DOI: 10.31038/CST.2017233
Commentary Article
Uterine sarcomas comprise a group of rare tumors with differing tumor biology, natural history and response to clinical treatment. Diagnosis is often made following surgery for presumed benign disease. Currently pre-operative imaging does not reliably distinguish between benign leiomyomas (LMAs) and other malignant pathology. Human uterine leiomyosarcoma (Ut-LMS) is neoplastic malignancy that typically arises in tissues of mesenchymal origin. The identification of novel molecular mechanism leading to human Ut-LMS formation and the establishment of new clinical therapies has been hampered by several critical points. Our research group earlier reported that mice with a homozygous deficiency for Proteasome beta subunit (Psmb)9/ β1i, an interferon (IFN)-γ inducible factor, spontaneously develop Ut-LMS. The use of research findings obtained from the research experiments with mouse model has been successful in increasing our knowledge and understanding of how alterations, in relevant oncogenic, tumor suppressive, and signaling pathways directly impact sarcomagenesis. The IFN-γ pathway is physiological important for control of tumor progression, and has been implicated in several malignant tumors. In this study, the experiments with human clinical materials revealed a defective expression of PSMB9/β1i in human UtLMS that was traced to the IFN-γ pathway and the specific effect of somatic mutations of Janus kinase (JAK) 1 molecule and/or promoter region on the locus cording PSMB9/β1i gene. Understanding the biological characters of human Ut-LMS may lead to identification of new diagnostic candidates or therapeutic targets against human Ut-LMS.
Uterine mesenchymal tumors have been traditionally divided into benign tumor leiomyoma (LMA) and malignant tumor, i.e. leiomyosarcomas (LMS) based on cytological atypia, mitotic activity and other criteria. Uterine LMS (Ut-LMS), which is some of the most common neoplasms of the female genital tract, is relatively rare uterine mesenchymal tumor, having an estimated annual incidence of 0.64 per 100,000 women [1]. They account for approximately one-third of uterine sarcomas, of only 53% for tumors confined to the uterus [2, 3]. Generally, patients with Ut-LMS typically present with vaginal bleeding, pain, and a pelvic mass. Gynecological tumors, e.g. breast cancer and endometrial carcinomas, are strongly promoted by female hormones, but the rate of expression of hormone receptor in human Ut-LMS is reported to vary in comparison with normal myometrime. Importantly, in case of elder patients, low expressions of hormone receptors were found to unclearly correlate with the promotion of initial disease or with the overall survival of patients with Ut-LMS.
As Ut-LMS is resistant to chemotherapy and radiotherapy, and thus surgical intervention is virtually the only means of clinical treatment for this malignant tumor, however, molecular targeting therapies against tumors have recently shown remarkable achievements [4-8]. It is noteworthy that, when adjusting for stage and mitotic count, human Ut-LMS has a significantly worse prognosis than carcinosarcoma; developing an efficient adjuvant therapy is expected to improve the prognosis of the disease [9]. A trend towards prolonged diseasefree survival is seen in patients with matrix metalloproteinase (MMP)-2-negative tumors [10]. Although typical presentations with hypercalcemia or eosinophilia have been reported, this clinical abnormality is not an initial risk factor for human Ut-LMS. To the best of our knowledge, little is known regarding the biology of human UtLMS; therefore, the risk factors that promote the initial development of human Ut-LMS and regulate their growth in vivo remain poorly understood.
