Abstract
Introduction: Dentine hypersensitivity (DH) is one of the most common clinically encountered conditions globally, affecting up to 74%. It has been described as a short, sharp pain resulting from changes in the fluid flow of exposed dentinal tubules, in response to physical and chemical stimuli.
Objective: To compare the effectiveness of oxalate containing desensitizing products in reducing both dentine permeability and tubular occlusion vs. a control product using a recognized in vitro model.
Methods: Three oxalate containing products were tested (Listerine® Advanced Defence Sensitive [LADS] mouth rinse, a 3% oxalate solution and an oxalate containing herbal toothpaste), vs. an artificial saliva control. The permeability of the acid-etched dentine discs was measured by hydraulic conductance (Lp). Dentine discs were examined using scanning electron microscopy and energy dispersive X-ray spectroscopy. After establishing the baseline permeability of the acid-etched dentine discs, discs (n=4) were randomly treated with the desensitizing products together with the addition of artificial saliva for 2 mins, followed by rinsing with distilled water (60 s). Permeability was measured at 30 s intervals for a total of 150 s. The occluded discs were acid challenged to assess tubular occlusion stability following the application of both the test and control products.
Results: The oxalate containing desensitizing products in combination with artificial saliva significantly occluded the dentinal tubules by up to 65%, in comparison to the artificial saliva control that occluded ≤21% of the dentinal tubules. The occlusion associated with the oxalate containing desensitizing agents was substantially more stable in resisting an acid challenge compared to the control as determined by hydraulic conductance. Furthermore, the SEM images of the oxalate containing desensitising agents and control were consistent with the hydraulic conductance data. Of interest was that the oxalate containing herbal toothpaste deposited more precipitation on the surface than inside the tubules. The EDX analysis confirmed the presence of oxalates, calcium, and other ingredients of toothpaste. The results from the present study are in broad agreement with those of a previous study in that an oxalate containing mouth rinse provided a more stable tubular occlusion which was more resistant to an acid challenge compared to the other test products.
Conclusion: Oxalate containing desensitizing agents were significantly more effective in occluding the dentinal tubules vs. an artificial saliva control. These results are of clinical significance as they demonstrate that oxalate containing desensitizing agents provide both significant and stable tubular occlusion of the open dentinal tubules following an acidic challenge.
Keywords
Oxalate based desensitizing products, Dentine hypersensitivity, Tubular occlusion, Hydraulic conductance
Introduction
Oxalate is a dianion with several derivatives including salts of oxalic acid, calcium oxalate, sodium oxalate and potassium oxalate and occurs naturally in plants, rhubarb, parsley, spinach, and cocoa. 2-3% potassium oxalate in the form of professionally applied products gels, sealants and whitening strips have become a widely accepted form of treatment for Dentine Hypersensitivity (DH) by practitioners in the USA [1-3]. The incorporation of oxalate products into dental products was based on in vitro studies that reported the dentine discs treated with oxalates showed significantly reduced hydraulic conductance [4- 7]. Other studies have been conducted both in vitro [8-12] and in vivo [13-17] using various oxalate products, with mixed results. However, most of the in vitro studies reported that oxalate products interacted with the exposed dentine tubules to form precipitates of calcium and phosphate, which in turn reduced hydraulic conductance through tubule occlusion. A systematic review comparing data achieved from studies on humans with DH, evaluating oxalate interventions vs. a placebo, concluded that a 3 monohydrogen-monopotassium oxalate treatment was effective against DH [18]. However, it was evident from this review that several studies did not provide a direct comparison between oxalate containing products and a suitable placebo. The mechanism of action of oxalates is not conclusive although, there is some evidence that promotes the theory of oxalate reacting with free calcium ions of saliva or dentine to form calcium oxalate crystals inside and on the surface of dentine tubules [19]. This reaction provides a sealing effect, occluding the tubules to reduce fluid flow within the dentinal tubules, and thus reducing DH. However, it has been shown that the effects of oxalate crystals diminish over time due to the removal of calcium oxalate by brushing or dietary acids [14,15]. Conversely, studies have shown that this can be improved by initially acid etching the dentine, to enhance the penetration of calcium oxalate crystals further within the tubules [20].
