Abstract
Gold nanoparticles (AuNPs) were synthesized in situ on cotton, one of the most popular cellulose materials, to achieve functionalization. The localized surface plasmon resonance of the AuNPs imparted the cotton fabric with colors, showing good colorfastness to washing and rubbing. Characterization of the surface morphology and chemical composition of the modified cotton fabric confirmed synthesis and coating of the AuNPs on cotton fibers. The relationship between the morphology of the AuNPs and the optical properties of the cotton fabric was analyzed. Acid condition enabled in situ synthesis of AuNPs on cotton. Cotton with AuNPs exhibited significant catalytic activity for reduction of 4-nitrophenol by sodium borohydride, and could be reused in this reaction. Treatment with AuNPs substantially improved the ultraviolet (UV)-blocking ability of the fabric and resulted in cotton with remarkable antibacterial activity. Traditional reactive dyes were applied to the cotton with AuNPs to enhance its color features. The catalytic properties of the AuNPs on the fabric were not influenced by dyeing with traditional dyes. AuNP-treated cotton fabric used as a flexible active substrate showed improved Raman signals of dyes on the fabric.
Keywords
Gold nanoparticles, Cotton, Coloration, Catalysis, Surface-enhanced Raman Scattering, Antibacterial
Introduction
Modification of cellulose fibers, in particular cotton products, using functional nanomaterials has attracted extensive attention, with the aim of imparting properties such as antibacterial, flame retardancy, and hydrophobic properties [1-7]. Many strategies have been developed to obtain such combinations of fabric and nanoparticles, including plasma treatment, electrostatic assembly, chelation by active groups, and in situ synthesis [8-12]. Anisotropic silver nanoparticles (AgNPs) have been assembled on cotton fabric via electrostatic interaction between nanoparticles and cotton fibers, endowing the textile with bright colors due to their unique optical property, i.e., localized surface plasmon resonance (LSPR) [13]. Cotton fabric with AgNPs was also modified, followed by treatment with fluorinated decyl polyhedral oligomeric silsesquioxane (FPOSS), to fabricate colored fabric with durable antibacterial and self-healing superhydrophobic properties. AgNPs have been combined with cotton under the binding effect of branched poly(ethylenimine) (PEI) [13]. Poly-butylacrylategrafted carbon nanotubes has been applied to cotton fabric using a common dipping–drying–curing finishing procedure [14]. The modified cotton showed various functions, such as enhanced mechanical properties and extraordinary flame retardancy. Core–shell-structured silica dioxide@zinc oxide (SiO2@ZnO) nanoparticles have been prepared and adhered to cotton fabric with the assistance of (3-Aminopropyl)triethoxysilane (APTES) or vinyltriethoxysilane (VTES) [15], resulting in textiles with antibacterial activity, UVprotection properties, and high durability. Titanium dioxide (TiO2) nanoparticles have also been used for functionalization of cotton to obtain fabric with selfcleaning and UV-blocking properties [16,17]. Among functional nanoparticles, gold nanoparticles (AuNPs) have received considerable attention from researchers, owing to their promising optical, electronic, magnetic, catalytic, and biomedical applications [18,19]. As the most stable metal nanoparticles, AuNPs can be prepared using straightforward routes and are resistant to oxidation, facilitating diverse applications. AuNPs present significant catalytic activity in various reaction systems. They exhibit unique optoelectronic features and possess excellent biocompatibility with appropriate ligands as well as high surface-to-volume ratio. The properties of AuNPs including the LSPR optical effect can be readily tuned by controlling their size, shape, and surroundings. Moreover, AuNPs have been widely used as effective active substrate materials for surface-enhanced Raman scattering (SERS) analysis. The Raman signals of molecules adsorbed on AuNPs can be extremely enhanced. Modification of textile materials with AuNPs can transfer some of these important properties to the resulting textile products. Cotton is the most widely used natural fibrous material for textile and clothing production. Modification of cotton is driven by growing consumer demand for enhanced functions of conventional textile products. In situ synthesis of nanoparticles on fabric/fibers is a simple and effective route to achieve functional modification of textile materials; For example, A hydrophobic hierarchical structure has been fabricated on cotton fabric by in situ growth of silica nanoparticles [20,21]. AgNPshas been synthesized in situ on cotton fabric by adjusting the pH value at room temperature. The as-synthesized AgNPs imparted vivid colors and strong antibacterial properties to the treated fabric. TiO2 nanoparticles have been synthesized with anatase structure using ultrasonic irradiation at low temperature and loaded them onto cotton fabric [22]. The TiO2-loaded cotton fabric exhibited UV-protection and self-cleaning features. ZnO nanoparticles have been synthesized in situ in the cellulosic pores of cotton fabric by reacting zinc nitrate and sodium hydroxide to obtain fabric with antibacterial and UV-protection properties [23]. In the previous research, AuNPs were synthesized in situ on silk fabric by heat treatment [24]. This silk fabric treated with AuNPs showed not only vivid colors but also enhanced Raman signals for use as an active SERS substrate for detection of trace analytes [25]. These results inspired us to develop cotton functionalized with AuNPs by in situ synthesis. In this study, cotton fabric was modified by AuNPs synthesized in situ through heat treatment. The optical properties of the resulting colorful fabric treated with AuNPs were analyzed based on color strength (K/S) curves and ultraviolet–visible (UV–Vis) diffuse reflectance absorption spectroscopy. The surface morphology of the cotton fabric before and after modification with AuNPs was investigated by scanning electron microscopy (SEM). The influence of pH on the in situ synthesis of the AuNPs was investigated. The catalytic activity and antibacterial features of the AuNP-treated cotton fabric were evaluated. Complex coloration of cotton fabric using both AuNPs and traditional dyes was also explored. Furthermore, cotton fabric with AuNPs was used as a flexible active substrate to enhance the Raman signals of dyes on the fabric.
