DOI: 10.31038/AFS.2024614
Abstract
Aquaponics is based on the principle of integrating the cultivation of aquatic organisms with plant farming to minimize waste and maximize productivity. However, certain aspects of conventional aquaponics can pose health risks to aquatic species. A novel technique that combines Biofloc technology with hydroponics offers an alternative approach to aquaponics for addressing these challenges. Biofloc-based aquaponics employs a dense microbial co-culture that enhances nutrient cycling, typically reducing feed requirements and minimizing the need for mechanical and biological filters. The technical advantages of BFT can lower costs compared to conventional intensive methods. However, this technique is still in its early stages of research, primarily due to inconsistencies in experimental design and system configuration. This article focuses on the cultivation of aquatic animal and plant species using BFT. The review analyzes effective development of BFT in aquaponics, guiding future research to make this method economically viable and improve sustainable aquaculture production.
Keywords
Aquaponics, Biofloc technology, Aquaculture sustainability
Introduction
Climate change poses a major threat to global food security, poverty reduction efforts, and sustainable development. Therefore, reforming the aquaculture sector is crucial [1]. The substantial potential of aquaculture has become increasingly evident through the advancement of hyper-intensive and intensive production systems [2]. There is an increasing need for the sustainable intensification of aquaculture production systems to meet rising food demands and address environmental challenges [3]. As a result, several studies have documented innovative production systems. Aquaponics is a method that integrates soil-less plant growth (hydroponics) with the cultivation of fish and crustaceans in a recirculating water system [4].
Aquaponics is a technique for producing food that involves the simultaneous cultivation of aquatic animals and terrestrial plants, enabling the sharing of water and nutrients among the various species involved [4,5]. In intensive animal aquaculture systems, waste nutrients are generated, as aquatic animals typically assimilate only a fraction of the feed provided [6,7]. For instance, marine shrimps typically improve about 22–32% of nitrogen and 11–14% of phosphorus [8]. However, this waste can be beneficial for plants, as they can utilize these nutrients, thereby reducing or eliminating the need for nutrient solutions, as seen in traditional hydroponic systems [9,10]. Furthermore, this enables the system to diversify and enhance its total production while simultaneously mitigating net environmental effects. By utilizing a water supply that is typically dedicated to plant production, it may also be used for the cultivation of aquatic animals [5].
BFT has been proven to be a viable aquaculture technique for aquaponics. This process comprises the transformation of harmful nitrogenous waste produced by animals into less harmful forms, such as nitrate, and the growth of bacterial biomass inside the same unit. Unlike conventional systems that rely on water renewal or recirculating aquaculture systems that use external biofilters, this method utilizes microbial processes like immobilization and nitrification to achieve this conversion. The bioflocs, which are formed by the combination of organic matter and microbes, are kept suspended in the water and can serve as an additional food source for the cultured animal. This facilitates the conversion of aquatic animal nutritional wastes into biomass via the microbial loop route. Furthermore, this system enhanced biosecurity eliminates the necessity of continuously sourcing water from the natural environment [11-15].
Aquaponics systems that utilize BFT can achieve high productivity for both fish and plants, while also offering significant ecological benefits through nutrient recycling, the elimination of pesticides, and efficient use of water and space [16]. Recent research suggests that combining hydroponics with BFT can enhance the production of marine shrimp [17] and tilapia [18]. FLOCponics is an evolution of traditional aquaponics in which the aquaculture subsystem utilizes BFT instead of RAS [19,20]. The BFT fundamentally relies on the growth of specific microbial communities that act as biological filters, facilitating the cycling of nutrients. FLOCponics research is becoming increasingly popular because it requires less fish feed, enables continuous nutrient cycling, and eliminates the need for complex filters compared to RAS production methods [16,17]. Despite the theoretical benefits of FLOCponics systems, [19] found that 63% of the plants grown using water from a mature biofloc-based fish system exhibited unappealing visual characteristics for the market. These findings raise concerns about the economic viability of FLOCponics [15]. When investing in new technologies, it is crucial to assess the financial feasibility of the enterprise [19,20].
Diversifying and sustaining aquaculture production through new technologies like FLOCponics necessitates profitability for producers [21]. Therefore, it is essential to assess the profitability of integrating biofloc technology (BFT) with hydroponics, as well as the impact of plant visual quality on FLOCponics production [22,23]. This study provides a thorough analysis on cultivation of aquatic animal and plant species using biofloc technology in Aquaponics system. The review analyzes effective development of BFT in aquaponics, guiding future research to make this method economically viable and improve sustainable aquaculture production.
History and Basics of Aquaponics System
The term “aquaponics” was coined in the late 1970s and early 1980s by Mark McMurtry and researchers at the New Alchemy Institute and North Carolina State University in the United States. This system, known as the “Integrated Aqua-Vegeculture System” (IAVS), combines aquaculture and hydroponics. The origins of aquaponics may be traced back to ancient civilizations such as the Aztec Chinampas, Egypt, Babylon, and Far Eastern countries like China and Thailand. These societies used a combination of fish and vegetable growing [24]. According to [25] William McLarney, Nancy Todd, and John Todd recreated the Aztec aquaponics system prototype in 1969, leading to the creation of the first commercially effective aquaponics system. In 1981, Dr. James Rakocy and his colleagues at the University of the Virgin Islands pioneered modern commercial-scale aquaponics. Most of the research on aquaponics began in the early 1970 [26].
Aquaponics is a kind of farming that combines the production of aquatic creatures in tanks with hydroponics. This technology utilizes microbial processes to convert nutrient-rich wastewater from aquaculture into valuable resources for plant nourishment and irrigation [27]. Aquaponics systems utilize resource reutilization and recycling to produce healthy food while minimizing or eliminating the need for chemicals such as fertilizers, pesticides, and antimicrobials. Consequently, compared to traditional aquaculture and hydroponics systems, aquaponics offers several advantages and has been designed to serve as a more sustainable and cyclical method of food production [5,23].
