Abstract
In the experiment was studied the effect of the addition of sodium humate to feed mixtures on carcass characteristics, relative weight of organs, fermentation process in caecum and dropping quality. Overall, one hundred one-day-old broiler chicks were assigned in two equal groups. Birds of the experimental group were fed with diets supplemented with sodium humate (in amount 5 g.kg-1 during the first two weeks and 7 g.kg-1 from the 3rd to the 6th week). The experimental period lasted 6 weeks. The carcass yield and relative weights of the monitored internal organs were not statistically significantly affected compared to the control group. The addition of sodium humate led to an increase in the proportion of abdominal fat, significantly in cocks (P<0.05). The level of short-chain fatty acids (except for butyric acid) and the pH value in the caecum content were not significantly influenced by the addition of sodium humate. The content of butyric acid was significantly higher in the experimental group than in the control group (P˂0.05). The dry matter content of chicken droppings was not significantly affected, but the addition of sodium humate led to a significant decrease in the content of crude protein (P<0.01), which may contribute to reducing the environmental burden from poultry farms.
Keywords
Humic substances, Carcass yield, Caecal fermentation, Dropping quality, Poultry
Introduction
In recent years, interest in humic substances and their possible use in animal nutrition have increased in research. These are substances of natural origin occurring in rock sediments, peat, brown coal and lignite. Humic substances are products of chemical and biological degradation of dead plant and animal tissues. Humic acids, fulvic acids and humin are considered the main fractions of humic substances [1]. Humic acids form the highest quality fraction of humic substances [2]. The ability to bind ions is given by their polyanionic character [3]. Together with calcium and magnesium, they form calcium and magnesium humates that are insoluble in water, which affect the favorable technological properties of soils. With sodium and potassium, they form sodium and potassium humate, which are characterized by good solubility in water. They have the ability to bind a number of heavy metals (e.g. Cd, Pb, Zn, Hg), with which they form difficult-to-dissolve compounds and thus limit their movement in the soil and uptake by plants [4].
Nowadays, humic substances are used in agriculture (both in plant and animal production), in industry, in veterinary and human medicine, pharmacology and in the field of environmental protection. In plant production, they are mainly used as fertilizer in the form of humates [3]. Humic acids and their sodium salts are permitted for oral use in horses, ruminants, swine and poultry for the treatment of diarrhoea, dyspepsia and acute intoxications [5]. The results of various studies show that the addition of humic substances to diets or water can lead to an increase in the intensity of animal growth, to an improvement in feed conversion ratio, to a decrease in mortality [6-11], to increase carcass yield [6,12] and can also affect the chemical composition of the meat [13-15]. Their positive influence may consist in increasing the use of nutrients from the feed through the stabilization of the intestinal microflora [16,17] or through increasing the height of the villi of the intestinal mucosa, which leads to an increase in the absorption surface [6,7]. Their positive effects on animal immunity were also recorded [18-20]. However, it follows from the results of various studies that the influence of humic substances can be different depending on the composition and amount of administered humic substances, on the method of their application (in feed or water) or on the type of animals used.
The objective of this experiment was to study the influence of sodium humate on carcass characteristic, processes of digestive tract and dropping quality in broiler chicks.
Materials and Methods
Animals and Experimental Design
One hundred one-day-old unsexed chickens (ROSS 308) were included in the experiment, which were divided into two groups (n = 50) and placed on deep litter while observing standard environmental conditions. Lighting was continuous throughout the whole experimental period. The experiment was carried out in accredited stables of the Department of animal nutrition and husbandry at the University of Veterinary Medicine and Pharmacy in Košice in compliance with the EU regulations concerning the protection of experimental animals. The experiment was carried out with the consent of the institutional Animal Care and University Ethics Committee.
During the experiment, the chickens were fed with complete feed mixtures based on corn, wheat and soybean meal according to the growth phases: starter diet (1st-2nd week), grower diet (3rd-5th week), and finisher diet (6th week). No antibiotic growth promoters or anticoccidial drugs were used in the diets.