Technical Details
The following chemicals were used
Tetrachloroauric(III) acid trihydrate, acetic acid, sodium hydroxide, 4-nitrophenol, and sodium borohydride, Cellulose powder, CI Reactive Red 3 and CI Reactive Red 195
The textile material used was Knitted cotton fabric
The instruments used for measurement are as follows
SEM
Plasma atomic emission spectrometer
Raman microscope system
Color i7 spectrophotometer
Liquor to fabric ratio was 50: 1
The following have been followed
Colorfastness to washing
Colorfastness to rubbing
Catalytic activity
Antibacterial testing against Gram negative bacterium (Figure 1)
Figure 1: Modification of cellulose fibers
Preparation and Characterization of Gold Nano-particles
Figure 2 shows a photograph of the treated cotton fabric samples. The cotton fabric treated in 0.025 mM HAuCl4 solution (Cot-Au-1) was light red, implying presence of AuNPs on the cotton fibers. The color of the cotton fabric with AuNPs changed from light red to red to dark red as the initial concentration of HAuCl4 was increased from 0.025 mM to 0.125 mM. Based on ICP-AES, the gold content of the cotton fabric with AuNPs was measured to be 0.386, 0.725, 0.921, 1.638, and 1.849 mg g-1 for Cot-Au-1, Cot-Au-2, Cot-Au-3, Cot-Au-4, and Cot-Au-5, respectively.

Figure 2: Treated cotton fabrics
The gold content of the samples increased as the concentration of HAuCl4 was increased. K/S curves were obtained to analyze the color changes. The peak of the K/ S curves for the treated cotton fabric remained unchanged, being located at 540 nm, with increasing gold content. However, the maximum value of the treated cotton fabric increased as the gold content was increased, consistent with its deepening color. UV– Vis diffuse reflectance absorption spectra of cotton fabric treated with AuNPs were measured. As shown in Figure 1c, a single absorption band located at 534 nm appeared in the UV–Vis absorption spectrum of CotAu-1, assigned to the characteristic LSPR mode of AuNPs. The LSPR band of cotton fabric treated with AuNPs red-shifted from 534 nm to 547 nm as the initial concentration of HAuCl4 was increased from 0.025 mM to 0.125 mM, along with an increase in the absorption band intensity. These changes in the color and LSPR property of the AuNP-treated cotton fabric may be related to the gold content, and the morphology and density of AuNPs on the cotton. SEM was employed to observe the surface morphology of the treated cotton fabric (Figure 3).
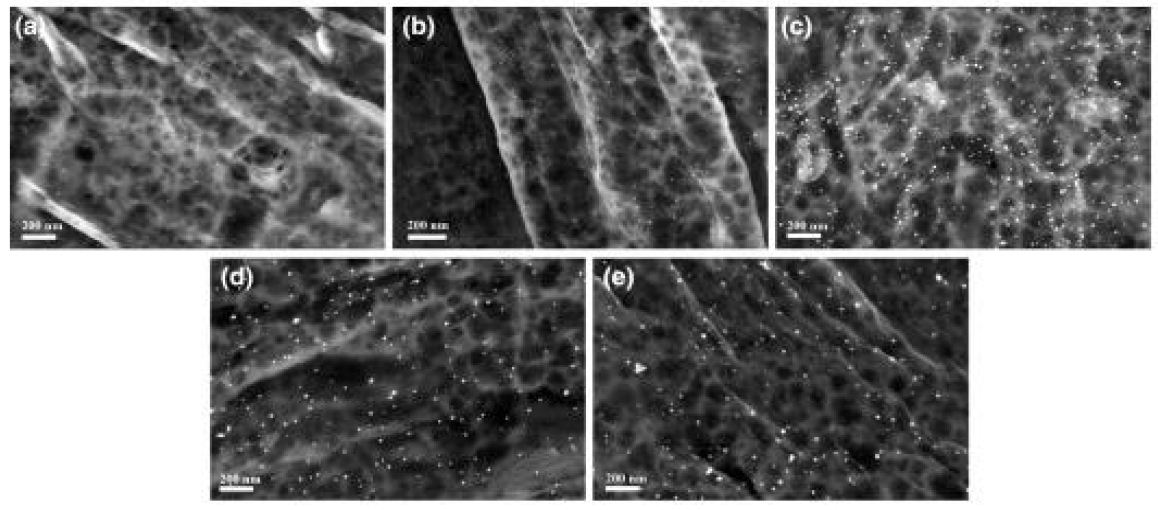
Figure 3: SEM images of a Cot-Au-1, b Cot-Au-2, c Cot-Au-3, d Cot-Au-4, and e Cot-Au-5
A number of nanoparticles were seen over the surface of fibers (Figure 2), demonstrating that AuNPs were synthesized in situ on the cotton. The size of the AuNPs on cotton was measured to be 8.7 ± 1.2, 8.6 ± 1.3, 14.1 ± 3.0, 17.4 ± 3.0, and 20.5 ± 3.8 nm for Cot-Au-1 to CotAu-5, respectively. Although the sizes for Cot-Au-1 and Cot-Au-2 were similar, Cot-Au-2 with higher LSPR intensity had higher density of AuNPs on fiber surfaces in comparison with Cot-Au-1. The size of AuNPs increased as the gold content of the samples increased as the Au ion concentration was changed from 0.05 mM to 0.125 mM. It was found that nearly all the nanoparticles on the cotton fibers were spherical for low gold content (Cot-Au-1, Cot-Au-2, and CotAu-3), whereas a few anisotropic AuNPs, such as triangular nanoplates, appeared when the gold content was increased to high level (Cot-Au-4 and Cot-Au-5). The anisotropy of the AuNPs led to the red-shift of the LSPR band observed in the UV– Vis diffuse reflectance absorption spectra of the AuNPtreated cotton fabric. As seen from the SEM images, the density of AuNPs reduced when the Au ion concentration was changed from 0.075 to 0.10 and 0.125 mM, due to generation of larger and anisotropic AuNPs. All these effects on the morphology (shape and size) and density of the AuNPs on cotton led to the changes in the LSPR property of the different samples. XPS was used to analyze the cotton fabric treated with AuNPs. XPS peaks assigned to O 1s and C 1s as the normal components of cellulose were seen in the XPS spectrum of Pri-Cot fabric. XPS peaks ascribed to Au element appeared in the XPS spectra after the cotton fabric was treated. The XPS spectrum for Cot-Au-4 displayed two principal bands at 82.2 and 85.8 eV, attributed to binding energies of 4f7/2 and 4f5/2 of metallic Au, respectively [26]. These XPS results indicate that AuNPs were successfully synthesized on the cotton fabric.