Nitrifying bacteria in aquaponics systems convert aquaculture effluents into plant-available nutrients, allowing plants to grow and use their feed to its maximum potential [13,22]. To maintain a stable operation of the aquaponics system, it is necessary to establish a nitrogen cycle. Ammonia is discharged and transformed into nitrite by ammonia-oxidizing bacteria during the process of fish breeding. Nitrite is subsequently converted into nitrate by oxidation by nitrite-oxidizing bacteria, mainly Nitrospira sp. and Nitrobacter sp. [28,29].
The nitrates produced in aquariums serve as nutrients for plants, promoting their growth. Efficient circulation can only be achieved when there is a balanced equilibrium between the production of nitrates by aquatic animals and the amount of plant biomass in the system [30]. Furthermore, unlike conventional aquaponics systems, integrated systems offer the benefit of fulfilling plant’s nutritional requirements without the use of fertilizers. This is achieved by supplying plants with a diverse range of nutrients (such as phosphorus, nitrogen, potassium, calcium, Sulphur, iron, magnesium, copper, manganese, zinc, molybdenum, boron, and aluminum) through the utilization of food and excrement from cultured organisms [31]. Aquaponics reduces the need for fertilizer in hydroponics and minimizes water treatment in RAS systems, leading to nutrient recovery and increased profitability by allowing the simultaneous production of two cash crops within the same system. [16]
DAPS (Decoupled Aquaponic System)
The primary obstacle to the profitability of conventional aquaponics is its capacity to provide an ideal environment for the development of both fish and plants [27]. To address this issue, a decoupled aquaponic system was created. This system separates the hydroponic components and aquaculture and allows for the adjustment or adaptation of the environmental conditions of each component to meet the individual needs of the fish and plants [32]. To maximize the growth efficacy of the system, the compromise between pH, temperature, and nutrient needs must be minimized [33]. The decoupled aquaponic system consisted of two independent loops, one for the RAS and another for the hydroponic components [34]. This arrangement enabled the recirculation of process water within each component, allowing for better control of the system tailored to the specific needs of the species involved [32,33].
The mineralization loop utilizes microorganisms to break down sludge, enhancing the availability of dissolved nutrients for plants [35]. Additionally, the acidification process lowers the pH, producing an ideal environment for plant development [36]. The demineralization loop is used in hydroponics to separate the nutrients and dissolved salts from the process water. The process water is then returned to the hydroponic unit, while the demineralized water is circulated back to the RAS [37]. This helps to improve control over the nutrient concentration in the respective subsystem of Dynamic Aquaponics System [38]. Decoupled aquaponics involves a reduction in the strength of interconnections between subsystems, resulting in the autonomous construction of the system in accordance with the nutritional needs of plants [27,33]. This results in a one-way flow where fish do not receive any benefits from the plants. Nevertheless, a connection is being established between the two subsystems, and the remineralization unit and other loops work together to maintain water quality [39]. This creates a virtuous cycle wherein fish, bacteria, plants, retained water, and fish all benefit from each other [27]. The phrase “decoupled” refers to the process of restructuring software systems by breaking down a huge monolithic system into smaller, independent pieces [37]. The DAPS design enables independent control of water recirculation in aquaculture and hydroponic systems, ensuring optimal water quality for promoting healthy growth of both plants and fish [40].
In contrast to a traditional aquaponic system, [40] asserted that the enhanced production of the hydroponic component was a result of the decoupled system’s superior control over water quality. African catfish and basil exhibited satisfactory growth in the decoupled aquaponic system when the feeding rate was reduced by 30% of the fish’s actual feeding rate [41]. In a decoupled aquaponic system, increased plant production was the result of higher fertilizer concentrations in hydroponics, whereas enhanced water quality for fish was the consequence of lower nutrient levels in RAS. When comparing DAPS to traditional aquaponics, found that improved pH and fertilizer control led to a 36% increase in tomato fruit output. Compared to hydroponics, the DAPS achieved the same yield while using 100% less freshwater and 62.8% fewer mineral nutrients for production [36].
The decoupled system’s net present value (NPV) was shown to be higher than that of traditional aquaponics in the cost-benefit analysis [40]. Contrarily, [36] proposed using the decoupled system for large-scale production and using conventional aquaponics to cultivate plants with lower nutrient requirements and fruiting vegetables with higher nutrient requirements. While DAPS offers improved sub-system control and increased productivity, it is much more sophisticated than connected aquaponics and hydroponic systems, requiring a far larger initial investment [41]. Additionally, the extra loops lessen the system’s economic viability for small-scale entrepreneurs; instead, they are appropriate for commercial production systems operating on a large scale or in regions with access to energy sources. Therefore, to expand DAPS, or on-demand coupled aquaponic systems, on a commercial scale, substantial research should be adopted [40,27].
Ecological Prospectives of Aquaponics
Due to the growing global population, limited resources, and advancements in production technology, aquaponics is seen as a promising ecological solution to the global food crisis and its environmental impacts [42,43]. Aquaponics possesses the capacity to significantly augment ecological and sustainable intensification in agriculture through the following mechanisms: reduction of resource consumption (e.g., water and land), optimization of wastewater and nutrient reuse efficiency, generation of zero waste, attainment of high productivity, and obviation of chemical fertilizer requirements. Because of this, it contributes significantly to climate-smart agriculture and the circular economy [44,45].
Aquaponics uses 90% less water than soil-based systems, with water consumption amounting to only 1% of that used in pond aquaculture. The extensive claims about the sustainability of aquaponics and its potential to reduce environmental costs have raised questions, leading to increased interest in evaluating the validity of these assertions (Chen et al., 2020) [45]. Life cycle assessment has become a comprehensive method for evaluating the sustainability of aquaponics and measuring the direct and indirect environmental effects of processes or products over their entire life cycle, from inception to disposal [43,45].