Recently, a novel desensitising mouthwash containing 1.4% potassium oxalate (Listerine Advanced Defence Sensitive) was produced as an improved form of delivery for desensitising products [21]. A randomised clinical trial evaluated the efficacy of this product compared to a positive control (Sensodyne Original containing 5% potassium nitrate) and a negative control (Crest toothpaste containing sodium fluoride) over a 4-week period [22]. These investigators reported that the positive control significantly reduced DH compared to the negative control and in turn, the potassium oxalate containing mouthwash significantly reduced DH compared with the negative control. Sharma et al. [23] also demonstrated that multiple in vitro applications of potassium oxalate containing mouth rinses sequentially reduced hydraulic conductance of dentine. The question whether using oxalates in the form of mouth rinses, gels etc., as a long-term solution for treating DH, however, lacks supporting evidence since very few well controlled studies have been conducted. The present study aims to 1) evaluate the effectiveness of selected oxalate based desensitising products, in reducing fluid flow through dentine by tubular occlusion and 2) determine whether oxalates are more effective as a mouthwash or a toothpaste when immersed in artificial saliva.
Aim
The aim of the present study therefore, was to evaluate the ability of oxalate based desensitising products to occlude dentinal tubules and reduce dentine hypersensitivity (DH) by 1) measuring hydraulic conductance and fluid flow within the dentinal tubules using dentine discs following different treatments, 2) demonstrating occlusion of the dentinal tubules openings on the dentine disc sections using Scanning Electron Microscopy and 3) comparing the selected oxalate based desensitising products with other controlled or placebo products (Figures 1 and 2).
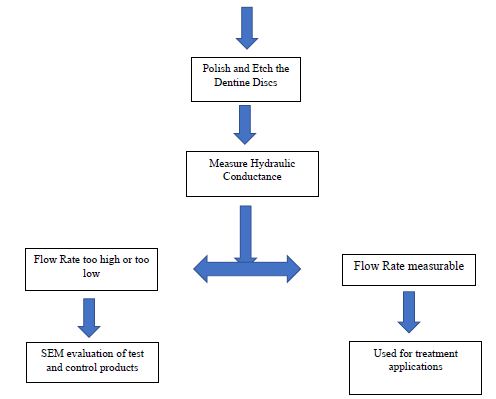
Figure 1: Flow criteria for the selection of dentine discs
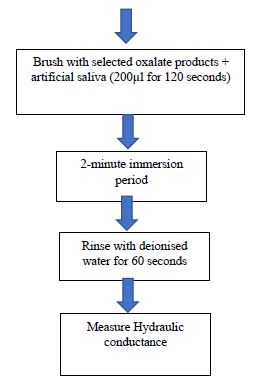
Figure 2: Flow chart steps used to measure hydraulic conductance following treatment with different oxalate based desensitising products and artificial saliva
Material and Methods
Materials
Oxalate Base Desensitising Products
Three commonly used oxalate based desensitising products and one controlled product tested in this study are as follow
- Listerine advanced defence sensitive mouthwash
- Herbal toothpaste containing oxalates
- 3 % oxalic acid solution
- Artificial saliva as control
Methods
Preparation of Dentine Discs
- Cutting of dentine discs. Dentine discs were prepared from 29 extracted human molars and premolars teeth obtained from Royal London Dental Hospital, London after approval from the local Ethics Board/IRB (QMREC2011/99). The teeth were stored in sodium hypochlorite solution. A Struers Accutom-5 diamond cutting was used to cut the dentine discs. The teeth were moulded with impression compound (Kerr’s impression compound) and placed in the holder of the machine to make sure they were stable in the holder and in a direction perpendicular to the cutting diamond blade. The teeth were cut in mesio-distal directions to obtain dentine discs of 900 µm thickness. Discs from the mid coronal section of the crown with no visible defects were selected and stored in a 3 % ethanol solution.
- Sanding and Polishing of Discs. The selected discs were sand blasted on both sides with silicon carbide paper (Buehler-Met) of P1000 and P 2500 grit The discs were then polished using a polishing machine (Kemet 300L lapping Machine) to reduce the thickness of the discs and remove the smear layer. A micrometre gauge was used to measure the final thickness of the discs which were stored in ethanol for future use.
Preparation of Artificial saliva
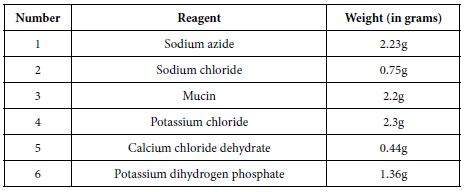
All reagents were weighed in quantities as shown above (Table 1) on a digital weighing machine and were dissolved separately in small amount of deionised water (approximately 20 ml water per reagent) in clean beakers. 400 ml deionised water was boiled in a clean kettle, 400 ml room temperature deionised water was added into it to reduce the temperature, and overall, 800 ml mixed deionised water was placed on magnetic stirrer (Stuart SB 162-3 Hot plate stirrer). Reagents were dissolved separately and added one by one in small quantities until they mixed completely. Deionised water was added to make a 1 L solution and the pH of the artificial saliva was measured to be 5.5 using a pH meter (Oakten 11 series PH meter), the pH was adjusted to a pH of 6.5 by adding 0.5 M potassium hydroxide drop by drop and stored in a fridge at temperature of 2°C.