The first group designated as the control group, was without the addition of the monitored substances. In the second group, sodium humate (dry matter 84.8%, humic acids 63.2%, ash 36.9%) was added to the mentioned feed mixtures at the expense of wheat in the amount of 5 g.kg-1 of diet in the first phase and 7 g.kg-1 of diet in the second and third phase of fattening. Diets and drinking water were provided ad libitum over the whole experimental period. Composition of diets used in respective experimental periods is shown in Table 1.
Table 1: Composition of the experimental diets
|
Control group |
Sodium humate group | |||||
| Starter | Grower | Finisher | Starter | Grower |
Finisher |
|
| Ingredients (g.kg-1) | ||||||
| Maize |
435 |
500 | 500 | 435 | 500 | 500 |
|
Wheat |
121 | 90 | 104 | 116 | 83 |
97 |
| Soybean meal |
360 |
330 | 310 | 360 | 330 | 310 |
|
Vegetable oil |
40 | 40 | 50 | 40 | 40 |
50 |
| Limestone |
20 |
16 | 15 | 20 | 16 | 15 |
|
Vitamin-mineral premix |
201 | 202 | 203 | 201 | 202 |
203 |
| Lysine |
4 |
4 | 1 | 4 | 4 | 1 |
|
Sodium humate |
5 | 7 |
7 |
|||
| Chemical analysis | ||||||
| Dry matter (g) |
897 |
900 | 894 | 898 | 897 | 906 |
|
Crude protein (g.kg-1 DM) |
250 | 231 | 219 | 249 | 232 |
222 |
| Crude fibre (g.kg-1 DM) |
37 |
44 | 43 | 40 | 37 | 38 |
|
Crude ash (g.kg-1 DM) |
82 | 67 | 66 | 74 | 66 |
69 |
| Ether extract (g.kg-1 DM) |
70 |
72 | 80 | 72 | 69 | 78 |
|
Calculated analysis |
||||||
| ME (MJ/kg DM) |
13 |
13 | 14 | 13 | 13 |
14 |
DM: Dry Matter; ME: Metabolizable Energy
1Vitamin and Mineral premix (per kg): Ca 95 g, P 135 g, Na 75 g, Mg 5 g, DL-methionine 80 g, vit.A 600,000 IU, D3 135,000 IU, E 900 mg, K3 150 mg, panthotenic acid 600 mg, niacin 4000 mg, cholin chloride 20,000 mg, B6 150 mg, B12 900 μg, biotin 3000 μg, folic acid 76,000 μg, vit. C 2000 mg, Fe 1500 mg, Cu 500 mg, Zn 3000 mg, Mn 5000 mg, I 25 mg, Se 23 mg, Co 10 mg;
2Vitamin and Mineral premix (per kg): Ca 100 g, P 135 g, Na 75 g, Mg 5 g, DL-methionine 80 g, vit. A 425,000 IU, D3 84,000 IU, E 900 mg, K3 100 mg, pantotenic acid 420 mg, niacin 3400 mg, cholin chloride 14,200 mg, B6 100 mg, B12 640 μg, biotin 2150 μg, folic acid 54,500 μg, vit.C 1400 mg, Fe 1500 mg, Cu 500 mg, Zn 3000 mg, Mn 5000 mg, I 25 mg, Se 23 mg, Co 10 mg;
3Vitamin and Mineral premix (per kg): Ca 110 g, P 145 g, Na 75 g, Mg 9 g, DL-methionine 55 g, vit. A 370,000 IU, D3 135,000 IU, E 900 mg, K3 95 mg, panthotenic acid 370 mg, niacin 3880 mg, cholin chloride 14,000 mg, B6 95 mg, B12 560 μg, biotin 1850 μg , folic acid 47,000 μg, vit.C 1240 mg, Fe 1500 mg, Cu 500 mg, Zn 3000 mg, Mn 5000 mg, I 25 mg, Se 23 mg, Co 10 mg.
Sampling and Measurements
Internal organs (liver, heart, spleen, bursa of Fabricius, and pancreas) were obtained on the 14th and 35th days of the experiment from eight chickens from each group after they were weighed and killed. The relative weight of internal organs is expressed as a percentage of the live body weight of chickens. On the 35th day of the experiment, the contents of the caecum were obtained from seven chickens from each group, in which the pH and concentration of short-chain fatty acids (acetic, propionic, butyric, and lactic acid) were determined. The pH value of caecum contents was determined by pH-meter (Consort C830, Belgium). The concentration of short-chain fatty acids was analysed by isotachophoresis using a two-capillary isotachophoretic analyser (EA100, VILLA LABECO, Slovak Republic).
