Abstract
The opening of a solvus curve around their critical point starts with a singularity. At this singularity, an element distribution forms, described by a Lorentzian-type curve, showing the highest concentration of some trace and primary elements at the critical point, corresponding to the most elevated temperatures, and with cooling, this Lorentzian curve opens and snuggles up to the solvus curve. This combination is strong proof of supercritical transition to critical and under-critical conditions. Furthermore, we show here that remnants of an older mineralization are present, demonstrating that the current picture must not be correct. Supercritical fluids can have a significant influence on mineralization as a whole.
Keywords
Supercritical fluids, Element enrichment, Solvus- and Lorentzian-curves, Tin deposit Ehrenfriedersdorf
Introduction
Since the beginning of the studies on melt inclusion in pegmatite quartz from the tin deposit Ehrenfriedersdorf, particularly on the pegmatites from the Sauberg mine, in the years around the turn of the century, we often found very water-rich melt inclusions. Water content and temperature appear to be a characteristic relationship from the beginning: a characteristic solvus curve [1]. After that, such curves were also found for many other pegmatites and evolved granites worldwide (Figure 1) [2].
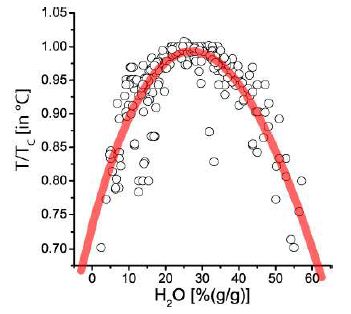
Figure 1: Pseudo-binary solvus curve for 19 different evolved granites and pegmatites worldwide. Note: each point represents the arithmetic mean of measurement on up to 100 melt inclusions. Values at T/TC (in °C)=1.0 correspond to the solvus curve’s critical point. The abscissa (analytically determined water content) first approximates the melt density. Note here that the point scattering is, in a first approximation, the result of the complex interaction of volatiles (H2O, F, H3BO3), which sometimes work additively.
For the origin of such curves, a clear answer could not given. At this time, the necessary analytical technique was still in it’s infancy-however, the evidence of the characteristic relationship between water content and temperature increases significantly year to year. Applying reduced parameters (T/TC) displays relatively good comparability for different granite and pegmatite systems (Figure 1) see also Figure 2 in Thomas and Davidson, 2015) [3]. So, a more universal relationship is probably. However, demonstrating such curves was the first step to solving this puzzle because a deeper origin is behind the solvus curves (temperature versus water concentration). Here, we explicitly use the water concentration because the density decreases and is not steady with the increase in the melt’s water content (especially at or near the solvus crest). We have shown [4] that at the solvus crest, the concentration of volatiles can obtain extreme values far away from the solvus crest water concentration (including F, B, C, P, and high concentrations of alkali elements, Be, Sn, and others.
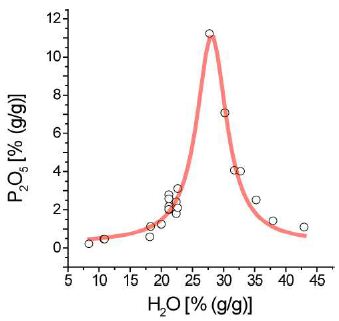
Figure 2: Lorentzian distribution of P versus H2O concentration of water-rich melt inclusions in pegmatite quartz from the Sauberg mine, Ehrenfriedersdorf. Note: each point represents the data mean on 5 to 10 melt inclusions.
Key Observations
Starting with the work on the enrichment of Be in granite-pegmatite systems in 2011 [5], it would, from case to case, be evident that the highest element enrichment is related to the solvus crest-the start point for the opening the solvus with the drop in temperature. According to our findings of diagnostic HP-HT minerals from mantle depths transported by supercritical fluids, it would be more evident that the solvus crest is the locus where the supercritical fluid changes into the critical/under critical state. Therefore, here, we mainly found the highest enrichment of the most scarce elements (Be, Cs, Zn, Sn, and many others). To our surprise, the strongly enriched trace and main elements show a characteristic Lorentzian distribution related to the water concentration, with the maximum corresponding to the solvus crest [2]. Some elements show a Gaussian distribution caused by overlapping two or more Lorentzian curves of different anion species. Mathematically speaking, the critical point here is a physicochemical singularity. During the transition from supercritical to critical and hydrothermal conditions, unusual processes are far from equilibrium, such as moissanite and beryl’s synchronous growth [6]. Typically, for technical processes, moissanite grows at temperatures above 1000°C. Also, typical rare elements in granitic systems show extreme enrichment in part. One such process is the excessive element enrichment, such as zinc [7]: 75.000 to 85.000 ppm Zn in a fluid inclusion trapped near the solvus crest with a water concentration of about 30%. Table 1 shows the extraordinarily enriched elements and their relationship to the Clarke values.
