not have a significantly different distribution according to ATM and SIRT1 expressions (OS p=0.4 and p=0.2, respectively; DFS p=0.56 and p=0.81, respectively).
In contrast, in the subgroup of patients with TRG 4-5, usually characterized by poor prognosis, the absence of ATM expression seemed to be a positive prognostic factor. The median DFS in patients whose tumor had TRG 4-5 and absent ATM expression was 32.1 months compared to 7.7 months in those with TRG 4-5 and positive ATM expression (p=0.055). Although not statistically significant, the median OS was numerically higher in the subgroup with ATM-absent than in the subgroup with ATM expression (55.0 months vs. 26.9 months, respectively; p=0.6) (Figures 5 and 6).
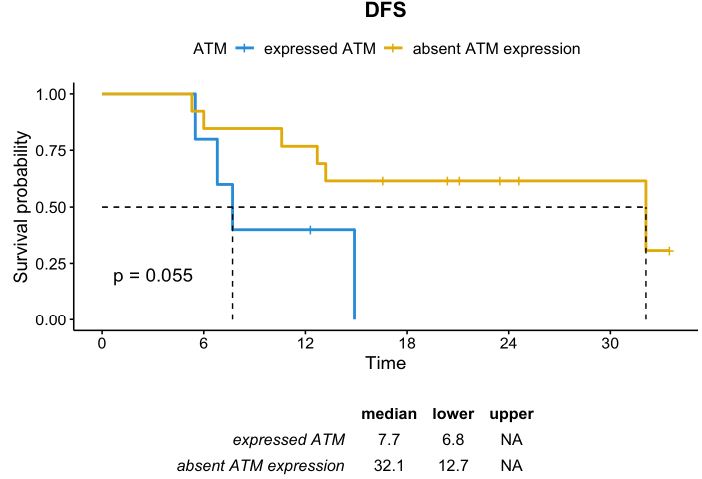
Figure 5: DFS distribution in patients whose tumor had TRG 4-5 according to ATM expression
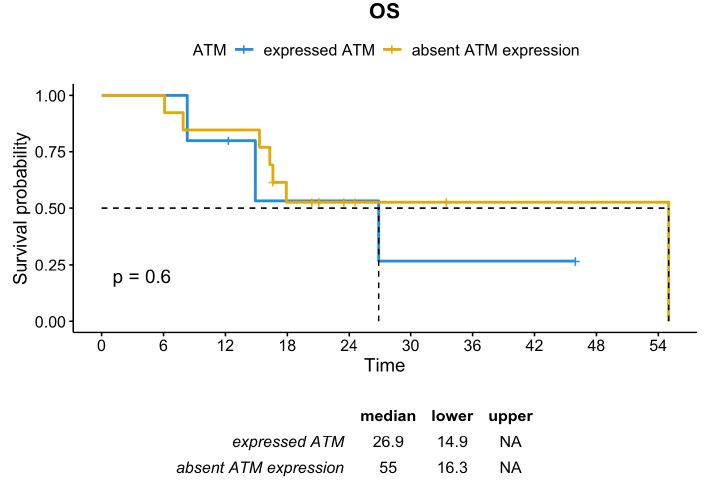
Figure 6: OS distribution in patients whose tumor had TRG 4-5 according to ATM expression
Furthermore, among patients with TRG-high and ATM-expressed, those with SIRT1 <10% had a median DFS of 12.7 months, which was significantly inferior if compared with the entire population (median DFS not reached; HR=0.31; CI=0.11-0.91; p=0.024) (Figure 7).