During the cutting of the dentine discs, a smear layer was created. To remove this smear layer and open the tubules, the discs were etched with a 6% citric acid solution for 180 seconds and then rinsed with deionised water for 60 seconds.
Table 1: Reagents used in the preparation of artificial saliva
Hydraulic Conductance
To obtain initial baseline values each disc was acid etched as indicated above and placed in a Pashley chamber sequentially and the flow rate of each disc was recorded using a modified hydraulic conductance machine (based on the original device by Pashley and co-workers) [4,6]. 27 discs were prepared from 29 human molar and premolar teeth and 25 were subsequently deemed suitable after measuring the baseline fluid flow rates. Initially the hydraulic conductance system was examined to ensure that it was bubble free before introducing an air bubble via a syringe and the movement of the air bubble was measured at a 30 second interval for a period of 150 seconds (2.5 minutes).
The following criterion was used to select the dentine discs for SEM evaluation and Hydraulic conductance.
After measuring the baseline flow rates, 20 discs were suitable for treatment with the selected desensitising agents and five discs were also selected for SEM to assess the tubular occlusion following application of the products.
The following criteria were used to measure hydraulic conductance:
- Twenty discs were used (with four discs per treatment group [n=5]) to determine the flow rate in dentinal tubules.
- The discs remained in a Pashley cell during the measurements to ensure that the disc orientation was the same to prevent any changes in the fluid flow rate. An electric toothbrush (Oral B toothbrush) was used to brush the discs with the selected treatments.
- The discs were then placed in 6% citric acid for 90 seconds to simulate an acid challenge, then rinsed with deionised water for 30 seconds prior to remeasuring the fluid flow rate to determine the impact of an acid challenge.
SEM Evaluation of the Dentine Discs
Initially, four discs were prepared for Scanning Electron microscopy to evaluate the occlusion of dentinal tubules due to the oxalate based desensitising products and control. The discs were sectioned into four parts using orthodontic pliers and the test and control treatments were applied as indicated (Figure 3).
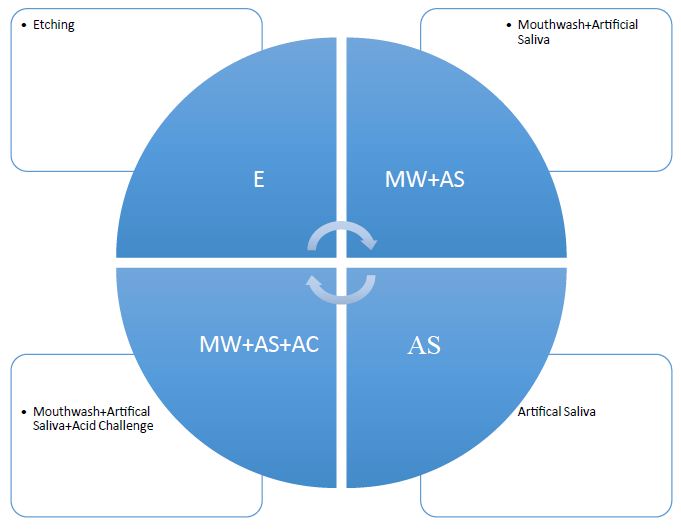
Figure 3: Sectioned dentine discs with the test and control products
EDX was also performed to analyse the elemental composition following the oxalate-based test and control treatments.
Statistical Analysis
The following formula and statistical tests were used to analyse the hydraulic conductance measurements.
Average Percentage Permeability Reduction for each Desensitising Agent
The average permeability reduction for each disc for a particular desensitising agent and control was measured and calculated using the formula below:
![]()
Results
Hydraulic Conductance Results
The average value of permeability reduction for four discs per treatment is shown in Table 2 and a graphical representation of the data with standard deviations is shown in Figure 4 with the degree of resistance (remaining tubular occlusion) provided by both the test and control treatments (Figure 5).
Table 2: Average percentage reduction in permeability following treatment
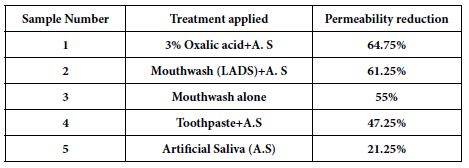
A paired T test was also applied to the HC data following treatment and exposing the discs to an acid challenge, to compare pairs of desensitising agents to determine statistical significance. Values obtained are shown in Tables 3 and 4.