Table 1: Extreme trace element enrichment in syngenetic fluid inclusions trapped at or near the solvus crest [7].
|
Element (in ppm) |
Melt inclusion 1 | Melt inclusion 2 | Clarke (ppm) | Enrichment |
|
Rb |
12.300 | 15.700 | 200 |
61.5-78.5 |
| Cs |
22.700 |
20.000 | 5 | 4540-4000 |
|
Zn |
80.000 | 45.000 | 50 |
1600-900 |
| Cd |
200 |
580 | 0.1 | 2000-5800 |
|
Sn |
240 | 2.200 | 3 |
80-733 |
| Sb |
900 |
740 | 0.26 | 3462-2846 |
|
Pb |
1.790 | 1.430 | 20 |
89.5-71.5 |
Clarke, according to Rösler and Lange (1975) [8]
At room temperature, Zn is, according to Raman spectroscopy, present as a well-transportable potassium tetrachlorocomplex: K2[ZnCl4]. Another unusual observation at the beginning of our research was the finding of extreme P-rich melt inclusions in pegmatite quartz Qu8 with 50.7 ± 3.5% P2O5 [8,9]. Because the phosphorus here was easily water-soluble, we were cautious during sample cleaning before any analytical studies. Later, we found melt inclusion in pegmatite quartz from the Sauberg mine with high P2O5 concentrations. Near the solvus crest, phosphorus shows strong enrichment in the form of the Lorentzian distribution (Figure 2). That is significantly higher than London (1998) [10] showed for P-rich peraluminous granites. This author interpreted higher P values as local build-up in boundary layers. Here, we tell another story: Enrichment of P by the transition of supercritical fluids into undercritical fluids during the interaction of the first one with the granites. Similar results arise also for the sulfate-anion and other elements.
In different publications, the authors [2,4,11] have shown that many trace and main elements are Lorentzian distributed. The proof was not straightforward because many pieces of evidence are nearly invisible because of the extreme postmagmatic hydrothermal intensity in the region, which blurs the primary processes. The remnants of strongly peralkaline mineralization prove such changes. The first author found strong peralkaline melts in the Ehrenfriedersdorf pegmatite Qu8, indicated by nepheline in quartz (Figure 3) [12]. That is an extraordinary observation because, usually, nepheline and quartz exclude themselves.
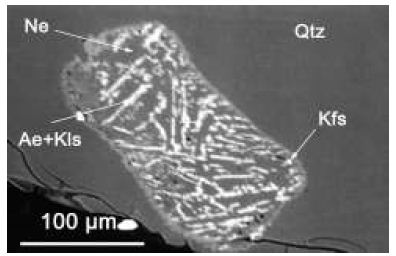
Figure 3: Nepheline in pegmatite quartz (Qu8) from Ehrenfriedersdorf, Sauberg mine, Germany. The nepheline is composed of nepheline [(Na, K)AlSiO4] and kalsilite [KAlSiO4]. Between the nepheline and quartz is a tiny K-feldspar rim.
Table 2 gives the microprobe results on nepheline and feldspar. The nepheline aggregate (Figure 3) resembles a corroded crystal according to the form and feldspar rim. However, most nepheline crystals in this sample are in K-feldspar. That means that the nepheline points to very different rock chemistry before the reworking of the whole deposit by intensive hydrothermal activity. Non-consideration can lead to entirely wrong conclusions. Figure 4 shows another nepheline crystal in K-feldspar.