Influence of pH Value
We investigated the influence of the pH value on the in situ synthesis of AuNPs on the cotton fabric. The original pH value of the HAuCl4 aqueous solutions at 0.025–0.125 mM was around 4. The pH value of the reaction systems was adjusted by addition of acetic acid or NaOH aqueous solutions. The K/S curves and UV–Vis diffuse reflectance absorption spectra of cotton fabric treated has been obtained with 0.10 mM HAuCl4 solution at different pH values (3–6). As can be seen, the cotton fabric treated at pH 3 showed the highest K/S value of 1.71 among the different cotton samples. The K/S value of the AuNP-treated cotton fabric decreased as the pH value of the reaction system was increased. The maximum K/S decreased to 0.28 when the pH value was increased to 6. Vivid cotton fabric was obtained when using 0.10 mM HAuCl4 in the pH range of 3–6, although the cotton fabric changed slightly in color after heat treatment in HAuCl4 solution (0.10 mM) at pH 7 or above, implying that nearly no AuNPs were produced. The UV–Vis diffuse reflectance absorption bands of the cotton fabric treated with AuNPs at different pH values were centeredaround 540 nm. The intensity of the absorption bands decreased with increase in the pH value, consistent with the change trend of the K/S values. It can be inferred that acid condition facilitated in situ synthesis of AuNPs on cotton fabric, similar to the case of in situ preparation of AuNPs on ramie fibers [27]. It is well documented in literature that the pH value of the reaction system plays a vital role in formation of AuNPs through reduction of HAuCl4 [28-33]. Au ion complexes with chloride and/or hydroxide as ligands were suggested to be AuCl4 – (pH 3.3), AuCl3(OH)- (pH 6.2), AuCl2(OH)2 – (pH 7.1), AuCl(OH)3 – (pH 8.1), and Au(OH)4 – (pH 12.9) ions, corresponding to different ranges of pH [34,35]. The reduction potential of Au ion complexes depends remarkably on the pH value, with decreased reactivity as the pH is increased, in the order AuCl4 -[AuCl3(OH)-[AuCl2(OH)2 -[AuCl(OH)3 – [Au(OH)4 -. In the present study, AuNPs were synthesized in presence of cotton under acid condition, whereas Au ions were not reduced to form AuNPs in neutral or basic solution. The present results are consistent with previous analyses of the influence of pH on the formation of AuNPs. Mechanism of in situ synthesis of AuNPs It is well known that cellulose is the dominant component of cotton, consisting of long chains of Dglucose units [36]. In situ synthesis of AuNPs on cotton fabric in this study could result from reduction of Au ions by cellulose. Pure cellulose powder has been employed to reduce Au ions according to the same experimental procedure with cotton fabric. As shown in Figure S3, purplish red and grayish purple cellulose powders were produced after heat treatment in HAuCl4 solution, implying synthesis of AuNPs by cellulose. This result indicates that the reducing effect of cellulose in cotton led to in situ formation of AuNPs. Cellulose materials have been reported to act as reducing agents to synthesize AuNPs and AgNPs, owing to their abundant hydroxyl groups [37,38]. It is suggested that hydroxyl groups of cellulose play a pivotal role in the in situ formation of metal nanoparticles. The primary hydroxyl groups with higher reactivity in cellulose could be oxidized by Au ions during in situ synthesis of AuNPs. The reducing ends of cellulose in cotton could also contribute to reduction of Au ions to form AuNPs [39]. As proposed in previous research, oxygen-containing groups on the surface of cotton, including carboxylate and hydroxyl groups, can serve as active sites that might combine with AuNPs through complexing or electrostatic interaction [40]. Zeta potential measurements in our previous work indicated that cotton powder carries negative charge. It is suggested that a complexing or electrostatic interaction could lead to effective combination of synthesized AuNPs with cotton fabric in the present study. Cotton acted as a reducing agent and a stabilizing agent to prepare AuNPs on the fiber surface. Assessment of colorfastness is one of the important parameters to assess the properties and performance of textile products. The colorfastness to washing of the cotton fabric treated with AuNPs was tested by washing in