Table 3: P-values from a students T test on comparison of pairs of desensitising agents following treatment
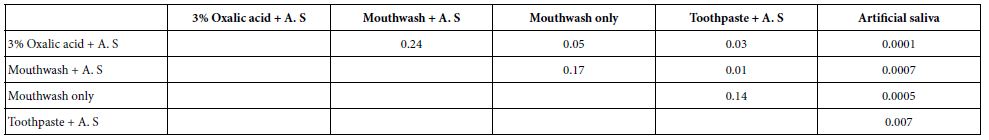
Table 4: P-values from a students T-test on comparison of pairs of desensitising agents following an acid challenge
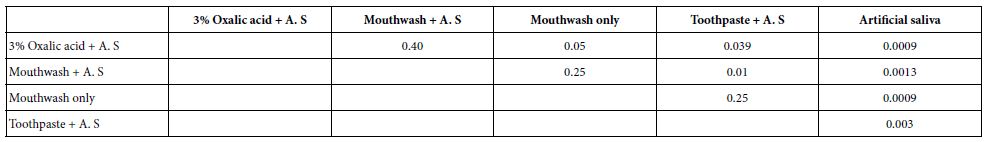
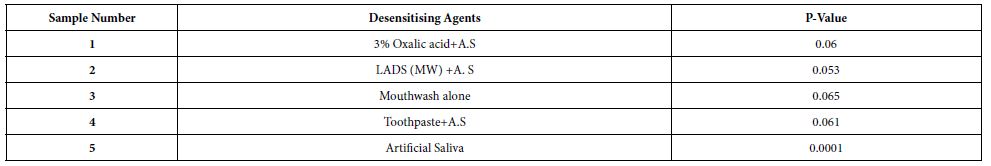
Paired student T tests were also applied to evaluate the HC data to statistically explore the relation between both the treated discs and immersing discs in 6% citric acid solution for each individual desensitising agent and the control. P-values obtained are shown in Table 5.
Table 5: p-values obtained on applying a paired students T-test on the treated discs before and after exposure to an acid challenge for each desensitising agent
Figures 6a-6d represent the hydraulic conductance data for one of the discs selected from the four discs tested. They compare the flow rates before treatment and after applying treatments.
SEM Imaging
Figures 7a-7e shows the images obtained after treating with different oxalate-based reagents and a control using SEM.
Energy Dispersive X-ray Analysis – EDX
The results of the EDX analysis of the oxalate-containing desensitising agents and the artificial saliva control are shown in Figures 8a-8d below. The high peaks of calcium carbon and oxygen were observed in almost all the desensitising agents, confirming the presence of oxalate(s) and that calcium was most likely originating from the addition of the artificial saliva as well as the available calcium already present within the dentinal tubules. EDX analysis of the discs treated with the herbal toothpaste showed small peaks of silicon phosphorous and silica, which confirmed that these were ingredients of the toothpaste. A small sodium peak was observed in the artificial saliva treated disc because of the addition of sodium chloride salt in the preparation stages of the artificial saliva.
Discussion
The hydrodynamic theory as proposed by Brännström [24,25] promotes two basic approaches for treating hypersensitive dentine, namely 1) occluding the patent (open) tubules and thereby reducing any stimulus-evoked fluid movements across dentine and 2) reduce the intradental nerve excitability, to prevent the nerves from responding to the stimulus-evoked fluid movements. Underpinning this mechanism is the importance of the presence of the open tubules and the size of their radius which is according to the Hagen-Poiseuille formula indicates is a direct function of fluid flow. In other words, if the radius of the dentinal orifice (opening) is reduced by tubular occlusion (following application of a desensitising agent) then the minute fluid flow movements within the tubules will be dramatically reduced to the power of r4 and a subsequent reduction in dentine hypersensitivity (DH) would be expected. Numerous desensitising products have been proposed and utilised in clinical practice and most of these products result in tubular occlusion except for potassium-based products where reduction of the response is by nerve desensitisation. Potassium oxalate may have a dual function of both tubular occlusion and nerve desensitisation although evidence for this is not forthcoming. There has however been some concern regarding the use of high concentrations of oxalates in the daily food intake as this can result in kidney stones [26]. It should be noted however those high concentrations of oxalates are often consumed as ingredients of food products as well as seeds and leafy plants such as spinach and rhubarb. For example, the concentration of oxalates in spinach is 794 mg/100 g and in chocolate, it is 60.4 mg/g [27]. The concentration of oxalate in the LADS mouthwash used by Sharma et al. [22,23] was 1.4%, which is, considerably less as compared to the concentration of oxalates found in spinach. One of the main aims of the present study was to determine the reduction in the rate of fluid flow using a hydraulic conductance device following the application of several oxalate containing desensitising products onto an exposed dentine disc compared to a control. In the Sharma et al, [23] the investigators used a Listerine Advanced Defence Sensitive (LADS) mouthwash without the addition of artificial saliva, however in the present study the LADS mouthwash was used with and without the addition of artificial saliva. The rationale for this methodological change was that potassium oxalate would require a source of calcium to form the calcium oxalate precipitate. Since the dentine disc was treated with citric acid and washed in distilled water it can be postulated that most of the available of calcium rich fluid would have been removed from the disc surface. Consequently, the only calcium available to react with the oxalate was the calcium present within the dentine tubules which may explain the observation that tubule occlusion is often not seen at the surface with oxalates [5,8] The inclusion of immersing the dentine disks in AS, however provides a copious supply of Ca to react with the oxalate.