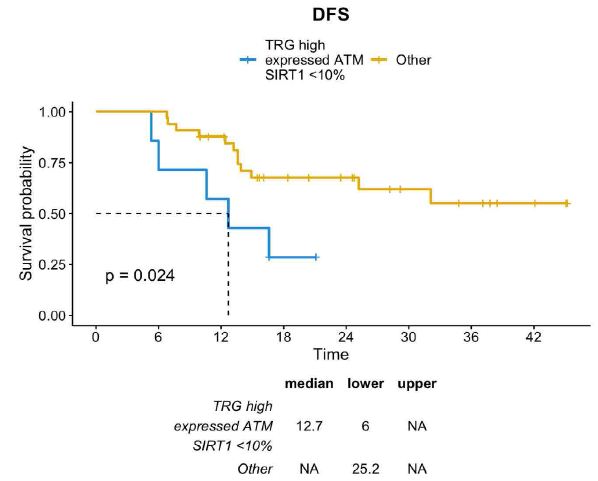
Figure 7: DFS distribution between TRG-high/ATM-expressed/SIRT1-absent profile vs other profiles
This difference was partially sustained by the positive prognosis of TRG-low patients. Therefore, excluding patients with TRG-low, the TRG-high/ATM-expressed/SIRT1-absent profile was associated with a trend toward a lower DFS compared with other TRG-high patients (median DFS 12.7 months vs. 32.1 months; p=0.32) (Figure 8).
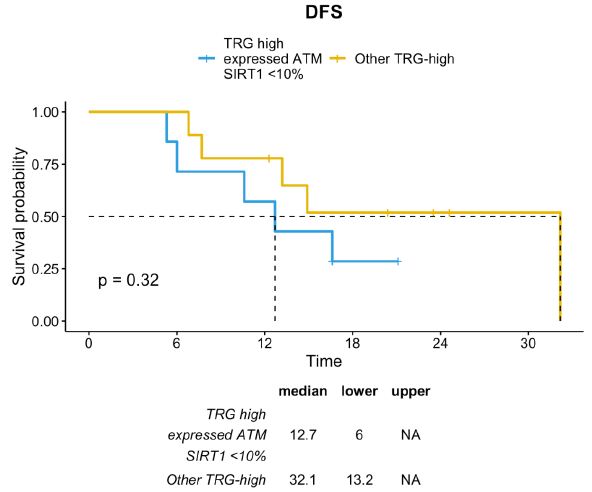
Figure 8: DFS distribution between TRG-high/ATM-expressed/SIRT1-absent profile vs TRG-high subgroup
Discussion
Since the perioperative treatments have become the standard of care for a vast majority of patients with cT2 or higher and/or nodal-positive resectable GC, the identification of new prognostic and predictive biomarkers is an urgent need. ATM expression has been extensively studied with conflicting results [19-21]. Kelmpner et al. did not show any association between ATM expression and clinico-pathological factors and any impact on prognosis from ATM profiles in a cohort of patients who were treated with first-line XELOX for advanced GC [20]. In contrast, in a study of Kim et al., a low ATM expression was associated with older age, advanced stage, MSI, and lower DFS and OS in patients who underwent radical surgery for resectable GC. In this study, the worst prognosis was exhibited by the subgroup which had low ATM expression and MSS [21]. Although the setting was similar to our study, the patient population of Kim and colleagues received upfront surgery followed by adjuvant CT in 50% of cases, while all our patients received neoadjuvant CT followed by surgery, and this may have contributed to a different ATM expression and prognosis. In our study, patients with ATM-expressed cancer after neoadjuvant CT were more frequently associated with TRG 4-5 and, consequently, had a worse prognosis. Although the real reason remains largely unknown, we can suppose that high expression of ATM, playing a crucial role in the repair of DSBs, may offer a highly efficient mechanism of repair from damages induced by CT, radiation, oxidative stress, and stochastic events [11]. As previously described, repair of DSBs involves an extensive network of signals, including a synergism between ATM and SIRT1 with epigenetic implications [11-13]. To date, the role of epigenetic alterations and changes during CT is debated. An extensive knowledge of epigenetic mechanisms underlying prognosis and treatment response could produce new promising epigenetic strategies for GC treatment [22]. SIRT1 contributes to several processes involving GC development, invasion, and metastatic spread. In preclinical studies, knockdown of SIRT1 promoted GC cell migration and invasion in vitro and metastasis in vivo. Among genes downregulated by SIRT1, ARHGAP5 has been identified as an independent prognostic marker of GC [23-25]. As previously described, SIRT1 has an ambiguous role acting both as tumor suppressor and tumor promoter [12,13]. An et al. explored the role of SIRT1 expression in chemoresistance of GC both in vitro and in vivo. They showed that SIRT1 had inhibitory activity on chemoresistance and eliminated cancer stem cell properties [17]. Several retrospective studies suggested a negative prognostic impact of SIRT1-high profile than SIRT1-low, but the prognostic role of SIRT1 expression remains unclear. In a study by Noguchi et al, patients with SIRT1-high GC had a shorter cancer-specific survival than patients with SIRT1-low GC [26,27]. Similarly, Zhang et al. showed that low