The mice with a targeted disruption of proteasome beta-subunit 9 (PSMB9)/β1i, which is interferon (IFN)-γ-inducible proteasome subunit, exhibited a defect in tissue- and substratedependent physiological function of immune-proteasome, and female PSMB9/ β1i-deficient mice shown to develop Ut-LMS, with a disease prevalence of 37% by 14 months of age [11,12]. Defective expression of PSMB9/β1i is likely to be one of the risk factors for the development of human Ut-LMS, as it is in PSMB9/β1i-deficient mice [12]. Recent report shows that stable expression of PSMB9/β1i contributes to cell proliferation, which directly correlates to the progressive deterioration with increasing stage and the tumor aggressive grade. As the importance and involvement of the IFN-γ pathway for the activation of shared-promoter of PSMB9/β1i and the transporter associated with antigen processing (TAP) 1 have been established, it is demonstrated that the defective expression of PSMB9/β1i is attributable to G871E somatic mutation in the adenosine triphosphate (ATP)-binding region of JAK1 molecule in SKN cell line, which is established from patient with Ut-LMS. It is furthermore likely that the expressions of PSMB9/β1i are significantly down regulated in human Ut-LMS tissues such like human Ut-LMS cell line. Our research group demonstrates that there are serious mutational defects in the factors on the IFN-γ pathway, which is the key signal cascade for PSMB9/β1i expression and promoter region of PSMB9/β1i gene, in human Ut-LMS. The somatic mutational defects in the IFN-γ pathway may induce the initial development of Ut-LMS. Recent advances in our understanding of the biological characters of Ut-LMS have concentrated on the impaired IFN-γ pathway. It is clear that somatic mutations in key regulatory genes alter the behavior of cells and can potentially lead to the unregulated growth seen in malignant tumor. Therefore, continued improvement of our knowledge of the molecular biology of Ut-LMS may ultimately lead to novel therapies and improved outcome.
The effects of IFN-γ on expression of PSMB9/β1i was examined using five cell lines [13]. Expressions of PSMB9/β1i were not markedly induced by IFN-γ treatment in human Ut-LMS cell lines, although cervical epithelial adenocarcinoma cell lines and normal human uterus smooth muscle cells underwent strong induction of PSMB9/β1i following IFN-γ treatment [13]. Furthermore, the immunohistochemistry (IHC) experiments revealed a serious loss in the ability to induce expression of PSMB9/β1i in human Ut-LMS tissues in comparison with normal myometrium tissues located in same tissue sections and 4 various mesenchymal tumor types. Of 58 Ut-LMS, 50 cases were negative for PSMB9/β1i, 4 cases were focally positive, 2 cases were weakly positive, and 2 cases were positive. IHC analyses showed positivity for ki-67/MIB1 and differential expression of estrogen receptor (ER), progesterone receptor (PR), tumor protein 53 (TP53), and calponin h1. In addition, the expression level of PSMB9/β1i was also examined in the skeletal muscle metastasis from human Ut-LMS, the histological diagnosis was consistent with metastatic LMS for skeletal muscle lesions. Pathological study of surgical human samples showed presence of a mass measuring 3 cm at largest diameter in lumbar quadrate muscle without a fibrous capsule. All lymph nodes were negative. In western blotting and RTPCR experiments, PSMB9/β1i was expressed in normal myometrium, LMA, and IFN-γ-post-treated HeLa cells, but not in human Ut-LMS.
The both research experiments strongly supported the research findings obtained from IHC experiments.
Most frequently, human Ut-LMS have appeared in the uterus, retroperitoneum or extremities, and although histologically indistinguishable, they have different clinical courses and chemotherapeutic responses. The molecular basis for these differences remains unclear, in addition, physiological significance of mutational defect is reportedly associated with progression of malignant tumors. Therefore, the molecular examinations of 23 human Ut-LMS tissue regions and normal tissue regions located in the tissue sections obtained from individual patients were performed to detect somatic mutations in the IFN-γ pathway, i.e. JAK1, JAK2, signal transducer and activator of transcription 1 (STAT1) and promoter region of PSMB9/β1i gene (Figure 1). As the catalytic domains of these IFN-γ signal molecules are most likely to harbor mutations that inactivate the gene product, we focused on stretches (exons) containing the kinase domains, transcriptional activation domains and enhancer/ promoter region. Over all, nearly 43.5% (10/23) of human Ut-LMS tissues had serious mutations in the ATP binding region or kinasespecific active site of JAK1; furthermore, 43.5% (10/23) of human Ut-LMS tissues had serious mutations in transcriptional activation sites of the promoter region of PSMB9/β1i gene, which is required for transcriptional activation of PSMB9/β1i gene. No somatic mutation in essential sites, e.g. Tyr701 and Ser727, which are required for physiological function of STAT1 as transcriptional activator, was elucidated in human Ut-LMS. Nearly 21.7% (5/23) of human Ut-LMS tissues unexpectedly had somatic mutations in the intermolecular region of STAT1, which is not yet reported to be important for biological function as transcriptional activation. No somatic mutation in the ATP-binding region and kinase-active site of JAK2 molecule was detected in human Ut-LMS. MOTIF Search profiling [14] and NCBI’s Conserved Domain Database and Search Service, v2.17 analysis also revealed that somatic mutations, which were identified in the catalytic domains of these genes, resulted in impaired physiological functions of tyrosine kinases or transcriptional factor [15]. In a recent report, a comparative genomic hybridization (CGH)-based analysis of human Ut-LMS using a high resolution genome-wide array gave genome-level information about the amplified and deleted regions that may play a role in the development and progression of human Ut-LMS. Other reports showed that among the most intriguing changes in genes were losses of JAK1 (1p31-p32) and PSMB9/β1i (6p21.3) [16,17]. It has also been demonstrated that a correlation exists between the development of malignant tumors and ethnic background, so we conducted CGH experiments with tissue samples obtained from Japanese patients in order to obtain genome-level information. Our results showed that human Ut-LMS having a clear functional loss at JAK1 (1p31-p32) and PSMB9/β1i (6p21.3) also harbored one nonsense mutation and one deletion, suggesting a possible homozygous loss of function. The discovery of these mutational defects in a key signal pathway may be important in understanding the pathogenesis of human Ut-LMS.