The results from the present study demonstrated than the application of the selected oxalate products resulted in a significant reduction in fluid flow rate for all the desensitising agents compared to a control as shown in Figures 4, 5 and Table 2. A 3% oxalate solution resulted in the highest average permeability reduction of 64.75%. These values of permeability reduction were calculated as an average of fluid flow rates for four treated discs against their flow rates calculated when the discs were only etched. One of the problems encountered with measuring fluid flow through dentine was the variation in the tubular orientation and density within each disc as can be observed with the standard deviations (Figures 4 and 5) which may impact on the results particularly in a relatively small sample size. An interesting observation from the results was the improvement in the flow rates when the samples were immersed in artificial saliva. For example, the LADS mouthwash with the addition of artificial saliva resulted in a greater average reduction in dentine permeability as compared to LADS mouthwash alone, 61.25% vs. 55% respectively Overall, the oxalate containing desensitising products 3% Oxalic Acid,, LADS mouthwash with or without AS and the Herbal toothpaste in combination with artificial saliva significantly occluded the dentinal tubules by up to 65%, in comparison to the artificial saliva control that occluded less than 21% of the dentinal tubules (Figures 4, 5, Table 2). The stability of tubule occlusion following an acid challenge demonstrated that while the flow rates increased for the oxalate containing products, indicating that there was some loss of the oxalate precipitate with a slight increase in the tubular radius, nevertheless the values were still favourable compared to the artificial saliva control (Figures 5 and 6a-6d).
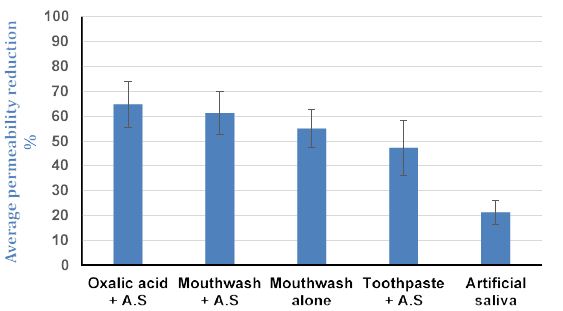
Figure 4: Average percentage reduction in permeability (fluid flow rate) following treatment
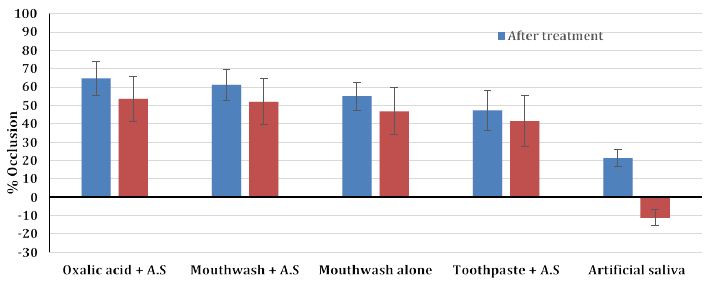
Figure 5: The stability of tubule occlusion after an acid challenge for the selected oxalate containing desensitising agents and control (% occlusion and standard deviations)
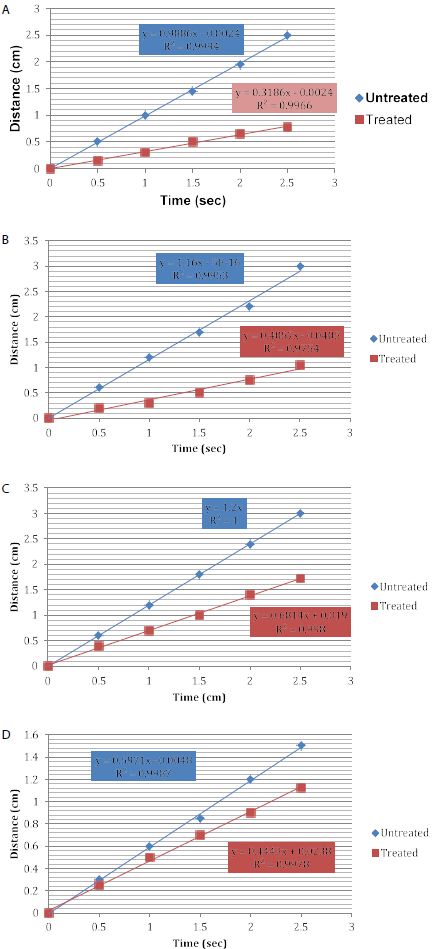
Figure 6a: Reduction in Hydraulic conductance following the application of a 3% oxalic acid solution
Figure 6b: Reduction in Hydraulic conductance after the application of LADS mouthwash and artificial saliva
Figure 6c: Reduction in Hydraulic conductance after application of the Herbal toothpaste and artificial saliva
Figure 6d: Reduction in Hydraulic conductance after application of the artificial saliva (control disc)