One possible way to describe aquaponics’ environmental performance is to use life cycle assessment to explicitly evaluate midway and endpoint impacts [46]. Endpoint impact analysis focuses on assessing the impact of a particular activity on quality, ecosystem resources, and human welfare [22]. In contrast, midpoint evaluation does not consider the environmental costs that occur earlier in the cause-effect chain, such as eutrophication, acidification, depletion of abiotic resources, and global warming potential. Compared to hydroponics, the midpoint impact of aquaponics is 1.7 times lower, and the endpoint impact is 50% lower [45]. Conversely, RAS incurs the highest environmental costs in terms of operation and infrastructure, primarily due to their high energy consumption and the technical infrastructure needed for water recycling. This significantly contributes to the exacerbation of global warming [34]. The environmental impact of aquaponics is considerably reduced in comparison to that of conventional hydroponics and aquaculture systems. The overall negative impacts on fish culture are mitigated by the coexistence of plant and fish production, which is achieved through climatic control, efficient use of water and resources, and plant biofiltration [46].
These variables help mitigate harm to the ecosystem and conserve resources [47]. A recent advancement in aquaponics research involves the development of customized aquafeeds tailored to different species. To achieve this, it is essential to modify the diet to meet the nutritional requirements of both the plants and fish within the system [48]. The primary goal was to reduce reliance on fishmeal and fish oil as protein sources and to identify viable alternatives. This approach aims to alleviate pressure on overexploited marine capture fisheries, decrease carbon emissions, protect biodiversity, and mitigate the environmental impacts of this sustainable production system, all within the framework of a circular economy [43].
From both socioeconomic and environmental perspectives, aquaponics encompasses a range of factors. This method recycles nutrients and wastewater, reduces pollution from aquaculture discharge, and supports economic development and food security. Additionally, it contributes to the reduction of greenhouse gas emission [46]. Moreover, as a tool for combating climate change, this climate-resilient system shows great promise. It can adapt to various environments by employing a method that meticulously controls abiotic factors [16]. When managed effectively, it becomes less susceptible to environmental fluctuations and climate change, leading to improved disease management, increased production, and reduced resource use [49].
Use of Biofloc Technology in Aquaponics for Culturing Aquatic Animals
The application of biofloc technology to aquaponic systems is a relatively recent phenomenon (Barbosa et al., 2022) [21]. Microorganisms constitute vital constituents within BFT systems [50,51]]. To maintain water quality, the bacterial population is managed to restrict the growth of autotrophic microorganisms. This is achieved by maintaining a high carbon-to-nitrogen ratio, as heterotrophic bacteria can efficiently consume nitrogenous by-products [14]. At the beginning of the culture cycles, a high carbon-to-nitrogen ratio is essential to promote optimal development of heterotrophic bacteria (Pinho et al., 2022) [16]. This energy is utilized by the bacteria for their maintenance and growth. Additionally, various species of microorganisms are crucial components of biofloc technology (BFT) systems. The population of chemoautotrophic bacteria, specifically nitrifying bacteria, stabilizes after approximately 20 to 40 days [7,50].
Approximately two-thirds of the ammonia absorption in the system may be attributed to these bacteria [52]. Therefore, it is important to reduce the amount of external carbon added, while replenishing the alkalinity consumed by the microbes with alternative carbonate and bicarbonate sources [11]. The dynamic interaction of diverse populations of naturally occurring species, including fungi, bacteria, nematodes, microalgae, rotifers, and protozoans determines the stability of zero or minimal water exchange [51]. Bioflocs, which are aggregates consisting of proteins and lipids, serve as a natural food supply. These bioflocs are available throughout the day because of the intricate interplay between physical substrate, organic matter, and a diverse array of microbes [14].
The production of microorganisms in tanks, raceways, or lined ponds serves three primary purposes: first, it helps maintain water quality by reducing nitrogen compounds and generating in-situ microbial protein. Second, it provides nutrition, which lowers feed costs and enhances the feasibility of the culture. Third, it helps combat harmful microbes [51]. In BFT, key concerns regarding water quality for cultured organisms include excess particulate organic matter, harmful nitrogen compounds, and oxygen levels, among other factors [53]. There are three ways that ammonia nitrogen is removed in this environment: first, photoautotrophic removal by algae; second, heterotrophic bacteria turn ammonia nitrogen into microbial biomass; and third, autotrophic bacteria turn ammonia into nitrate [51]. Plants grown in aquaponic systems can utilize nitrates and other nutrients, both macronutrients and micronutrients, that accumulate throughout the growth cycle as substrates [54]. In summary, the findings indicate that BFT is beneficial for shrimp and fish growth. BFT enhances fish yields and improves water quality compared to traditional aquaculture systems [55]. This improvement may be attributed to increased microbial activity, which enhances nutrient availability [13].
Biofloc serves as a high-quality food source for cultured organisms, resulting in significant cost reductions in aquaculture, where feed accounts for 40–60% of operational expenses [50]. Utilizing biofloc as a food source can improve feed efficiency by reducing protein requirements and increasing nitrogen utilization. Biofloc consists of various components, including proteins, lipids, carbohydrates, essential amino acids, essential fatty acids, antioxidants, and vitamins [7]. These elements contribute to positive outcomes such as enhanced growth, improved immunity, increased survival rates, and better reproductive performance in cultured organisms [56]. Additionally, the beneficial microorganisms present in the BFT play a crucial role in supporting aquaculture species. They compete with pathogenic bacteria in the environment, leading to a significant reduction in both the abundance and virulence of these harmful bacteria [57]. BFT eliminates the need for water exchange by utilizing microorganisms to naturally filter the water. This zero-water-exchange system enhances biological security by preventing the spread of pathogenic microorganisms that can occur during water exchange [57]. Additionally, this technology is vital for preventing pollution and ensuring biosecurity by stopping the transmission of diseases from aquaculture wastewater into the natural environment [11]. This system effectively prevents the escape of aquaculture organisms while maintaining optimal temperatures for aquaculture, all while minimizing energy consumption. This suggests that stable production of aquatic products is achievable through BFT [14].