The faeces were collected thrice a day every day during the second and fifth week. The collection of faeces from random chickens in each group was made on clean solid base immediately after excretion to eliminate any contamination with raw feed or feathers. Composite samples from each group in appropriate amounts were frozen and kept at-18 °C until analysis for dry matter and crude protein content.
At the end of the trial (42nd day), the birds were left for 10-12 h without feed, weighed and slaughtered, processed by decapitation, neck, feathers and feet removal and evisceration. Twenty birds per group (ten from each sex) were used for evaluation of carcass yield and abdominal fat pad (percentage carcass weight). The carcass yield is expressed as a percentage of the carcass weight from the body weight before slaughter.
The chemical compositions of the diets and faeces were determined analytical methods according to the EC Commission Regulation 152/2009 [21].
Statistical Analysis
Statistical evaluation of the effects of sodium humate on monitored parameters was done by unpaired t-test with the statistical software GraphPad Prism 8.0. For all statistical calculations, the significance was considered as a value of P < 0.05. Data are presented as means ± standard error of means (SEM).
Results and Discussion
The carcass yield of broiler chickens was not statistically significantly affected by the addition of sodium humate to feed mixtures (Table 2). These results agree with the results of other studies in which the effect of humic substances was observed in chickens [22-24] and quails [25]. El-Husseiny et al. [26] reported opposite results in their experiment, where the carcass yield of chickens that received a feed mixture with the addition of humic substances in a concentration of 0.25 and 0.125% was significantly higher than in the group without the addition of humic substances. A significantly higher carcass yield was also recorded in broiler chickens that were fed feed with the addition of humic acids in 0.6% concentration [12].
Table 2: Effect of sodium humate on carcass yield and abdominal fat pad
|
Treatments |
Carcass yield
(%) |
Abdominal fat (%) |
| Female | ||
| Control |
74.02 |
2.03 |
|
Sodium humate |
73.71 |
2.22 |
| SEM |
0.297 |
0.148 |
|
P-value |
0.624 |
0.534 |
| Male | ||
| Control |
73.74 |
1.38a |
|
Sodium humate |
73.57 |
2.09b |
| SEM |
0.278 |
0.154 |
|
P-value |
0.769 |
0.016 |
SEM: Pooled standard error of the mean
Values marked with a different superscript in the same column are statistically significantly different (abP < 0.05).
A higher percentage of abdominal fat was recorded in the sodium humate-supplemented group than in the control group (Table 2). A statistically significant difference was found in cocks (P < 0.05). Ozturk et al. [27] also noted an increase in abdominal fat under the influence of humic substances in broiler chickens.
The results of present study are not in agreement with the findings of El-Husseiny et al. [26], who reported that the addition of humates to feed can lead to a reduction in abdominal fat in broiler chickens. A decrease in the percentage of abdominal fat due to the addition of humic substances to the feed was also recorded in Japanese quail [6].
The relative weight of the internal organs was not statistically significantly affected by the addition of the monitored substance compared to the control group (Table 3).