Statistically, there was a significant difference between all the oxalate containing desensitising agents and the artificial saliva control, in terms of the reduction in permeability (flow rate) following application of the oxalate containing desensitising agents and the acid challenge (Table 3 and 4; see also Figures 6a-6d). Significant differences were also observed the herbal toothpaste vs. the 3 % oxalic acid solution and the LADS mouthwash with artificial saliva where the p value was 0.03 and 0.01 respectively.
The application of oxalate containing desensitising agents with the addition of artificial saliva, therefore resulted in the enhanced formation of calcium oxalate precipitates which occluded the dentine tubules and surface and reduced the fluid flow rates. The calcium oxalate crystals formed by the oxalate containing desensitising agents were very stable to an acidic challenge and retained most of their occlusion compared to the control where all the crystals formed by the artificial saliva were completely washed away. The SEM images of the oxalate containing desensitising agents and control were consistent with the hydraulic conductance data (Figures 7a-7e). Of interest was the oxalate containing herbal toothpaste where more precipitation was evident on the surface rather than inside the tubules. EDX analysis confirmed the presence of oxalates, calcium, and other ingredients of this toothpaste formulation. The results from the present study are also in broad agreement with those of Sharma et al. [23] in that an oxalate containing mouth rinse provided a more stable tubular occlusion which was more resistant to an acid challenge compared to the other test products. However, it should be noted that the Sharma et al. study used multiple applications of the oxalate mouthwash to obtain a reduction in hydraulic conductance, whereas in the present study this effect was achieved using only one application of oxalate.
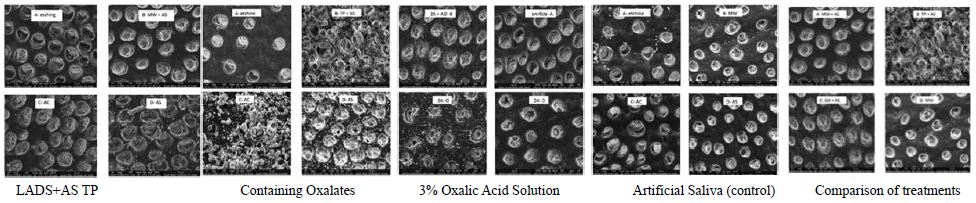
Figure 7a: SEM images of a quartered dentine disc treated with Listerine Advanced Defence Sensitive mouthwash +Artificial Saliva: A) Etching-showing opening of dentine tubules. B) Treated with LADS+AS showing significant occlusion of tubules by calcium oxalate crystals. C) Acid Challenge: showing that the oxalate crystals are very stable to acid challenge due to their sustained tubular occluding properties D) The control: disc treated with Artificial Saliva showing relatively low levels tubular of occlusion and almost fully opened tubules.
Figure 7b: SEM images of a quartered dentine disc treated with toothpaste (TP) +Artificial Saliva. A) Etching showing the opening of dentine tubules. B) Treated with TP+AS showing significant numbers of oxalate crystals on the surface with partially occluded tubules. C) Acid Challenge showing that the oxalate crystals are very stable to an acid challenge due to their sustained tubular occluding properties D) The control disc treated with Artificial Saliva showing relatively low levels of tubular occlusion and almost fully opened tubules.