FLOCponics: Combination of Biofloc Technology and Aquaponics
Aquaponics and aquaculture based on BFT are considered environmentally friendly methods of food production. Both are intensive aquaculture systems that prioritize water conservation and nutrient recycling [59]. FLOCponics shares similar characteristics. By integrating the principles of aquaponics and bioflocs, FLOCponics has the potential to serve as an additional tool in addressing the challenges of the global sustainable food supply [16]. Hydroponics and BFT are combined in FLOCponics, an integrated biofloc-based food production system [60]. Based on the same ideas of maximizing and recycling nutrients, water, energy, and land, it is a subset of aquaponics [58]. Plants grown in water can utilize the nutrients present to their advantage, which is why hydroponic loops are being integrated into biofloc-based farms [27]. This integration helps diversify production and provides aquaculture growers with additional products to sell [61]. Microbial interactions in BFT can enhance nutrient recycling and promote greater fish development, making it a viable alternative to RAS for aquaculture producers [11,62]. In addition to the advantages already mentioned, the use of bioflocs in shrimp and fish production may result in more efficient and sustainable use of water and nutrients [27].
Research on FLOCponics has primarily focused on comparing its yields to those of other production systems, as well as evaluating its nutritional profile and water quality [51,46]. Although the studies covered a wide range of topics, their overarching goal was to enhance FLOCponics and facilitate its market entry. FLOCponics has demonstrated promise not only in producing fish and shrimp and recovering nutrients but also in meeting sustainability standards [62]. However, some studies have reported operational challenges when implementing FLOCponics in a permanently connected setup [16,62]. Large-scale farmers are more inclined to adopt FLOCponics for educational and social purposes compared to RAS-based aquaponics, which are primarily used for commercial objectives [63]. One drawback of FLOCponics is the high cost of the infrastructure needed to maintain the biofloc bacteria and keep the system operational, making it challenging to implement the technology for social initiatives [64]. In terms of real-world applications, FLOCponics is still in its early stages. The private sector typically does not share data, resulting in limited information on the commercial uses of FLOCponics [62]. However, there have been documented instances of BFT farmers integrating hydroponic subsystems into their production units to conduct small-scale experiments with FLOCponics [46]. Optimal system design, target species to be cultivated, and overall economic viability are determined by environmental factors, production goals and scale, and management tactics [54,16]. FLOCponics typically operates in a closed setup, requiring very little land and water to produce nutritious food, which gives it an advantage over aquaponics and BFT systems [62].
Aquatic Animal Species Cultured in FLOCponics
In a study done by [65] it was shown that among 256 aquaponic participants, 70% utilized Tilapia (Oreochromis niloticus) [18], and 27% utilized Catfish (Siluriformes) [66] in their commercial operations. Additional fish species frequently utilized in commercial aquaponics are Rainbow trout (Oncorhynchus mykiss) [67], Common carp (Cyprinus carpio) [68], Largemouth bass (Micropterus salmoides) [69], Barramundi (Lates calcarifer) [70], Pacu (Piaractus mesopotamicus) [65-71], and Murray cod (Maccullochella peelii) [5]. An essential attribute for the successful cultivation of aquatic organisms in aquaponics is their capacity to endure elevated population densities as well as substantial concentrations of total suspended solids, phosphorus, nitrogen, and potassium [69]. It is typically not recommended to exceed a fish stocking density of 0.07 kg/L. However, species that can flourish at this density level are well-suited for aquaponics [67,72]. Furthermore, these species should be feasible for culturing in highly intensive culture system (Figure 1) [69].
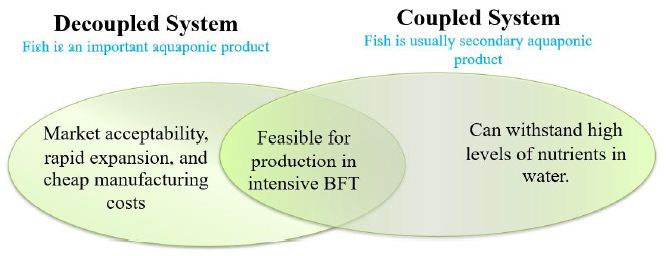
Figure 1: The general qualities that fish species need in both coupled and decoupled aquaponics systems to be productive.
In aquaponics, Nile tilapia is the most frequently used and conceivably most successful fish species, followed by carp and African catfish [18]. The literature review revealed that 44% of published works included tilapia species as the primary aquatic organism [54,73]. Tilapia thrives in aquaponic environments due to its remarkable resilience to suboptimal water conditions [73]. This fish is characterized by rapid growth, resistance to stress and disease, tolerance to diverse environmental conditions, and the ability to consume food from lower trophic levels [19,54].
The tilapia fish is a microphagous low-trophic omnivore that feeds on phytoplankton and other tiny organic particles [73]. Since tilapia have a low dissolved oxygen requirement as they don’t need a lot of room to grow. This makes them perfect for aquaponic systems that aim to meet plant nutrient demands [18,73]. The impact of excretion from different species on nutrient concentrations in the aquaponic solution and subsequent plant production remains an open question in the current aquaponic literature [54]. For example, the water effluent from Nile tilapia, African catfish, and common carp contained nitrate levels ranging from 18 to 41.6 mg/L and phosphorus levels between 9.5 and 19 mg/L [66].
In their study, [74] found that using Common carp wastewater resulted in higher cucumber yields compared to tilapia effluent. However, tilapia effluent led to greater tomato yields. Tilapia fish have a higher level of metabolic feeding activity than carp, but the exact explanation for the superior development of tomatoes with tilapia effluent compared to carp effluent is still unknown [74,75]. This suggests that tilapia excreted a greater amount of feces into the water compared to carp. The authors also proposed that using various species might provide benefits in terms of achieving a more comprehensive nutrient water profile [18]. Certain shrimp species, such as Litopenaeus vannamei [46,76] and Penaeus monodon [77], have been utilized in aquaponics. However, the exploration of polyculture, which involves using different aquatic species in aquaponics to enhance plant development has not yet been thoroughly examined [16].