SIRT1 expression was associated with better outcomes both in patients with advanced GC and in those with early-stage GC [28]. In contrast, Kang et al. reported a positive prognostic effect from SIRT1 expression in a cohort of 452 patients who received surgery for GC. In this study, SIRT1-high profile was associated with more favorable clinicopathological features, including intestinal histotype, lower grade, and lower pT and pN stage [29]. In our study, among patients with poor prognosis (TRG-high and ATM-expressed), a numerically lower DFS was observed in the SIRT1 <10% group than in the SIRT1 ≥10% group. Although statistical significance was not reached, we can speculate that a complete loss of SIRT1 expression could be associated with inhibition in reversing chemoresistance and amplification of the negative prognostic effect of ATM-high profile which efficiently repairs chemotherapy-induced DNA damage. This hypothesis is consistent with a study by An et al. [17], in which silencing of SIRT1 facilitated resistance to 5-fluorouracil and cisplatin.
Regarding limitations, our study included only patients of European origin and the comparison with studies carried out in Asia might be precluded by the geographic heterogeneity in pathological features of GC. In addition, the limited sample size could have induced us to underestimate small differences or subgroup effects. Availability of pretreatment biopsies would have contributed to a better interpretation of the results.
In conclusion, the role of ATM and SIRT1 expression in resected GC patients has not been thoroughly explored and could be of interest for the generation of hypotheses that warrant a future prospective validation in larger clinical trials. This study confirms TRG as a surrogate of survival and suggests an association between ATM expression and TRG. The TRG-high/ATM-expressed/SIRT1-absent profile tends to be associated with a poor prognosis and merits study as a stratification marker after neoadjuvant CT.
Funding
No financial funding was received.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Competing Interests
The authors declare that they have no competing interests.
Abbreviations
ATM: Ataxia Telangiectasia Mutated; CT: Chemotherapy; DFS: Disease-Free Survival; FFPE: Formalin Fixed Paraffin-Embedded; FLOT: 5-Fluorouracil, Oxaliplatin, Docetaxel; GC: Gastric Cancer; HDAC: Histone Deacetylase; IHC: Immunohistochemistry; MSI: Microsatellite Instability; MSI-H: Microsatellite Instability-High; MSS: Microsatellite Stable; OS: Overall Survival; pCR: Pathological Complete Response; SIRT1: Sirtuin-1; TRG: Tumor Regression Grade
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. [crossref]
- Roviello G, Fancelli S, Gatta Michelet MR, et al. TAS-102 in gastric cancer: Development and perspectives of a new biochemically modulated fluroropyrimidine drug combination. Crit Rev Oncol Hematol [crossref]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. [crossref]
- Giommoni E, Lavacchi D, Tirino G, et al. Results of the observational prospective RealFLOT study. BMC Cancer. [crossref]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. [crossref]
- Nappo F, Fornaro L, Pompella L, et al. Pattern of recurrence and overall survival in esophagogastric cancer after perioperative FLOT and clinical outcomes in MSI-H population: the PROSECCO Study. J Cancer Res Clin Oncol. [crossref]
- Lavacchi D, Fancelli S, Buttitta E, et al. Perioperative Tailored Treatments for Gastric Cancer: Times Are Changing. Int J Mol Sci. [crossref]
- Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial.