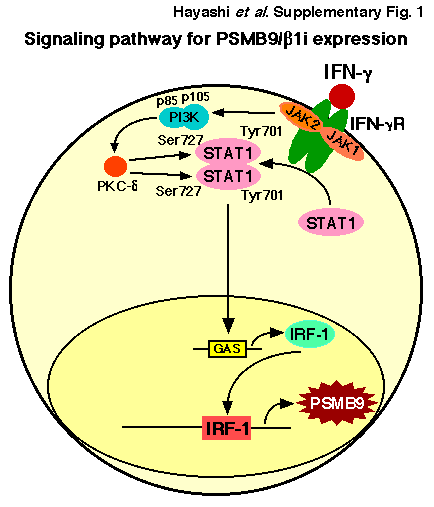
Uterine LMS are relatively rare mesenchymal tumors, having an estimated annual incidence of 0.64 per 100 000 women. They account for approximately one-third of uterine sarcomas and 1.3% of all uterine malignancies. They are the disease with extremely poor prognosis, considering aggressive malignancies with a 5-year survival rate of only 50% for tumors confined to the uterus. At present, surgical intervention is virtually the only means of treatment for Ut-LMS [4- 8]. Although adjuvant pelvic irradiation appears to decrease the rate of local recurrence, adjuvant therapy does not appear to significantly improve survival. Furthermore, gynecological cancer, for instance breast cancer and endometrial carcinomas, are strongly promoted by female hormones, but the rate of expression of estrogen receptor and progesterone receptor is reported to vary in human Ut-LMS compared with normal myometrium. In case of elder patients, low receptor expressions were found to not correlate with the promotion of initial disease or with the overall survival of patients with Ut-LMS; however, molecular targeting therapies against tumors have recently shown remarkable achievements [18]. To improve the prognosis of human Ut-LMS, research experiments were performed to identify the key role of pro- or anti-oncogenic factors that have an important function in their pathogenesis and that could serve as molecular targets for tumor treatment. For this purpose, several research facilities conducted a microarray procedure between human UtLMS and normal myometrium and showed that several known prooncogenic factors, such as brain-specific polypeptide PEP-19 and a transmembrane tyrosine kinase receptor, c-KIT, may be associated with the pathogenesis of human Ut-LMS [19-21]. However, in terms of the sarcomagenesis of human Ut-LMS, merely comparing the expression of potential pro-oncogenic factors between normal and malignant tissues is not sufficient because the results obtained may be the consequence of malignant transformation and, therefore, not necessarily the cause. In addition, dysregulation of apoptotic cascade has also been implicated in many human malignancies. Although the significant differential expression of apoptotic and cell cycle regulators in human Ut-LMS, such as B-cell Lymphoma-2 (BCL-2), BCL-2- Associated X protein (BAX), p16 inhibits CDK4 (P16/INK4a), p21 cyclin-dependent kinase inhibitor 1 (P21/CIP1), p27 kinase inhibitor protein 1 (P27/KIP1), cellular v-KIT Hardy-Zuckerman 4 Feline Sarcoma Viral Oncogene Homolog (c-KIT), mitogen-inducible gene-2 (MIG-2), MDM2, tumor protein 53 (TP53), have all been reported and compared to normal myometrium, there exists no scientific evidence to show that abnormal expression of these factors directly correlates to the initiation and promotion of human Ut-LMS. PSMB9/β1i-dificient mice were reported to be prone to the development of Ut-LMS, but not in their parental mice, C57BL/6 mice [12]. The percentage of mice with overt tumors increased with age after six months, with a cumulative prevalence of Ut-LMS in female mice of 37% by 14 months of age and no apparent plateau at this late observation time. Histopathological examinations of PSMB9/β1i-deficient uterine neoplasms revealed common characteristic abnormalities of Ut-LMS. In