Plant Species Cultured in FLOCponics
Traditionally, leafy vegetables have been cultivated in aquaponic systems due to their short growing seasons, low nutrient requirements, tolerance for nitrogen-rich environments, and high global demand [21]. However, despite their higher economic value compared to leafy greens, cultivating flowering crops in aquaponic systems poses greater challenges. These challenges stem from their increased demand for phosphate and potassium fertilizers, greater susceptibility to pests and diseases, and slower growth cycles (Chu and Brown, 2020) [15]. Furthermore, the same study found that profit does not always correlate with crop value. According to their research, Bibb (Boston) lettuce generated higher revenue per week per m2 ($8.50–9.50 USD) than basil ($4.90–5.90 USD), despite basil having the greatest value per kg ($8.50–10.03 USD) [78]. This was due to improved planting density and yield. The majority of aquaponic economic studies concluded that the system was lucrative by including green vegetables. found that an aquaponics farm producing barramundi and lettuce generated an annual economic return that was $22,850 higher than that of the two independent systems. Over the course of a year, the aquaponic farm saved $1,315 on phosphate and nitrogen fertilizers, $1,270 on wastewater disposal, and $3,390 on all variable expenditures [79].
Integrating a lettuce and basil NFT system with trout farm producing 20,680 kg annually would plants yield a return of 13.8% and increased profit due to lower water remediation costs and higher plant production revenue [78]. This might be the reason why leafy greens and herbs are the main crops grown in commercial aquaponic systems. Basil (81%), salad greens (77%) [78], Solanum lycopersicum (Tomatoes) (66%) (Nadia et al., 2023) [75], Lactuca sativa (69%) [80], Brassica oleracea (56%) [81], Beta vulgaris (53%) [82], Capsicum annuum (pepper) (48%) [83], and Cucumis sativus (Cucumbers) (47%) [40] were the most grown crops by commercial aquaponic growers. In addition, aquaponic systems have been developed to cultivate plants that are capable of flourishing in saline environments [1]. Salicornia persica is a significant plant species suitable for cultivation in aquaponics systems that use salt or brackish water [46]. Salicornia is a halophyte that demonstrates tolerance to high salinity levels and efficiently absorbs significant amounts of phosphate and nitrate [84]. The advancement of aquaponics has also broadened the range of plant species that can be successfully cultivated [75]. However, there is a lack of scholarly studies specifically addressing the use of aquaponics for cultivating flowering plants [78]. Further research should investigate the effectiveness of aquaponics in the floriculture sector [85].
Water Quality and Nutrient Recycling
The ability of BFT microbes to effectively recycle nutrients and maintain optimal water quality for farmed species is a crucial aspect of BFT [52]. Phytoplankton, nitrifying bacteria, and heterotrophic bacteria all participate in the ammonia-nitrogen cycle [50]. They convert harmful ammonia-nitrogen into nitrate or incorporate it into bacterial biomass. Various forms of nitrogen conversion typically occur simultaneously, with the prevalence of each form depending on the nutrient management practices of the system [8]. Furthermore, the physicochemical properties of the water must meet the specific requirements of the microbes. Specifically, it is essential to provide high levels of dissolved oxygen and alkalinity, along with a favorable carbon-to-nitrogen ratio [6,40]. Detailed information is needed regarding the precise water quality required for the development of BFT microorganisms, as well as the recommended values of water parameters that must be maintained in fish or shrimp tanks within BFT systems [7,50].
Most of the physical-chemical quality of water indicators are still within acceptable limits to produce fish or shrimp, according to the findings of studies conducted in FLOCponics systems with an emphasis on animal production [16]. The total volume of suspended solids (bioflocs) was an anomaly, falling below the acceptable level. For instance, the average volume of bioflocs in tilapia culture typically ranges from 2.5 to 4.8 ml/L, 0.4 ml/L, and 0.4 to 0.9 ml/L. These levels are significantly lower than the recommended minimum of 6 ml/L [71]. However, these low numbers do not appear to have affected the ability of microorganisms to recycle nitrogen or maintain water quality [16]. This suggests that the relationship between microbial activity and biofloc volume in both BFT monocultures and FLOCponics remains ambiguous and varies significantly [46]. Some chemical and physical properties of water, particularly those related to pH and the concentration of suspended particles in connected FLOCponics systems, do not always appear to be beneficial for plants [16,65]. Hydroponic production typically recommends a pH range of 5.5 to 7 to ensure optimal nutrient availability for plant uptake [86]. However, most documented FLOCponics systems have been operated at relatively neutral pH values [40].
There is no need to regulate the pH of the FLOCponics system due to the lack of significant impacts on plant development [40]. In hydroponic subsystems, it is essential to maintain a very low concentration of suspended solids to prevent the accumulation of bioflocs in the plant roots, which can impair the ability of plants to respire and absorb nutrients [4]. In contrast, FLOCponics systems are known to have higher solid content in their hydroponic tanks [42]. Maintaining low solids concentrations in the hydroponics subsystems while concurrently preserving the biofloc concentration in the fish tanks at ideal levels for animal production seems to be one of the trade-offs associated with connected FLOCponics [87]. All subsystems require optimal water quality conditions, which depend on the inflow of nutrients and their transformation by microbes [50]. In traditional aquaponics, most of the nutrients used to nourish plants are believed to come from RAS effluent, and this is also expected to hold true for FLOCponics [37].