Figure 7c: SEM images of a quartered dentine disc treated with Oxalic Acid (OA) +Artificial Saliva. A) Etching: showing the opening of dentine tubules. B) Treatment with OA+AS showing significant occlusion of tubules by calcium oxalate crystals. C) Acid Challenge: showing oxalate crystals are very stable to acid challenge due to their sustained tubular occluding properties D) The control: disc treated with Artificial Saliva showing relatively low levels of tubular occlusion and almost fully open tubules.
Figure 7d: SEM images of a quartered dentine disc treated with LADS mouthwash alone (LADSMW). A) Etching: showing the opening of dentine tubules. B) Treatment with LADSMW showing significant occlusion of the tubules by calcium oxalate crystals. C) Acid Challenge after Artificial Saliva treatment: showing that immersion in artificial saliva does not improve the stability of the oxalate crystals against an acid challenge. The openings of the dentine tubules remained opened. D) The control: disc treated with Artificial Saliva showing relatively low levels of tubular occlusion and almost fully opened tubules.
Figure 7e: SEM images of the quartered dentine discs illustrating a comparison between the different treatments based on the occlusion of dentine tubules. A) Treatment with Mouthwash+ Artificial Saliva showed reasonable occlusion of the tubules. B) Treatment with Toothpaste +Artificial Saliva showed moderate occlusion of tubules with numerous crystals formed on the surface. C) Treatment with Oxalic Acid + Artificial Saliva showed significant occlusion of dentine tubules with relatively few crystals deposited on the surface. D) Treatment with Mouthwash alone showing only partial occlusion of the tubules.
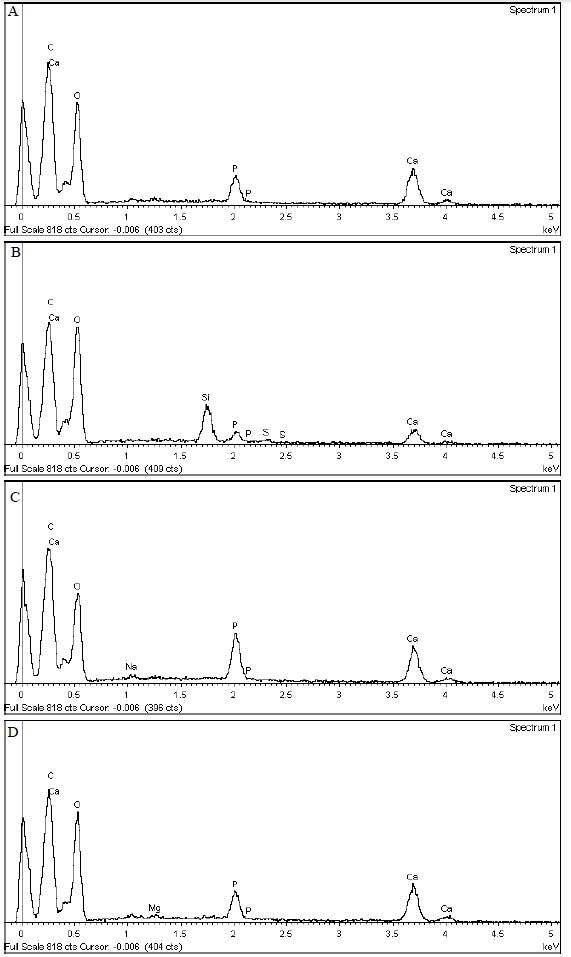
Figure 8a: EDX analysis of the LADS Mouthwash + Artificial Saliva treated disc
Figure 8b: EDX analysis of the Herbal Toothpaste + Artificial Saliva treated disc
Figure 8c: EDX analysis of the Artificial Saliva treated disc
Figure 8d: EDX analysis of the 3% Oxalic acid solution +Artificial Saliva treated disc
Conclusion
Within the limitations of the present in vitro study, it was observed that oxalate containing desensitizing agents were significantly more effective in occluding the dentinal tubules vs. an artificial saliva control. These results are of clinical significance as they demonstrate that oxalate containing desensitizing agents provide both significant and stable tubular occlusion of the open dentinal tubules following an acidic challenge.
References
- Cunha-Cruz J, Wataha J, Zhou L, et al. (2010) Dentin hypersensitivity: choice of treatments by dentists of the Northwest PRECEDENT network. J Am Dent Assoc 141: 1097-1105.
- Anderson CJ, Gerlach RW, Kugel G, et (2016) Use of Oxalate Strips in Dentistry: Overall Response and Case Studies with Recent and Longstanding Sensitivity. Compend Contin Educ Dent 37(1): e32-e37.