Table 3: Effect of sodium humate on relative weight of some internal organs
|
Treatments |
Liver
(%) |
Heart
(%) |
Spleen
(%) |
Bursa of Fabricius
(%) |
Pancreas (%) |
| On the 14th day | |||||
| Control |
3.493 |
0.681 | 0.066 | 0.226 | 0.389 |
|
Sodium humate |
3.493 | 0.745 | 0.061 | 0.257 |
0.395 |
| SEM |
0.076 |
0.017 | 0.003 | 0.011 | 0.024 |
|
P-value |
0.999 | 0.062 | 0.451 | 0.172 |
0.906 |
| On the 35th day | |||||
| Control |
2.015 |
0.588 | 0.099 | 0.266 | 0.210 |
|
Sodium humate |
2.079 | 0.538 | 0.087 | 0.258 |
0.204 |
| SEM |
0.082 |
0.025 | 0.004 | 0.016 | 0.009 |
|
P-value |
0.711 | 0.336 | 0.150 | 0.819 |
0.763 |
SEM: Pooled standard error of the mean
Similar results were recorded by Karaoglu et al. [22], Kaya and Tuncer [23] and Arif et al. [9]. Likewise, Rath et al. [28] reported no changes in the relative weights of heart, liver and spleen in broiler roosters receiving humic acid-enriched feed at 1.0 and 2.5% concentration compared to the control group, but the weight of the bursa of Fabricius was significantly higher in the group with 2.5% concentration of humic acid. This indicates a positive immunostimulating effect of humic acids. ELnaggar and El-Kelawy [10] also noted the enlargement of the bursa of Fabricius due to humic acids.
On the other hand, Abdel-Mageed [6], who investigated the effect of supplementing humic substances in the diet of Japanese quail, noted a significant increase in the relative weight of the liver.
Short-chain fatty acids produced by microbial fermentation of carbohydrates in the gastrointestinal tract are beneficial for the animal. They are used by the host organism as a source of energy, and their presence in the digestive tract leads to a decrease in pH of the intestinal content, which can inhibit pathogenic bacteria and can accelerate the proliferation of intestinal epithelial cells [29].
Feeding sodium humate in the concentration used had no significant effect on the concentration of acetic, propionic and lactic acid in the contents of the caecum (Table 4). However, the content of butyric acid, which has a positive effect on the growth of epithelial cells in the gastrointestinal tract [30], was significantly higher in the experimental group than in the control group (P ˂ 0.05). The pH value of the caecum content was not significantly affected.
Table 4: Effect of sodium humate on pH and concentration of short-chain fatty acids in the caecum content
|
Treatments |
pH | Acetic acid | Propionic acid | Butyric acid | Lactic acid |
| (mmol.L-1) | (mmol.L-1) | (mmol.L-1) |
(mmol.L-1) |
||
| Control |
6.93 |
145.95 | 27.22 | 8.78a | 29.18 |
|
Sodium humate |
6.75 | 145.00 | 20.82 | 12.89b |
35.50 |
| SEM |
0.056 |
4.713 | 1.943 | 0.936 | 2.866 |
|
P-value |
0.099 | 0.925 | 0.101 | 0.021 |
0.287 |
SEM: Pooled standard error of the mean
Values marked with a different superscript in the same column are statistically significantly different (abP < 0.05).
Our results are partly consistent with the results reported in the study by Shermer et al. [31]. The addition of humate in amounts of 5 and 10 g.kg-1 of the feed mixture had no significant effect on the concentration of acetic, propionic, isobutyric, isovaleric, valeric as well as butyric acid in the content of the caecum of broiler chickens. Similar results were recorded in broiler chickens that were given diets with the addition of natural humic substances in amounts of 5 and 7 g.kg-1 [32].
The dry matter content in chicken droppings was not significantly affected by the addition of sodium humate (Figure 1a). Although in the second week of the experiment a slightly higher content of crude protein in chicken droppings was detected in the experimental group (Figure 1b), in the fifth week a significantly lower crude protein content was recorded in this group than in the control group (P < 0.01).
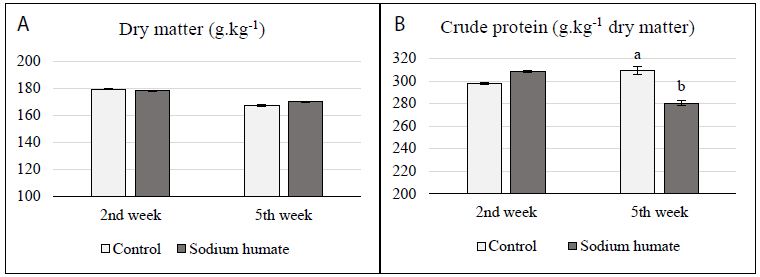
Figure 1: Effect of sodium humate on content of (a) dry matter and (b) crude protein in droppings (abP < 0.01)
We also recorded similar results in our earlier study, in which we investigated the use of natural humic substances in the fattening of broiler chickens [32]. This significant reduction in the content of nitrogenous substances in chicken droppings indicates a better utilization of nitrogenous substances from the feed. This leads to the decrease of volatile ammonia emerging by microbial fermentation in the litter. A higher concentration of ammonia in the air of stud areas negatively affects health and performance of animals as well as health of farm staff [33].