Feed typically serves as the primary source of nutrients in the aquaculture subsystem of RAS. In contrast, FLOCponics may provide a higher concentration of nutrients, as it integrates both organic and inorganic carbon sources [58]. According [52] both processes are frequently necessary to encourage the proliferation of BFT bacteria. Due to the limited knowledge of the specific features of the nutrient supply used in the FLOCponics systems, it is difficult to accurately estimate the quantity of nutrients that will be accessible for plant growth [46]. Furthermore, the precise rates of nutrient recycling and nutrient intake by the BFT bacteria remain unknown, leading to significant uncertainty in projections. Examining the nutritional composition of the plant biomass enables us to identify the nutrients delivered in the smallest amounts [88].
FLOCponics water often has lower fertilizer concentrations compared to hydroponic solutions [89]. However, in contrast to traditional aquaponics utilizing RAS, the practice of external carbon additive in aquaponics resulted in elevated levels of K, P, S, Ca, and Fe [71]. The process of converting RAS-sludge into minerals utilizing bioreactors and efficiently using the resulting liquid as fertilizer in multi-loop aquaponics [39]. There is limited documentation on the utilization of mineralized solids as a nutrition source for plants in FLOCponics [89]. Investigations in FLOCponics research have explored the use of plants as filters to eliminate nutrients from water as part of nutrient recycling methods. The investigations have mostly focused on the recovery of nitrogen and phosphorus and their conversion into plant biomass [58]. [46] conducted a study on the extraction of nitrogen and phosphorus from BFT effluent using halophyte plants. Combining shrimp and plant production may eliminate between 24.2% and 39.4% of nitrogen (N) and between 14.6% and 19.5% of phosphorus from the to feed input, as indicated by their findings. It is noteworthy that both nutrients often build up in BFT water [68]. When present in large quantities, these compounds can be harmful to the animals being raised. Additionally, if they are released into aquatic ecosystems, they can contribute to water eutrophication [7].
Productive Results of Aquatic Animals Cultivated in FLOCponics
Most of the research utilized Pacific white shrimp (Litopenaeus vannamei) or Nile tilapia (Oreochromis niloticus), except for [90] who cultivated South American catfish (Rhamdia quelen). According to [52] Pacific white shrimp and Tilapia are the predominant species in biofloc-based cultures. Both species exhibit a notable resilience to adverse environmental circumstances, including elevated levels of suspended particles and nitrogenous chemicals in water. This is the primary factor contributing to their ability to thrive in such environments. In addition, their morphological modifications allow them to efficiently exploit bioflocs as an extra food source [91].
The most used species for the nursery period was tilapia, with an initial weight ranging from 0.4 to 4.2 g [18]. Nevertheless, in the context of shrimp farming, the growth-out phase involved the production of shrimp, starting from an initial weight of 1.6 g until they reached an approximate weight of 13 g [76]. Research on the efficacy of FLOCponics for aquatic creature development has examined a wide range of factors. Some examples of treatments include: (i) adjusting the trophic levels of the BFT or the carbon source to evaluate alternative nutrient inputs [90], (ii) varying levels of salt in the water [46], (iii) the impact of using BFT in conjunction with hydroponics [92], (iv) how shrimp performance is affected by plant production-specific management [93] and (v) how classical aquaponics utilizing RAS [71], compares to FLOCponics systems in terms of plant and fish development.
[93] described that tilapia fed with a feed containing 40% protein and no fertilizer addition performed better than those fed with a food containing greater protein content and fertilizer supplement in the FLOCponics system [18]. Using bioflocs from an ex-situ BFT led to better tilapia output and feed conversion ratio [51]. A comparative study between traditional aquaponics and FLOCponics systems was conducted to assess the production of tilapia juveniles. The results showed that the FLOCponics system yielded a higher growth rate, greater final weight, and a lower FCR compared to the traditional aquaponics system. [71]. The authors noted that the average volume of bioflocs in the tank was below the required level for BFT cultivation. A larger supply of natural food in the tank could have improved the performance of the fish. [94] also discovered a similar pattern of low biofloc volume and its effect on fish development in their study, which involved the use of linked systems. The authors of this study did not see any significant statistical differences in Rhamdia quelen production between aquaponics and FLOCponics. Both experiments indicated that enhancing system design might optimize the interaction of BFT with hydroponics.
The reported density of tilapia by Fimbres-Acedo et al., (2020) [93] which is 23 kgm−3, is considerably lower than the maximum density of 50 kgm−3 seen in BFT or the density of 70 kgm−3 achievable in the growth-out phase of commercial aquaponics with RAS [58]. The nursery phase values, ranging from 6.9 to 8.8 kgm−3, are within the expected range of 7 to 9 kgm−3 in BFT systems. Commencing the growth-out phase with a stocking density of 260 to 520 juveniles per cubic meter in shrimp production can lead to the development of marketable shrimp weighing more than 20 grams and achieving yields of 6 to 8 kg/m3 [17]. The FLOCponics shrimp studies employed comparable stocking densities, resulting in lower yields ranging from 2.2 to 2.9 kg/m3 [46]. Connecting a hydroponics system to biofloc tanks affects solids and bioflocs, as discussed earlier. With reduced biofloc, there is less natural food available, which may alter microbial activity. This is likely the reason for FLOCponics’ lower yields compared to monocultures reliant on biofloc [16]. The findings indicate that improving the system’s design and carrying capacity may be able to address issues with yield performance and solids management. This would make FLOCponics work better and get it closer to commercial aquaponics with RAS [46,16].
Productive Results of Plant Cultivated in FLOCponics
One of the main components of FLOCponics systems is the use of nutrient rich BFT effluents to feed hydroponic plants. Nevertheless, there is disagreement among researchers over whether FLOCponics increases or decreases plant yields [46]. Plant growth in this system should be compared with crops in hydroponics, conventional aquaponics utilizing RAS obtain definitive conclusions on the influence of BFT waste on plant productivity [4]. Standardizing the nutritional input composition across all systems may also be accomplished concurrently with this comparison. There were several reviews that contrasted FLOCponics with hydroponics or conventional aquaponics, but none that did so with soil-based techniques [40].