presence of ECE reference detergent at 50oC for 45 min in each washing cycle. The DE values of the treated fabric before and after washing have been determined. After the first washing cycle, the DE values were measured to be 0.7 and 2.6 for Cot-Au-4 and Cot Au-5, respectively, revealing that color fading occurred for the cotton fabric treated with AuNPs during washing. However, the DE of the treated fabric increased slightly after the third washing cycle. These results demonstrate that the cotton fabric colored with AuNPs exhibited reasonably good colorfastness to washing. In addition, the colorfastness to rubbing of the treated cotton fabric was tested. The gray scale rating for the DE values of Cot-Au-2, Cot-Au-4, and Cot-Au-5 under dry and wet rubbing conditions was assessed. The dry rubbing colorfastness was rated as 5, 4–5, and 4–5 for Cot-Au-2, Cot-Au-4, and Cot-Au-5, respectively. The rating of the wet colorfastness of Cot-Au-2, Cot-Au-4, and Cot-Au-5 was estimated to be 4–5, 4, and 4, respectively. These results show that the cotton fabric treated with AuNPs exhibited good colorfastness to dry or wet rubbing. Investigation of catalytic activity AuNPs have been widely used as catalysts for various reactions [41-43]. In the present research, AuNPs were bound to cotton fibers after in situ synthesis. As the cotton fabric acts as a support for the nanoparticles (Scheme 1), they can be readily separated from the reaction system, enabling reuse of the catalyst. Reduction of 4-NP has been commonly used as a model catalytic reaction to evaluate the catalytic activity of metal nanoparticles [44]. The catalytic activity of the cotton fabric treated with AuNPs was assessed by monitoring the UV–Vis absorption spectra of aqueous solution during reduction of 4-NP using NaBH4. The color of the 4-NP solution changed from light yellow to green–yellow after addition of NaBH4. Nitro compounds are inert to NaBH4 in absence of catalyst, whereas metal nanoparticles can act as an electronic relay agent for electron transfer from NaBH4 to nitro compounds to accelerate the reduction reaction [45]. A new UV–Vis absorption peak for 4-NP solution appeared at 400 nm after NaBH4 was added, due to formation of 4-nitrophenolate ions. The evolution of the UV–Vis absorption spectra of 4-NP solution after mixture with NaBH4 in presence of pristine cotton fabric was recorded. The intensity of the absorption peak at 400 nm for 4-NP decreased only slightly, revealing that pristine cotton fabric showed no catalytic activity. Time-resolved UV–Vis absorption spectra of 4-NP solution with NaBH4 in presence of AuNP-treated cotton fabric have been obtained (Cot-Au-1 to Cot-Au-5). The absorption peak at 400 nm of 4-NP solution decreased distinctly in intensity after addition of NaBH4 in presence of all the fabric samples treated with AuNPs. Meanwhile, a new absorption peak arose at 300 nm due to the reduction process of 4-NP, implying formation of 4-AP [46,47]. The intensity of the absorption peak at 400 nm was plotted as a function of time to determine the reduction rate of 4-NP. The rapid intensity decrease of the peak at 400 nm indicates that the cotton fabric treated with AuNPs exhibited notable catalytic activity. It was found that the reduction system with Cot-Au-4 exhibited the highest reaction rate among the AuNPtreated cotton fabric samples. Reduction of 4-NP is generally considered to be a pseudo-first-order kinetic reaction on account of excess NaBH4 [48,49]. Plots of ln(At/A0) versus time have been obtained, where At and A0 denote the absorption intensity at 400 nm at time t and the initial stage, respectively. The linear correlation between ln(At/A0) and time confirms this pseudo-first-order hypothesis. The apparent rate constant (Kapp) of the catalytic reaction can be estimated from the linear slope of ln(At/A0) versus time. The Kapp value of the reduction reaction was found to be 1.89 9 10-2, 1.76 9 10-2, 2.29 9 10-2, 4.32 9 10-2, and 2.55 9 10-2 min-1 with Cot-Au-1, Cot-Au-2, CotAu-3, Cot-Au-4, and Cot-Au-5, respectively. The Kapp values obtained in this study are comparable to related literature results for AuNPs [50,51]. Comparing these Kapp values for the AuNP cotton fabric samples, CotAu-4 with the largest Kapp value showed the highest catalytic activity, although the gold content of CotAu-4 was lower than that of Cot-Au-5. The catalytic properties of the treated cotton