addition, recent research reports show the loss in the IFN-γ-inducible ability of PSMB9/β1i expressions in SKN cell line and other primary Ut-LMS cells established from patients. The histopathological experiments revealed serious loss in the ability to induce the expression of PSMB9/ β1i in human Ut-LMS tissues in comparison with normal myometrim tissues located in same tissue sections. IFN-γ treatment markedly induced the expression of PSMB9/β1i, a subunit of the proteasome, which alters the proteolytic specificity of proteasomes. Sequence analysis demonstrated that the loss of IFN- γ responsiveness in the human Ut-LMS cell line was attributable to the inadequate kinase activity due to a G781E somatic mutation in the ATP-binding region of JAK1 molecule [13]. The defect was localized to JAK1 activation, which acts upstream in the IFN-γ pathway since IFN-γ treatment could not strongly induce JAK1 kinase activity in human Ut-LMS cell lines. Genetic alterations in tyrosine kinases have previously been firmly implicated in tumorigenesis, but only a few serine/threonine kinases are known to be mutated in human malignant tumors [22-27]. For instance, mice carrying homozygous deletion of Phosphatase and tensin homolog deleted from chromosome 10 (Pten) alleles developed widespread smooth muscle cell hyperplasia and abdominal LMS [28], and JUN oncogene amplification and over-expression block adipocytic differentiation in highly aggressive sarcomas. Most frequently, LMS have appeared in the uterus, retroperitoneum or extremities, and although histologically indistinguishable, they have different clinical courses and chemotherapeutic responses. The molecular basis for these differences remains unclear, therefore, the examination of human Ut-LMS tissues (23 Ut-LMS tissue sections and normal tissue sections located in the same tissue) was performed to detect somatic mutations in the IFN-γ signal molecules. In a recent report, highresolution genome wide array comparative genomic hybrydization (CGH) analysis of human Ut-LMS cases gave gene-level information about the amplified and deleted regions that may play a role in the development and progression of human Ut-LMS. Among the most intriguing genes, whose copy number sequence was revealed by CGH, were loss of JAK1 (1p31-p32) and PSMB9/β1i (6p21.3) [16,17]. The discovery of these mutational defects in a key cell-signaling pathway may be an important development in the pathogenesis of human UtLMS. The growth of JAK1-deficient cell lines is reportedly unaffected; similarly, the cell cycle distribution pattern of freshly explanted tumor cells derived from JAK1-deficient tumors shows no response to IFN-γ signaling [29]. The growth of the original SKN cells, which had defective JAK1 activity, was unaffected by IFN-γ treatment. In contrast, the growth of JAK1-transfected SKN cells, which had strong exogenous JAK1 activity, was prevented by IFN-γ treatment. Interestingly, when PSMB9/β1i-transfected SKN cells, which have marked the expression of PSMB9/β1i, were analyzed, expression of exogenous PSMB9/β1i resulted in cell growth inhibition. Conversely, the growth of PSMB9/β1i-transfected SKN cells was unaffected by IFN-γ signal pathway. Taken together, IFN-γ response to cell growth inhibition may be attributable to the physiological significance of PSMB9/β1i.