The quantity and type of nutrients supplied to the hydroponics subsystem varied among the treatments and systems in experiments comparing FLOCponics with other approaches. Most studies on FLOCponics have focused on the output of lettuce or salicornia. Leafy plants like lettuce are often used in conventional aquaponics systems due to their rapid growth cycle and low nutritional requirements [4]. Among the studies comparing lettuce grown in FLOCponics to lettuce cultivated in other systems, 19% found that FLOCponics performed better, 13% indicated that traditional aquaponics was more effective, 25% reported better results with hydroponics, and 44% found no significant differences between the systems [60]. The researchers evaluated the production of lettuce using BFT effluents, either treated with filtering devices or left untreated, but neither scenario showed any modifications in plant development. Solids and bioflocs were observed on plant roots, particularly in the absence of filtration systems; therefore, the scientists proposed the development of effective mechanical filters to prevent the accumulation of solids. They evaluated the effects of supplementing the hydroponics subsystems of the FLOCponics treatments with fertilizer on lettuce growth within the same experiment. The authors found that, due to the added fertilizer, the lettuce grew similarly in both the hydroponics and FLOCponics systems [40]. The halophyte salicornia is a very valuable commodity. Researchers in these experiments failed to evaluate FLOCponics in comparison to other methods of crop production. Most of them emphasized how salicornia production and BFT may work together for the better (Table 1) [46].
Table 1: An overview of productive results of aquatic organisms and plant species cultivation in FLOCponics.
|
Plant |
Animal | Results |
Reference |
| Lettuce (Lactuca sativa L.) | Tilapia (Oreochromis niloticus) | Lettuce cultivated in FLOCponics showed noticeably poorer growth performance and visual quality compared to lettuce grown in traditional aquaponics. In contrast, juvenile tilapia demonstrated significantly enhanced zootechnical performance in the FLOCponics system. | Pinho et al. (2021) |
| Cucumber (Cucumis sativus L.) | Tilapia (Oreochromis niloticus | Variations in pH influenced the availability of macro and micronutrients. However, they did not significantly affect the growth rate of cucumbers. Both cucumbers and tilapia showed significant growth rate in BFT supported aquaponics. | Blanchard et al. (2020) |
| Cherry tomato (Solanum lycopersicum var. cerasiforme) | African cichlid (Melanochromis sp.) | Tomatoes and fish grown in FLOCponics water exhibited a remarkable 20% increase in growth rate compared to those cultivated in a traditional hydroponics system. This demonstrates the enhanced efficacy of the FLOCponics approach in promoting growth for both crops and aquatic species. | Castro-Castellón et al. (2020) |
| Jalapeño pepper (Capsicum annum) | Tilapia (Oreochromis niloticus) | Tilapia demonstrated enhanced productivity in tanks employing BFT. However, there were no significant differences in plant productivity among the assessed systems for the pepper plants. | Martinez-Cordova et al. (2020) |
| Tomato (Lycopersicon esculentum) | Tilapia (Oreochromis niloticus) | The growth performance of tomatoes was not enhanced in BFT supported aquaponics. In contrast, the growth and survival rates of tilapia showed significant improvement. | Martinez-Cordova et al. (2020) |
| Cherry tomato (Solanum lycopersicum var. cerasiforme) | Tilapia (Oreochromis niloticus) | The cherry tomato ‘Favorita’ yielded similarly in FLOCponics and hydroponics before fish harvest, whereas the tomato ‘Goldita’ yielded more in hydroponics. Both cultivars grew better in hydroponics after the fish harvest. | Pickens et al. (2020) |
| Perennial glasswort
(Sarcocornia ambigua) |
Pacific white shrimp (Litopenaeus vannamei) | The combined production of L. vannamei and S. ambigua in FLOCponics was recommended at 16–24 psu since the shrimp performed well and the plants grew and removed nitrogen and phosphate compounds. | Pinheiro et al. (2020) |
| Perennial glasswort
(Sarcocornia ambigua) |
Tilapia (Oreochromis niloticus) and pacific white shrimp (Litopenaeus vannamei) | Compared to BFT, the FLOCponics system’s IMTA produced a better yield. The presence of S. ambigua did not affect the consumption of phosphorus or nitrogen, despite the reduction in nitrate levels. | Poli et al. (2019) |
| Asparagus (Sarcocornia ambigua) | Pacific white shrimp (Litopenaeus vannamei) | The growth performance of Litopenaeus vannamei and Sarcocornia ambigua cultivated together in FLOCponics was significantly enhanced. | Soares et al. (2022) |
| lettuce (Lactuca sativa L.) | Tilapia (Oreochromis niloticus) | Growing lettuce in freshwater FLOCponics resulted in a greater harvest than in brackish water. | Zappernick et al. (2022) |
| Lettuce (Lactuca sativa L.) | Silver catfish (Rhamdia quelen) | Compared to traditional aquaponics, FLOCponics methods utilizing silver catfish wastewater as fertilizer significantly enhanced lettuce growth. | Rocha et al. (2017)
|
| lettuce (Lactuca sativa L.) | Tilapia (Oreochromis niloticus) | Lettuce grown using BFT effluent demonstrated greater productivity compared to that cultivated in conventional aquaponics. Among the various types of lettuce examined, butter lettuce exhibited the most favorable growth characteristics, highlighting its suitability for BFT systems. | Pinho et al. (2017) |
| Perennial glasswort
(Sarcocornia ambigua) |
Pacific white shrimp (Litopenaeus vannamei) | S. ambigua absorebed maximum nutrients from shrimp waste and improved the growth rate. While shrimp growth was not improved by the combination of S. ambigua with shrimp production, while using BFT in aquaponics. | Pinheiro et al. (2017) |
The BFT trophic level can have an impact on the performance of different plant species, including spinach, lettuce, pak-choi, rocket, basil, and others. Their findings emphasized the significance of determining the species’ suitability for a particular production scenario. [89] conducted a study comparing tomato growth in FLOCponics and hydroponics systems, both before and after fish harvest. Following the fish harvest, the researchers found that the FLOCponics system produced fewer tomatoes than the hydroponics system. This difference was attributed to a lack of nutrients in the water, which hindered the growth of the remaining tomatoes. Although nitrogen levels in the BFT effluent were considered low, the elemental composition of cucumber leaves remained within acceptable ranges [93].