fabric depend on the AuNPs on the surface of cotton fibers. It is believed that the catalytic activity is related to the shape, size, and density of AuNPs on the fabric. To evaluate the reusability of the catalyst, treated cotton fabric (CotAu-4) was separated from the reaction system and reused in repeated reduction reactions of 4-NP. The peak intensity at 400 nm versus reaction time for each complete conversion is plotted in Figure 6h. The treated fabric still exhibited strong catalytic activity even after seven cycles, indicating that the treated cotton fabric exhibited durable catalytic effect. UV-protection and antibacterial properties Treatment with AuNPs endowed the cotton fabric with additional functions. The UV transmittance and UV protection factor (UPF) values of the different fabric samples have been determined. Coating with AuNPs reduced the average transmittance values of the cotton fabric. Transmittance values in both the UVA (315–400 nm) and UVB (280–315 nm) regions showed a decreasing trend with increasing gold content on the cotton fabric. The UPF value of pristine cotton fabric was measured to be 65.1, while in situ synthesis of AuNPs on the cotton fabric increased the UPF value to 109.3, suggesting that the AuNPs improved the UV-blocking ability of the cotton fabric. The antibacterial properties of AuNPs have attracted great interest, and various potential antibacterial applications have been explored [52-54]. The antibacterial activity of cotton fabric treated with AuNPs was evaluated against Gram-negative bacterium E. Coli. The bacteria on the pristine cotton fabric and treated cotton fabric have been studied (CotAu-4). A full lawn of bacteria was seen on the plates corresponding to the pristine cotton fabric, whereas no bacteria colonies were found on the agar medium of the AuNP-treated cotton fabric, revealing that presence of AuNPs on the fabric inhibited growth of bacteria. These results demonstrate that the cotton fabric with AuNPs possessed significant antibacterial activity. Dyeing of AuNP-treated cotton fabric with traditional dyes to improve the color range and saturation of the fabric, traditional dyes including R3 and R195 were used to dye the AuNP-treated cotton fabric. Dyeing with traditional dyes imparted deeper color to the cotton fabric than that achieved based on the LSPR optical effect of the AuNPs. The K/S values of the cotton fabric observably increased after dyeing with R3 and R195. Due to the light color of AuNPs at low gold content (0.386 mg g-1), the K/ S curves of Cot-R3 were almost the same as for CotAu-R3-1. The K/S curves of Cot-R195 and Cot-Au-R195-1 were also the same. However, coating with more AuNPs gave rise to higher K/S values for the cotton fabric colored with AuNPs and traditional dyes (R3 and R195). The AuNPs and dyes played a combined role in the color of the cotton fabric, even though the traditional dyes dominated the optical properties of the colored cotton fabric. Coloration using traditional dyes did not influence the catalytic properties of the cotton fabric with AuNPs. The UV–Vis adsorption spectra of 4-NP solution with Cot-R195 changed very little after addition of NaBH4, demonstrating no evident catalytic activity of Cot-R195, consistent with the case of PriCot. The intensity of the UV–Vis absorption band at 400 nm of 4-NP solution in presence of Cot-Au-R195-4 decreased sharply after NaBH4 addition, revealing that the cotton fabric after complex coloration with AuNPs and traditional dyes retained strong catalytic activity. Moreover, the cotton fabric with AuNPs and R195 could be reused in the catalytic reaction, showing significant catalytic activity after seven cycles. These results attest that the catalytic properties of AuNPs on cotton fabric are retained after coloration with traditional dyes. SERS enhancement by AuNP-treated cotton fabric AuNPs have been widely used as active substrates for enhancing Raman signals due to their LSPR effect. Raman scattering spectra were obtained to investigate the SERS enhancement effect of the AuNP-treated cotton fabric. No distinct bands were seen in the Raman scattering spectrum of pristine cotton fabric. However, visible bands were found in the Raman scattering spectra after AuNPs were coated on the cotton fabric. Evident vibrational bands were seen at 1604, 1378, 1121, 1096, 519, 457, 435, 380, and 257 cm-1 in the Raman scattering spectra