Conclusion
The carcass yield and relative weights of the observed internal organs were not significantly affected by the addition of sodium humate to the diets. However, a higher proportion of abdominal fat was recorded in the experimental group than in the control group (significantly in cocks), significantly higher the content of butyric acid in the contents of the caecum and significantly lower content of crude protein in chicken droppings. The significant decrease in the content of crude protein in the dry matter of chicken droppings indicate that sodium humate can contribute to reducing the burden on the environment from poultry farms.
Acknowledgment
This work was supported by Slovak project VEGA No. 1/0402/20.
References
- Stevenson FJ (1994) Humus Chemistry: Genesis, Composition, Reactions. Wiley-Inter-Science: New York, NY, USA. 34-41.
- Islam KMS, Schuhmacher A, Gropp J M (2005) Humic acid substances in animal agriculture. Pakistan Journal of Nutrition 4: 126-134.
- Veselá L, Kubal M, Kozler J, Innemanová P (2005) Structure and properties of natural humic substances of the oxyhumolite type. Chemické listy 99: 711-717.
- Vrba V, Huleš L (2006) Humus-soil-plant (2) Humus and soil. cz, Available from https://biom.cz/cz/odborne-clanky/humus-puda-rostlina-2-humus-a-puda (Last modified November 14, 2006), ISSN: 1801-2655 (in Czech).
- EMEA (1999) Committee for veterinary medicinal products. Humic acids and their sodium salts. Available from emea.eu.int/pdfs/vet/mrls/055499en.pdf (Last modified April 21, 2008. Accessed February 1999).
- Abdel-Mageed MAA (2012) Effect of dietary humic substances supplementation on performance and immunity of Japanese quail. Egyptian Poultry Science Journal 32: 645-660.
- Taklimi SMS, Ghahri H, Isakan MA (2012) Influence of different levels of humic acid and esterified glucomannan on growth performance and intestinal morphology of broiler chickens. Agricultural Sciences 3: 663-668.
- Mirnawati YR, Marlida Y (2013) Effects of humic acid addition via drinking water on the performance of broilers fed diets containing fermented and non-fermented palm kernel cake. Archiva Zootechnica 16: 41-53.
- Arif M, Rehman A, Saeed M, Abd El-Hack MZ, Arain MA, et al. (2016) Impacts of dietary humic acid supplementation on growth performance, some blood metabolites and carcass traits of broiler chicks. Indian Journal of Animal Sciences 86: 1073-1078.
- ELnaggar AS, El-Kelawy MI (2018) Effect of humic acid supplementation on productive performance, blood constituents, immune response and carcass characteristics of sasso chicken. Egyptian Journal of Animal Production 55: 75-84.
- Hammod AJ, Zeny ZAH, Mahdi AS, Alfertosi KA (2021) Probiotic and humic acid as feed additives and their effects on productive and economic traits of broiler. Indian Journal of Ecology 48: 35-37.
- Marcinčáková D, Mačanga J, Nagy J, Marcinčák S, Popelka P, et al. (2015) Effect of supplementation of the diet with humic acids on growth performance and carcass yield of broilers. Folia Veterinaria 59: 165-168.
- Ozturk E, Ocak N, Coskun I, Turhan S, Erener G (2010) Effects of humic substances supplementation provided through drinking water on performance, carcass traits and meat quality of broilers. Journal of Animal Physiology and Animal Nutrition 94: 78-85. [crossref]
- Semjon B, Marcinčáková D, Koréneková B, Bartkovský M, Nagy J, et al. (2020) Multiple factorial analysis of physicochemical and organoleptic properties of breast and thigh meat of broilers fed a diet supplemented with humic substances. Poultry Science 99: 1750-1760. [crossref]
- Gálik B, Hrnčár C, Gašparovič M, Rolinec M, Hanušovský O, et al. (2023) The effect of humic substances on the meat quality in the fattening of farm pheasants (Phasianus colchicus). Agriculture 13: 295.