Table 2: Shows the composition of nepheline and K-feldspar by EMP analyses
|
Nepheline |
K-feldspar-rim | |
|
SiO2 |
45.8 ± 1.4 |
68.5 |
| Al2O3 |
31.8 ± 0.4 |
16.6 |
|
FeO |
0.3 ± 0.1 |
0.4 |
| Na2O |
17.1 ± 0.1 |
3.2 |
|
K2O |
3.8 ± 0.3 |
9.1 |
| Rb2O |
0.2 ± 0.04 |
0.2 |
|
P2O5 |
~0.02 |
0.02 |
| Sum |
99.02 |
98.0 |
|
n |
10 |
1 |
| XNe |
0.86 |
|
| XKls |
0.14 |
|
| XOr |
0.65 |
|
| XAb |
0.35 |
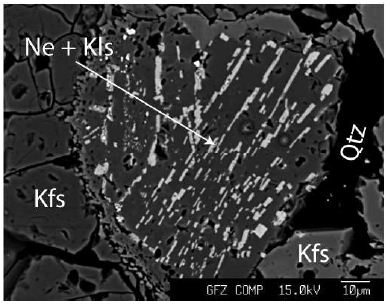
Figure 4: Nepheline (Ne) and Kalsilite (Kls) in K-feldspar (Kfs) in pegmatite Qu8. Quartz (Qtz) is secondary.
After re-homogenization, in the same sample are larger aggregates of silicate glass (~ 5mm) with a very low ASI (aluminum saturation index): ASI = 0.418 ± 0.009. The origin of this rock (now glass) is unclear. In the glass are small remnants of albite, K-feldspar, and nepheline. Table 3 shows the composition of this glass, determined with the microprobe SX50.
Maybe this rock (glass) is the reaction product of the supercritical fluid with the granitic rock in the reaction room (in the crust). At this time, maybe till 2012, the sporadically found high concentration of some elements (also tin) was explained more by chance (see also the discussion by London and Evensen 2002) [13]. We have had no explanation for the nepheline and glass with the low ASI values. However, from then on, we found indications of regularities in the appearance of many trace elements. A prerequisite for this systematic study was the water determination of the melt inclusion in question. Surprising was the Lorentzian distribution of about 20 trace elements with the water content of the corresponding melt inclusions [2].
Table 3: Composition of the re-homogenized silicate glass (700°C, 3.0 kbar) in the sample with nepheline in quartz and K-feldspar.
|
Mean |
± 1σ | |
|
SiO2 |
71.39 |
0.25 |
| TiO2 |
~0.01 |
<0.02 |
|
Al2O3 |
7.17 |
0.13 |
| P2O5 |
0.05 |
0.02 |
|
FeO |
0.21 |
0.04 |
| MnO |
<0.03 |
– |
|
CaO |
0.00 |
– |
| MgO |
<0.01 |
– |
|
Na2O |
7.64 |
0.10 |
| K2O |
4.14 |
0.04 |
|
Rb2O |
0.15 |
0.01 |
| Cs2O |
<0.04 |
– |
|
F |
0.00 |
– |
| Cl |
<0.01 |
|
|
H2O |
9.00 |
0.25 |
| Sum |
99.85 |
|
|
ASI |
0.42 |
0.02 |
Mean from 72 determinations, σ: Standard deviation, ASI: Aluminum Saturation Index, water is the difference to 100% and corresponds to the Raman spectrometric determined water content.
Interpretation
The combination of the pseudo-binary melt-water solvus with the extreme element enrichment in the shape of the typical Lorentzian distribution is, together with the HP-HT mineral relicts (diamond, graphite, moissanite, reidite, coesite, and others), a solid-proof of the interaction of supercritical fluids coming from mantle depths with rocks in the upper crust. Furthermore, the extraordinary element parageneses in the Ehrenfriedersdorf case imply an older subducted deposit in the mantle region already postulated by Schütze et al. (1983) [14] according to isotope, element-geochemical and radio-geochronological studies.
Our latest studies, which brought unambiguous proofs of the interaction between supercritical fluids coming from mantle depths and granitic rocks in the upper crust, the following points are essential:
- The most spherical crystals, often tiny, of diamond, graphite, moissanite, and others in crustal minerals prove that a supercritical fluid transported these crystals very fast from mantle depths to the crust.
- All such minerals are spherical, which means that during transport from the mantle to the crust, elements of these crystals go into the supercritical fluid by partial solution.
- The “chemical way” from mantle to crust differs from point to point. By that, the composition of the supercritical fluid is also not uniform.
- The transition from the supercritical state into the critical and under-critical state is related to the processes far from equilibrium.
- The fast-moving supercritical fluids are also not in an energetic equilibrium with the surroundings, bringing much water and energy into the crustal region.
- In this highly excited state, processes happen that are conventionally not feasible (moissanite whiskers grow in beryl at significantly lower temperatures and pressures.
- The decisive element enrichment in the Lorentzian distribution shows impressively that enrichment processes occur, which, under hydrothermal conditions, are impossible.