of the AuNP-treated cotton fabric, being characteristic Raman bands of cotton fibers [55,56]. Some of these bands are assigned to vibrations of b-1,4-glycosidic ring linkages between D-glucose units in cellulose. It was found that CotAu-5 exhibited the strongest Raman signal of cellulose among the AuNP-treated cotton fabric samples, which may be due to the optimal morphology and corresponding LSPR effect of the nanoparticles. Noble-metal nanoparticles on textiles can enhance the Raman signals of dyes used for coloration of fibers, leading to promising applications of SERS in the field of identification of cultural heritage, forensic analysis, and textile dyeing [57-61]. In the present study, we investigated the SERS enhancement of R3 on the AuNP-treated cotton fabric. The cotton fabric dyed with R3 without AuNPs showed unclear Raman bands from cellulose units and dye on the fabric. However, enhanced Raman bands were obtained from R3-dyed cotton fabric treated with AuNPs. The R3-dyed cotton fabric with higher gold content (Cot-Au-R3-4 and Cot-Au-R3-5) showed unambiguous enhanced Raman bands. The Raman scattering spectrum of pure R3 powder has been determined. Compared with the normal Raman scattering spectrum of R3, the SERS bands from Cot-Au-R3-4 and Cot-Au-R3-5 at 284, 385, 478, 1000, 1032, 1123, 1274, 1473, and 1589 cm-1 can be ascribed to R3 dye on the AuNP-treated cotton fabric, although tiny wavenumber shifts occurred due to interactions of the dye and cotton fibers as well as AuNPs. The AuNPs on the fabric enhanced the Raman signal of the dye on the fibers, facilitating nondestructive analysis of dyes on textiles and providing insights into the dyeing mechanism of fibers.
Conclusions
Cotton fabric was functionalized by AuNPs synthesized in situ by a heating method. The fabric was colored by the AuNPs by virtue of their LSPR optical effect. The intensity of the LSPR band of AuNPtreated fabric increased with increasing gold content in the cotton samples. The treated fabric showed good colorfastness to washing and rubbing. SEM and XPS investigations confirmed the synthesis and combination of AuNPs on cotton. The mechanism for in situ synthesis of AuNPs on cotton was investigated. The fabric with AuNPs exhibited notable catalytic activity, as shown by monitoring reduction of 4-NP to 4-AP. The cotton fabric with AuNPs showed improved UVprotection and excellent antibacterial properties. Traditional dyes were combined with the AuNP-treated cotton, revealing improved color properties. The fabric with such complex coloration still exhibited prominent catalytic activity. Cotton fabric with AuNPs can also act as a SERS substrate for analysis of dyes on the fabric.
References
- El-Shishtawy RM, Asiri AM, Abdelwahed NAM, Al-Otaibi MM (2011) In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 18: 75-82.
- Mohamed AL, El-Naggar ME, Shaheen TI, Hassabo AG (2017) Laminating of chemically modified silan based nanosols for advanced functionalization of cotton textiles. Int J BiolMacromol 95: 429-437. [crossref]
- Dhineshbabu NR, Arunmetha S, Manivasakan P, Karunakaran G, Rajendran V (2016) Enhanced functional properties of cotton fabrics using TiO2/SiO2 nanocomposites. J Ind Text 45: 674-692.
- Shaheen TI, El-Naggar ME, Abdelgawad AM, Hebeish A (2016) Durable antibacterial and UV protections of in situ synthesized zinc oxide nanoparticles onto cotton fabrics. Int J BiolMacromol 83: 426-432. [crossref]
- Alongi J, Malucelli G (2015) Cotton flame retardancy: state of the art and future perspectives. RSC Adv 5: 24239-24263.
- Alongi J, Carosio F, Malucelli G (2014) Current emerging techniques to impart flame retardancy to fabrics: an overview. PolymDegrad Stab 106: 138-149.
- Leng BX, Shao ZZ, de With G, Ming WH (2009) Superoleophobic cotton textiles. Langmuir 25: 2456-2460. [crossref]
- Cady NC, Behnke JL, Strickland AD (2011) Copper-based nanostructured coatings on natural cellulose: nanocomposites exhibiting rapid and efficient inhibition of a multidrug resistant wound pathogen, A. baumannii, and mammalian cell biocompatibility in vitro. AdvFunct Mater 21: 2506-2514.
- Dong H, Hinestroza JP (2009) Metal nanoparticles on natural cellulose fibers: electrostatic assembly and in situ synthesis. ACS Appl Mater Interfaces 1: 797-803. [crossref]
- Gorjanc M, Bukosek V, Gorensek M, Mozetic M (2010) CF4 plasma and silver functionalized cotton. Text Res J 80: 2204-2213.