- Pietrantonio F, Miceli R, Raimondi A, et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J Clin Oncol. [crossref]
- Raimondi A, Palermo F, Prisciandaro M, et al. TremelImumab and Durvalumab Combination for the Non-OperatIve Management (NOM) of Microsatellite InstabiliTY (MSI)-High Resectable Gastric or Gastroesophageal Junction Cancer: The Multicentre, Single-Arm, Multi-Cohort, Phase II INFINITY Study. Cancers (Basel). 2021 Jun 7;13(11): 2839. doi: 10.3390/cancers13112839. PMID: 34200267; PMCID: PMC8201030.
- Choi M, Kipps T, Kurzrock R. ATM Mutations in Cancer: Therapeutic Implications. Mol Cancer Ther. 2016 Aug;15(8): 1781-91. doi: 10.1158/1535-7163.MCT-15-0945. Epub 2016 Jul 13. PMID: 27413114.
- Alves-Fernandes DK, Jasiulionis MG. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int J Mol Sci. [crossref]
- Dobbin MM, Madabhushi R, Pan L, et al. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat Neurosci. [crossref]
- Kang B, Guo RF, Tan XH, et al. Expression status of ataxia-telangiectasia-mutated gene correlated with prognosis in advanced gastric cancer. Mutat Res. [crossref]
- Kim HS, Kim MA, Hodgson D, et al. Concordance of ATM (ataxia telangiectasia mutated) immunohistochemistry between biopsy or metastatic tumor samples and primary tumors in gastric cancer patients. Pathobiology. 2013;80(3): 127-37. doi: 10.1159/000346034. Epub 2013 Jan 15. PMID: 23328638.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. [crossref]
- An Y, Wang B, Wang X, et al. SIRT1 inhibits chemoresistance and cancer stemness of gastric cancer by initiating an AMPK/FOXO3 positive feedback loop. Cell Death Dis. [crossref]
- Miller RM, Nworu C, McKee L, et al. Development of an Immunohistochemical Assay to Detect the Ataxia-Telangiectasia Mutated (ATM) Protein in Gastric Carcinoma. Appl Immunohistochem Mol Morphol. [crossref]
- Bhangoo MS, Luu HY, Kim ST, et al. Low ATM expression is associated with improved progression-free and overall survival in advanced gastric cancer patients treated with platinum-based chemotherapy [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2018; 2018 Apr 14-18; Chicago, IL. Philadelphia (PA): AACR; Cancer Res 2018;78(13 Suppl):Abstract nr 5543.
- Klempner SJ, Bhangoo MS, Luu HY, et al. Low ATM expression and progression-free and overall survival in advanced gastric cancer patients treated with first-line XELOX chemotherapy. J Gastrointest Oncol. [crossref]
- Kim JW, Im SA, Kim MA, et al. Ataxia-telangiectasia-mutated protein expression with microsatellite instability in gastric cancer as prognostic marker. Int J Cancer. [crossref]
- Sato H, Niimi A, Yasuhara T, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. [crossref]
- Gigek C, Wisnieski F. Epigenetic mechanisms in gastric cancer. Epigenomics (2012) 4(3), 279–294.
- Canale M, Casadei-Gardini A, Ulivi P, et al. Epigenetic Mechanisms in Gastric Cancer: Potential New Therapeutic Opportunities. Int J Mol Sci. [crossref]
- Vaziri H, Dessain S, Eaton EN, et al. hSIR2(Sirt1) functions as an NAD-dependent p53 deacetylase. 2021. Cell 107(2): 149-59. Doi: 10.1016/S0092-8674(01)00527-X.
- Dong G, Wang B, An Y, et al. SIRT1 suppresses the migration and invasion of gastric cancer by regulating ARHGAP5 expression. Cell Death Dis 9, 977 (2018). https://doi.org/10.1038/s41419-018-1033-8.
- Noguchi A, Kikuchi K, Zheng H, et al. SIRT1 expression is associated with a poor prognosis, whereas DBC1 is associated with favorable outcomes in gastric cancer. Cancer Med. [crossref]
- Zhang S, Huang S, Deng C, et al. Co-ordinated overexpression of SIRT1 and STAT3 is associated with poor survival outcome in gastric cancer patients. [crossref]
- Kang Y, Jung WY, Lee H, et al. Expression of SIRT1 and DBC1 in Gastric Adenocarcinoma. Korean J Pathol. [crossref]