Discussion
Apart from our work, other colleagues have not found such correlations (a combination of natural solvus curves and Lorentzian element distributions). Here, we see a beautiful field of activity that can bring us completely new views into geological processes related to supercritical fluids. An excellent experimental starting point demonstrates [15]. For example, Zn is highly soluble in high-temperature fluids. Therefore, a longer transportway is possible. That means, not far from the Ehrenfriedersdorf tin deposit, there is also a Zn deposit possible. In reverse, a larger Sn deposit is also possible under the famous Pb-Zn deposit in the Freiberg area because we have found there spherical remnants of REE-rich tveitite-Y crystals [Ca14Y5F43], typical for evolved tin-granites from Zinnwald, E-Erzgebirge.
Acknowledgment
We thank the many people who contributed to the supercritical fluids work over the last 25 years. A special thanks go to James (Jim) D. Webster (1955-2019), who initiated the intense work on evolved granites and pegmatites.
References
- Thomas R, Webster JD, Heinrich W (2000) Melt inclusions in pegmatite quartz: complete miscibility between silicate melts and hydrous fluids at low pressure. Mineral. Petrol. 139: 394-401.
- Thomas R, Davidson P, Rericha A, Voznyak DK (2022) Water-rich melt inclusions as “frozen” samples of the supercritical state in granites and pegmatites reveal extreme element enrichment resulting under non-equilibrium conditions. Mineralogical Journal (Ukraine) 44: 3-15.
- Thomas R, Davidson P (2015) Comment on “A petrologic assessment of internal zonation in granitic pegmatites” by David London (2014). Lithos 212-215: 462-468.
- Thomas R, Rericha A (2023) The Variscan tin deposit Ehrenfriedersdorf, Germany: To the solubility of tin in supercritical and near-supercritical fluids. Geology, Earth and Marine Sciences 5: 1-5.
- Thomas R, Webster JD, Davidson P (2011) Be-daughter minerals in fluid and melt inclusions: implications for the enrichment of Be in granite-pegmatite systems. Mineral. Petrol. 161: 483-495.
- Thomas R, Recknagel U, Rericha A (2023a) A moissanite-diamond-graphite paragenesis in a small beryl-quartz vein related to the Variscan tin-mineralization of the Ehrenfriedersdorf deposit, Germany. Aspects in Mining & Mineral Sciences 11: 1310-1319.
- Borisova AY, Thomas R, Salvi S, Candaudap F, Lanzanova A, et al. (2012) Tin and associated metal and metalloid geochemistry by femtosecond LA-ICP-QMS microanalysis of pegmatite-leucogranite melt and fluid inclusions: new evidence for melt-melt-fluid immiscibility. Mineralogical Magazine 76: 91-113.
- Rösler HJ, Lange H (1975) Geochemische Tabellen. Second Edition. VEB Deutscher Verlag fürGrundstoffindustrie Leipzig, pg: 675.
- Thomas R, Webster JD, Rhede D (1998) Strong phosphorus enrichment in a pegmatite-forming melt. Acta Universitatis Carolinae-Geologica. 42: 150-164.
- London D (1998) Phosphorus-rich peraluminous granites. Acta Universitatis Carolinae-Geologica 42: 64-68.
- Thomas R, Davidson P, Appel K (2019) The enhanced element enrichment in the supercritical states of granite-pegmatite systems. Acta Geochim 38: 335-349.
- Thomas R, Webster J.D., Rhede D, Seifert W, Rickers K, et al. (2006) The transition from peraluminous to peralkaline granitic melts: Evidence from melt inclusions and accessory minerals. Lithos 91: 137-149.
- London D, Evensen JM (2002) Beryllium in silicic magmas and the origin of beryl-bearing pegmatites. In: Beryllium-Mineralogy, Petrology, and Geochemistry. Edited by E.S. Grew. Reviews in Mineralogy & Geochemistry 8: 445-486.
- Schütze H, Stiehl G, Wetzel K, Beuge P, Haberland R, et al. (1983) Isotopen-und elementgeochemische sowie radiogeochronologische Aussagen zur Herkunft des Ehrenfriedersdorfer Granits-Ableitung erster Modellvorstellungen. ZFI-Mitteilungen 76: 232-254.
- Sun Y, Liu X, Lu X (2023) Structures and transport properties of supercritical SiO2-H2O and NaAlSi3O8-H2O fluids. American Mineralogist in press.