- Tang B, Zhang M, Hou X, Li J, Sun L, Wang X (2012) Coloration of cotton fibers with anisotropic silver nanoparticles. IndEngChem Res 51: 12807-12813.
- Tang B, Kaur J, Sun L, Wang X (2013) Multifunctionalization of cotton through in situ green synthesis of silver nanoparticles. Cellulose 20: 3053-3065.
- Wu MC, Ma BH, Pan TZ, Chen SS, Sun JQ (2016) Silvernanoparticle-colored cotton fabrics with tunablecolors and durable antibacterial and self-healing superhydrophobic properties. AdvFunct Mater 26: 569-576.
- Liu YY, Wang XW, Qi KH, Xin JH (2008) Functionalization of cotton with carbon nanotubes. J Mater Chem 18: 3454-3460.
- El-Naggar ME, Hassabo AG, Mohamed AL, Shaheen TI (2017) Surface modification of SiO2 coated ZnO nanoparticles for multifunctional cotton fabrics. J Colloid Interface Sci 498: 413-422. [crossref]
- Bozzi A, Yuranova T, Guasaquillo I, Laub D, Kiwi J (2005) Self-cleaning of modified cotton textiles by TiO2 at low temperatures under daylight irradiation. J PhotochemPhotobiol A Chem 174: 156-164.
- El-Naggar ME, Shaheen TI, Zaghloul S, El-Rafie MH, Hebeish A (2016) Antibacterial activities and uv protection of the in situ synthesized titanium oxide nanoparticles on cotton fabrics. IndEngChem Res 55: 2661-2668.
- Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104: 293-346.
- Saha K, Agasti SS, Kim C, Li X, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112: 2739-2779. [crossref]
- Komeily-Nia Z, Montazer M, Latifi M (2013) Synthesis of nano copper/nylon composite using ascorbic acid and CTAB. Colloids Surf A PhysicochemEng Asp 439: 167-175.
- Chen XQ, Liu YY, Lu HF, Yang HR, Zhou XA, Xin JH (2010) In-situ growth of silica nanoparticles on cellulose and application of hierarchical structure in biomimetic hydrophobicity. Cellulose 17: 1103-1113.
- Sadr FA, Montazer M (2014) In situ sonosynthesis of nano TiO2 on cotton fabric. UltrasonSonochem 21: 681-691. [crossref]
- Prasad V, Arputharaj A, Bharimalla AK, Patil PG, Vigneshwaran N (2016) Durable multifunctional finishing of cotton fabrics by in situ synthesis of nano-ZnO. Appl Surf Sci 390: 936-940.
- Tang B, Sun L, Kaur J, Yu Y, Wang X (2014) In-situ synthesis of gold nanoparticles for multifunctionalization of silk fabrics. Dyes Pigm 103: 183-190.
- Liu J et al (2016) Surface enhanced Raman scattering (SERS) fabrics for trace analysis. Appl Surf Sci 386: 296-302.
- Medina-Ramirez I, Gonzalez-Garcia M, Palakurthi S, Liu J (2012) Application of nanometals fabricated using green synthesis in cancer diagnosis and therapy. In: Kidwai M (ed) Green chemistry—environmentally benign approaches. InTech, Rijeka.
- Tang B et al (2015b) Functional application of noble metal nanoparticles in situ synthesized on ramie fibers. Nanoscale Res Lett. [crossref]
- Bastu´s NG, Comenge J, Puntes V (2011) Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: size focusing versus Ostwald ripening. Langmuir 27: 11098-11105. [crossref]
- Chakraborty A, Chakraborty S, Chaudhuri B, Bhattacharjee S (2016) Process engineering studies on gold nanoparticle formation via dynamic spectroscopic approach. Gold Bull 49: 75-85.
- Ji X, Song X, Li J, Bai Y, Yang W, Peng X (2007) Size control of gold nanocrystals in citrate reduction: the third role of citrate. J Am ChemSoc 129: 13939-13948. [crossref]
- Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A (2006) Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B 110: 15700-15707. [crossref]
- Yu Chang S-S, Lee C-L, Wang CRC (1997) Gold nanorods: electrochemical synthesis and optical properties. J Phys Chem B 101: 6661-6664.
- Zhang P, Li Y, Wang D, Xia H (2016) High-yield production of uniform gold nanoparticles with sizes from 31 to 577 nm via one-pot seeded growth and size-dependent SERS property. Part PartSystCharact 33: 924-932.
- Goia D, Matijevic´ E (1999) Tailoring the particle size of monodispersed colloidal gold. Colloids Surf A 146: 139-152.
- Wuithschick M et al (2015) Turkevich in new robes: key questions answered for the most common gold nanoparticle synthesis. ACS Nano 9: 7052-7071. [crossref]
- Edwards HGM, Farwell DW, Webster D (1997) FT Raman microscopy of untreated natural plant fibres. SpectrochimActaA 53: 2383-2392.