In conclusion, it is clear that in this challenging clinical group of diseases early recognition and diagnosis of human Ut-LMS is critical in order to improve patient outcomes. The down regulation of expression of major histocompatibility complex (MHC)-related factors, including the TAP1 and PSMB9/β1i genes, is one of the biological mechanisms tumor cells use to evade host immune surveillance [30- 32]. Recently, the incidence of IFN-γ unresponsiveness in human tumors was examined in several malignant tumors, and revealed that approximately 33% of each group exhibited a reduction in IFN-γ sensitivity [33]. Nevertheless, the expression of PSMB9/β1i, rather than providing an escape from immune surveillance, seems to play an important role in the negative regulation of human Ut-LMS cell growth. Defective expression of PSMB9/β1i is likely to be one of the risk factors for the development of human Ut-LMS, as it is in the PSMB9/β1i-deficient mouse. Thus, gene therapy with PSMB9/β1i expression vectors may be a new clinical treatment for Ut-LMS that exhibits a defect in the expression of PSMB9/β1i. Because there is no effective therapy for unresectable human Ut-LMS, our results may bring us to specific molecular therapies to treat this disease [34-39].
Disclosure: The Authors report no conflicts of interest.
Acknowledgments
We sincerely appreciate the generous donation of PSMB9/β1ideficient breeding mice and technical comments by Dr. Van Kaer L, Vanderbilt University Medical Center. We thank Isamu Ishiwata for his generous gift of the Ut-LMS cell lines. This work was supported by grants from the Ministry of Education, Culture, Science and Technology, the Japan Science and Technology Agency, the Foundation for the Promotion of Cancer Research, Kanzawa Medical Research Foundation, and The Ichiro Kanehara Foundation.
References
- Zaloudek C, Hendrickson MR (2002) Mesenchymal tumors of the uterus, in Kurman RJ. (ed): Blaustein‘s Pathology of the Female Genital Tract (ed 5). Springer-Verlag: 561-578.
- Gadducci A, Landoni F, Sartori E, Zola P, Maggino T, et al. (1996) Uterine leiomyosarcoma: analysis of treatment failures and survival. Gynecol Oncol 62: 25-32. [crossref]
- Nordal RR, Thoresen SO (1997) Uterine sarcomas in Norway 1956-1992: incidence, survival and mortality. Eur J Cancer 33: 907-911. [crossref]
- Brooks SE, Zhan M, Cote T, Baquet CR (2004) Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma Gynecol Oncol 93: 204-208.
- Dusenbery KE, Potish RA, Argenta PA, Judson PL (2005) On the apparent failure of adjuvant pelvic radiotherapy to improve survival for women with uterine sarcomas confined to the uterus. Am J Clin Oncol 28: 295-300. [crossref]
- Wu TI, Chang TC, Hsueh S, Hsu KH, Chou HH, et al. (2006) Prognostic factors and impact of adjuvant chemotherapy for uterine leiomyosarcoma. Gynecol Oncol 100: 166-172.
- Leitao MM, Soslow RA, Nonaka D, Olshen AB, Aghajanian C. et al. (2004) Tissue microarray immunohistochemical expression of estrogen, progesterone, and androgen receptors in uterine leiomyomata and :leiomyosarcoma. Cancer 101: 1455-1462.
- Perez EA, Pusztai L, Van de Vijver M (2004) Improving patient care through molecular diagnostics. Semin Oncol 31: 14-20. [crossref]
- Miettinen M, Fetsch JF (2006) Evaluation of biological potential of smooth muscle tumours. Histopathology 48: 97-105. [crossref]
- Bodner-Adler B, Bodner K, Czerwenka K, Kimberger O, Leodolter S, et al. (2005) Expression of p16 protein in patients with uterine smooth muscle tumors: an immunohistochemical analysis. Gynecol Oncol 96: 62-66.
- Van Kaer L, Ashton-Rickardt PG, Eichelberger M, Gaczynska M, Nagashima K, et al. (1994) Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity 1: 533-541. [crossref]
- Hayashi T, Faustman DL (2002) Development of spontaneous uterine tumors in low molecular mass polypeptide-2 knockout mice. Cancer Res 62: 24-27. [crossref]
- Hayashi T, Kobayashi Y, Kohsaka S, Sano K (2006) The mutation in the ATP binding region of JAK, identified in human uterine leiomyosarcomas, results in defective interferon-gamma inducibility of TAP1 and LMP2. Oncogene 25: 4016-4026.
- MOTIF Search profiling. http://motif.genome.jp
- NCBI’s Conserved Domain Database and Search Service, v2.17 analysis.
- http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml
- Larramendy ML, Kaur S, Svarvar C, Bo¨hling T, Knuutila S (2006) Gene copy number profiling of soft-tissue leiomyo- sarcomas by array comparative genome hybridization. Cancer Genet Cytogen 169: 94-101.