- Pejmon A, Melanie M, Paul Sage, et al. (2016) Comparative Effects of 1.5% Oxalate Strips Versus 5% Potassium Nitrate Dentifrice on Dentin Hypersensitivity. Compend Contin Educ Dent 37(1).
- Greenhill J and Pashley D (1981) The Effects of Desensitizing Agents on the Hydraulic Conductance of Human Dentin in vitro. J Dent Res 60(3): 686-698.
- Pashley D and Galloway S (1985) The effects of oxalate treatment on the smear layer of ground surfaces of human Arch Oral Biol 30(10): pp.731-737.
- Pashley D, Livingston MJ, Reeder OW et al. (1978) Effects of the degree of tubule occlusion on the permeability of human dentine in vitro. Arch Oral Biol 23(12): 1127- 1133.
- Pashley DH, Andringa HJ, Eicnmiller F (1991) Effects of ferric and aluminium oxalates on dentine J Am Dent Assoc 4(3): 123-126.
- Ling T, Gillam D, Barber P, et al. (1997) An investigation of potential desensitizing agents in the dentine disc model: a scanning electron microscopy J Oral Rehabil 24(3): pp.191-203.
- Zhang Y, Agee K, Pashley D et al. (1998) The effects of Pain-FreeR desensitizer on dentine permeability and tubule occlusion overtime, in vitro. J Clin Periodontol 25(11): 884-891.
- Pillon F, Romani I and Schmidt E (2004) Effect of a 3% Potassium Oxalate Topical Application on Dentinal Hypersensitivity after Subgingival Scaling and Root Planning. J Periodontol 75(11): 1461-1464.
- Sauro S, Gandolfi M, Prati C et al. (2006) Oxalate-containing phytocomplexes as dentine desensitisers: An in vitro Arch Oral Biol 51(8): pp.655-664.
- Pereira J, Segala A and Gillam D (2005) Effect of desensitizing agents on the hydraulic conductance of human dentin subjected to different surface pre-treatments-an in vitro Dent Mater 21(2): pp.129-138.
- Muzzin K and Johnson R (1989) Effects of Potassium Oxalate on Dentin Hypersensitivity in J Periodontol 60(3): pp.151-158.
- Kerns DG, Scheidt MJ, Pashley DH, Horner JA, Strong SL, Van Dyke TE. Dentinal tubule occlusion and root J Periodontol 1991 Jul;62(7): 421-8.
- Gillam D, Coventry J, Manning R, et (1997) Comparison of two desensitizing agents for the treatment of cervical dentine sensitivity. Dent Traumatol 13(1): pp.36-39.
- Morris MF, Davis RD, Richardson BW (1999) Clinical efficacy of two dentin desensitizing agents. Am J Dent 12(2): 72-6.
- Santiago S, Pereira J and Martineli A (2006) Effect of commercially available and experimental potassium oxalate-based dentin desensitizing agents in dentin sensitivity. Dent Traumatol 13(1): 36-39.
- Cunha-Cruz J, Stout J, Heaton L et al. (2010) Dentin Hypersensitivity and J Dent Res 90(3): pp.304-310.
- Orchardson R and Gillam D (2006) Managing dentin hypersensitivity. J Am Dent Assoc 137(7): 990-998.
- Hongpakmanoon W, Vongsavan N, Soo-Ampon M (1999) Topical application of warm oxalate to exposed human dentine in J Dent Res 78: 300.
- Mantzourani M and Sharma D (2013) Dentine sensitivity: Past, present, and J Dent 41: pp. S3-S17.
- Sharma D, McGuire J, Gallob et al. (2013) Randomised clinical efficacy trial of potassium oxalate mouth rinse in relieving dentinal sensitivity. J Dent 41: pp. S40-S48.
- Sharma D, Hong C and Heipp P (2013) A novel potassium oxalate-containing tooth- desensitising mouth rinse: A comparative in vitro J Dent 41: pp. S18-S27.
- Brannstrom M, Astrom A (1972) The hydrodynamics of the dentine; its possible relationship to dentinal Int Dent J 22: 219-227.
- Brannstrom M, Linden L and Astrom, A (1967) The Hydrodynamics of the Dental Tubule and of Pulp Caries Res 1(4): pp.310-317.
- Mitchell T, Kumar P, Reddy T, et (2019) Dietary oxalate and kidney stone formation. Am J Physiol Renal Physiol 316(3): F409-F413.
- Holmes RP, Kennedy M (2000) Estimation of the oxalate content of foods and daily oxalate intake. Kidney Int 57: 1662-1667.