- Dong H, Hinestroza JP (2009) Metal nanoparticles on natural cellulose fibers: electrostatic assembly and in situ synthesis. ACS Appl Mater Interfaces 1: 797-803. [crossref]
- Montazer M, Alimohammadi F, Shamei A, Rahimi MK (2012) In situ synthesis of nano silver on cotton using Tollens’ reagent. CarbohydrPolym 87: 1706-1712.
- Pinto RJB, Marques P, Martins MA, Neto CP, Trindade T (2007) Electrostatic assembly and growth of gold nanoparticles in cellulosic fibres. J Colloid Interface Sci 312: 506-512. [crossref]
- Velleste R, Teugjas H, Valjamae P (2010) Reducing end-specific fluorescence labeled celluloses for cellulase mode of action. Cellulose 17: 125-138.
- Kumar A, Mandal S, Selvakannan PR, Pasricha R, Mandale AB, et al. (2003) Investigation into the interaction between surface-bound alkylamines and gold nanoparticles. Langmuir 19: 6277-6
- Corma A, Garcia H (2008) Supported gold nanoparticles as catalysts for organic reactions. ChemSoc Rev 37: 2096-2126. [crossref]
- Zhao Y, Huang YC, Zhu H, Zhu QQ, Xia YS (2016) Three-inone: sensing, self-assembly, and cascade catalysis of cyclodextrin modified gold nanoparticles. J Am ChemSoc 138: 16645-16654. [crossref]
- Herves P, Perez-Lorenzo M, Liz-Marzan LM, Dzubiella J, Lu Y, Ballauff M (2012) Catalysis by metallic nanoparticles in aqueous solution: model reactions. ChemSoc Rev 41: 5577-5587.[crossref]
- Barnes WL, Dereux A, Ebbesen TW (2003) Surface plasmon subwavelength optics. Nature 424: 824-830. [crossref]
- Liang M, Su R, Huang R, Qi W, Yu Y, et al. (2014) Facile in situ synthesis of silver nanoparticles on procyanidin-grafted eggshell membrane and their catalytic properties. ACS Appl Mater Interfaces 6: 4638-4649. [crossref]
- Tang B, Li JL, Fan LP, Wang XG (2015a) Facile synthesis of silver submicrospheres and their applications. RSC Adv 5: 98293-98298.
- Ai L, Yue H, Jiang J (2012) Environmentally friendly lightdriven synthesis of Ag nanoparticles in situ grown on magnetically separable biohydrogels as highly active and recyclable catalysts for 4-nitrophenol reduction. J Mater Chem 22: 23447-23453.
- Abdel-Fattah TM, Wixtrom A (2014) Catalytic reduction of 4-nitrophenol using gold nanoparticles supported on carbon nanotubes. ECS J Solid State SciTechnol 3: M18-M20.
- Panigrahi S et al (2007) Synthesis and size-selective catalysis by supported gold nanoparticles: study on heterogeneous and homogeneous catalytic process. J Phys Chem C 111: 4596-4605.
- Cui Y, Zhao Y, Tian Y, Zhang W, Lu X .et al (2012) The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 33: 2327-2333. [crossref]
- Emam HE, El-Hawary NS, Ahmed HB (2017) Green technology for durabformation of AuNPs. Int J BiolMacromol 96: 697-705.
- Wadhwani P, Heidenreich N, Podeyn B, Burck J, Ulrich AS (2017) Antibiotic gold: tethering of antimicrobial peptides to gold nanoparticles maintains conformational flexibility of peptides and improves trypsin susceptibility. BiomaterSci 5: 817-827. [crossref]
- Edwards HGM, Farwell DW, Webster D (1997) FT Raman microscopy of untreated natural plant fibres. SpectrochimActaA 53: 2383-2392.
- Zorko M, Vasiljevic J, Tomsic B, Simoncic B, Gaberscek M, et al (2015) Cotton fiber hot spot in situ growth of Stober particles. Cellulose 22: 3597-3607.
- Fateixa S, Wilhelm M, Nogueira HIS, Trindade T (2016) SERS and Raman imaging as a new tool to monitor dyeing on textile fibres. J Raman Spectrosc 47: 1239-1246.
- Leona M, Lombardi JR (2007) Identification of berberine in ancient and historical textiles by surface-enhanced Raman scattering. J Raman Spectrosc 38: 853-858.
- Leona M, Stenger J, Ferloni E (2006) Application of surfaceenhanced Raman scattering techniques to the ultrasensitive identification of natural dyes in works of art. J Raman Spectrosc 37: 981-992.
- Meleiro PP, Garcia-Ruiz C (2016) Spectroscopic techniques for the forensic analysis of textile fibers. ApplSpectrosc Rev 51: 258-281.
- Zaffino C, Ngo HT, Register J, Bruni S, Vo-Dinh T (2016) ‘‘Dry-state’’ surface-enhanced Raman scattering (SERS): toward non-destructive analysis of dyes on textile fibers. Appl Phys A Mater Sci Process.
- Meleiro PP, Garcia-Ruiz C (2016) Spectroscopic techniques for the forensic analysis of textile fibers. ApplSpectrosc Rev 51: 258-281.