- Agboola AF, Omidiwura BRO, Amole AO, Olanrewaju OA, Adeniran YE (2021) Influence of humic acid supplemented diets on intestinal microbiome and laying performance of egg-type chicken. Nigerian Journal of Animal Production 48: 276-286.
- Omidiwura BRA, Olajide OC, Olaniyan OS (2022) Potentials of pepper elder (Peperomia pellucida) and humic acid as feed additives in noiler chicken production. Nigerian Journal of Animal Production 49: 86-94.
- Mudroňová D, Karaffová V, Pešulová T, Koščová J, Maruščáková IC, et al. (2020) The effect of humic substances on gut microbiota and immune response of broilers. Food and Agricultural Immunology 31: 137-149.
- Mudroňová D, Karaffová V, Semjon B, Naď P, Koščová J, et al. (2021) Effects of dietary supplementation of humic substances on production parameters, immune status and gut microbiota of laying hens. Agriculture 11: 744.
- Bujňák L, Hreško Šamudovská A, Mudroňová D, Naď P, Marcinčák S, et al. (2023) The effect of dietary humic substances on cellular immunity and blood characteristics in piglets. Agriculture 13: 636.
- European Commission. Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 54: 1-130.
- Karaoglu M, Macit M, Esenbuga N, Durdag H, Turgut L, Bilgin ÖC (2004) Effect of supplemental humate at different levels on the growth performance, slaughter and carcass traits of broilers. International Journal of Poultry Science 3: 406-410.
- Kaya CA, Tuncer SD (2009) The effects of humates on fattening performance, carcass quality and some blood parameters of broilers. Journal of Animal and Veterinary Advances 8: 281-284.
- Jaďuttová I, Marcinčáková D, Bartkovský M, Semjon B, Harčárová M, et al. (2019) The effect of dietary humic substances on the fattening performance, carcass yield, blood biochemistry parameters and bone mineral profile of broiler chickens. Acta Veterinaria Brno 88: 307-313. [crossref]
- Sahin T, Aksu Elmali D, Kaya I, Sari M, Kaya O (2011) The effect of single and combined use of probiotic and humate in quail (Coturnix coturnix Japonica) diet on fatttening performance and carcass parameters. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 17: 1-5.
- El-Husseiny OM, Abdallah AG, Abdel-Latif KO (2008) The influence of biological feed additives on broiler performance. International Journal of Poultry Science 7: 862-871.
- Ozturk E, Ocak N, Turan A, Erener G, Altop A, Cankaya S (2012) Performance, carcass, gastrointestinal tract and meat quality traits, and selected blood parameters of broilers fed diets supplemented with humic substances. Journal of the Science of Food and Agriculture 92: 59-65. [crossref]
- Rath NC, Huff WE, Huff GR (2006) Effects of humic acid on broiler chickens. Poultry Science 85: 410-414.
- Van der Wielen PW, Biesterveld S, Notermans S, Hofstra H, Urlings BA, et al. (2000) Role of volatine fatty acids in development of the cecal microflora in broiler chickens during growth. Applied and Environmental Microbiology 66: 2536-2540. [crossref]
- Canani RB, Di Costanzo M, Leone L (2012) The epigenetic effects of butyrate: potential therapeutic implications for clinical practice. Clinical Epigenetics 4: 1-7. [crossref]
- Shermer CL, Maciorowski KG, Bailey CA, Byers FM, Ricke SC (1998) Caecal metabolites and microbial populations in chickens consuming diets containing a mined humate compound. Journal of the Science of Food and Agriculture 77: 479-486.
- Hreško Šamudovská A, Bujňák L, Zigo F (2022) Carcass characteristic, caecal metabolites and dropping quality in broiler chickens fed diets containing a humic substances. Asian Journal of Agriculture and Food Sciences 10: 133-138.
- Abd El-Hakim AS, Cherian G, Ali MN (2009) Use of organic acid, herbs and their combination to improve the utilization of commercial low protein broiler diets. International Journal of Poultry Science 8: 14-20.