- Svarvar C, Larramendy ML, Blomqvist C, Gentile M, Koivisto-Korander R, et al. (2006) Do DNA copy number changes differentiate uterine from nonuterine leiomyosarcomas and predict metastasis? Modern Pathol 19: 1068-1082.
- Hayashi T, Horiuchi A, Sano K, Hiraoka N, Ichimura T, et al. (2014) Potential diagnostic biomarkers: LMP2/?1i and Cyclin B1 differential expression in human uterine mesenchymal tumors. Tumori 100: 509-516.
- Kanamori T, Takakura K, Mandai M, Kariya M, Fukuhara K, et al. (2003) PEP-19 overexpression in human uterine leiomyoma. Mol Hum Reprod 9: 709-717. [crossref]
- Wang L, Felix JC, Lee JL, Tan PY, Tourgeman DE, et al. (2003) The proto-oncogene c-kit is expressed in leiomyosarcomas of the uterus. Gynecol Oncol 90: 402-406. [crossref]
- Ylisaukko-oja SK, Kiuru M, Lehtonen HJ, Lehtonen R, Pukkala E, et al. (2006) Analysis of fumarate hydratase mutations in a population- based series of early onset uterine leiomyosarcoma patients. Int J Cancer 119: 283-287.
- Futreal PA, Coin L, Marshall M, Down T, Hubbard T, et al. (2004) A census of human cancer genes. Nat Rev Cancer 4: 177-183. [crossref]
- Jones PA, Baylin SB (2007) The epigenomics of cancer. Cell 128: 683-692. [crossref]
- Lengyel E, Sawada K, Salgia R (2007) Tyrosine kinase mutations in human cancer. Curr Mol Med 7: 77-84. [crossref]
- Pajares MJ, Ezponda T, Catena R, Calvo A, Pio R, et al. (2007) Alternative splicing: an emerging topic in molecular and clinical oncology. Lancet Oncol 8: 349-357. [crossref]
- Ludmil B, Alexandrov LB, Ju YS, Haase K, Van Loo P, Martincorena I, et al. (2016) Mutational signatures associated with tobacco smoking in human cancer. Science 354: 618-622.
- Hayashi T, Kawano M, Ichimura T, Kasai M, Ida K, et al. Gene analysis of Interferon-? signal molecules in human uterine leiomyosarcoma. Wulfenia journal 23: 01-18.
- Post SM (2012) Mouse models of sarcomas: critical tools in our understanding of the pathobiology. Clin Sarcoma Res 2: 20. [crossref]
- Sexl V, Kovacic B, Piekorz R, Moriggl R, Stoiber D, et al. (2003) Jak1 deficiency leads to enhanced Abelson-induced B-cell tumor formation. Blood 101: 4937-4943. [crossref]
- Singal DP, Ye M, Ni J, Snider DP (1996) Markedly decreased expression of TAP1 and LMP2 genes in HLA class I-deficient human tumor cell lines. Immunol Lett 50: 149-154.
- Dovhey SE, Ghosh NS, Wright KL (2000) Loss of interferon-gamma inducibility of TAP1 and LMP2 in a renal cell carcinoma cell line. Cancer Res 60: 5789-5796. [crossref]
- Cabrera CM, Jimenez P, Cabrera T, Esparza C, Ruiz-Cabello F, et al. (2003) Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: beta2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens 61: 211-219.
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, et al. (1998) Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 95: 7556-7561.
- Parmar S, Platanias LC (2005) Interferons. Cancer Treat Res 126: 45-68. [crossref]
- Platanias LC (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5: 375-386. [crossref]
- Bardelli A, Parsons DW, Silliman N, Ptak J, Szabo S, et al. (2003) Mutational analysis of the tyrosine kinome in colorectal cancers. Science 300: 949. [crossref]
- Futreal PA, Coin L, Marshall M, Down T, Hubbard T, et al. (2004) A census of human cancer genes. Nat Rev Cancer 4: 177-183. [crossref]
- Hayashi T, Horiuchi A, Sano K, Hiraoka N, Kanai Y, et al. (2011) Potential role of LMP2 as tumor-suppressor defines new targets for uterine leiomyosarcoma therapy. Scientific Reports 1:180.