Researchers conducted further examination in FLOCponics research and obtained encouraging outcomes, assessing visual attributes, nutritional content, and stress indicators [57]. Their findings indicated that the growing conditions in FLOCponics did not lead to excessive plant stress. Some studies revealed that BFT had a positive impact on the visual quality of the plants, while others found no visible signs of nutritional deficiencies [54,89]. Research on FLOCponics often associates the presence of particulates or bioflocs on plant roots, as well as high water pH levels (above 7), with poor visual characteristics and inadequate plant growth. These factors can hinder the availability of nutrients in a form that plants can absorb [57]. Furthermore, nutritional imbalances and the consumption of nutrients in water by BFT microbes are additional factors that contribute to these issues [89]. However, the exact role of these bacteria in the processes of nutrient recycling and elimination remains unclear [95]. Additionally, the lack of effective waste management and the failure to optimize nutrients by reusing or demineralizing sediments and bioflocs exacerbate the issue [54].
Sustainability Aspects of FLOCponics
Researchers have developed emerging technologies to promote the transition of aquaculture toward more environmentally sustainable practices. Sustainability in aquaculture encompasses the need for systems to be both technically feasible and economically viable. The objective is to provide safe and nutritious food to meet the needs of current and future generations [59]. Conducting economic evaluations of different aquaculture operations can yield valuable information for implementing managing techniques that enhance the business’s resilience and longevity [89,57]. Sustainability assessments are essential for developing a comprehensive understanding of the social and ecological impacts of a new production system, considering its interconnectedness. This understanding is crucial for establishing effective public strategies that promote the sustainable growth of the industry. It encompasses biological, technical, and economic considerations [96]. Research utilizing Life Cycle Assessment has shown that the primary environmental impacts of aquaponics production are associated with infrastructure, energy consumption, and feed [97,98].
The positive aspects of aquaponics systems are frequently linked to their low water consumption and their potential to support cultural, recreational, educational, and tourism-related benefits, as well as to enhance the landscape [89,99]. While the carbon footprint associated with commercial shrimp production, as determined by life cycle assessment, does not significantly impact biofloc-based production, energy consumption does [59]. The literature reveals a lack of sustainability assessments for FLOCponics systems. This gap is likely due to the absence of a large and comprehensive database necessary for such analyses [16]. [58] described FLOCponics as a novel technique with the potential to mitigate certain unsustainable aspects of traditional aquaculture, despite the lack of available sustainability assessment data. Replacing the RAS with BFT can enhance the advantages and disadvantages of both biofloc-based systems and conventional aquaponics.
An environmentally friendly food production system, already recognized for its effectiveness, can integrate this substitution [16]. Additionally, the key sustainable benefits of FLOCponics systems include the ability to produce a variety of food items close to consumers, in compact urban areas, while minimizing environmental impact and providing social benefits [89]. Furthermore, FLOCponics is a highly significant system in food production, as it yields pesticide-free, nutritious products available to consumers in various forms, including fish and vegetables. A speculative commercial-scale FLOCponics system was modeled to incorporate Litopenaeus vannamei and S. ambigua, a halophyte, with a focus on its profitability. Even in the most pessimistic business projections, the authors assert that the system is financially viable due to the high market value of the species involved. Additionally, they found that FLOCponics requires expensive operational equipment, highly trained personnel, and significant deployment costs. It would be unwise to assume that FLOCponics will be profitable based solely on hypothetical outcomes in specific regions and with products [16,58].
It is crucial to recognize that if the productive capacity of FLOCponics is validated, the expenses could be mitigated by increased biomass production, addressing this economic concern [89,57]. For instance, the cost of electricity per kilogram of food produced in FLOCponics systems is expected to be lower than that in biofloc-based monocultures [85]. Incorporating renewable energy sources such as solar, wind, and biogas from biodigesters, along with durable infrastructure and equipment, could further enhance the environmental sustainability of FLOCponics systems [54,71]. Food production systems inherently affect the environment. Therefore, we recommend supporting systems that achieve high productivity with minimal negative impacts [100]. It is essential to evaluate the trade-offs between the benefits and drawbacks of FLOCponics, as well as to assess the long-term viability of actual systems. To accomplish these goals, we must develop a more comprehensive technical and economic database on FLOCponics, which can then be subjected to sustainability studies [85].
Challenges of Using BFT in Aquaponics
If the technological challenges are addressed, FLOCponics could serve as a viable alternative for investors looking to establish integrated agri-aquaculture farms. To effectively operate a FLOCponics system and achieve optimal results, a thorough understanding of several key subjects remains essential [16.95]. Additionally, the selection of the food production system must consider several elements, including market demand, climatic conditions, producer expertise, technical knowledge, input costs, and availability, among other considerations [100]. While recognizing the potential benefits of FLOCponics, it is essential to conduct a comprehensive review of the entire production process to select the most suitable method for a given situation [80]. The design and construction of FLOCponics systems are crucial elements that require alteration. The configuration of this system must be carefully designed to maximize the favorable environmental situations required for the growth of aquatic plants and organisms, as well as the nourishment of BFT bacteria [71]. The primary goal is to maintain optimal levels of suspended particles in the water to support the growth of both fish and plants. As previously mentioned, the high concentration of solids in FLOCponics systems appears to impede plant growth. Efforts to remove solid particles from the hydroponics subsystem have, however, diminished the availability of food and bioflocs for the animals in their natural environment [19,80]. A potential strategy to address this issue involves developing mechanical separators that efficiently separate the solid and liquid components of the BFT effluent. This would allow for the transfer of nutrients and water from the bioflocs to the hydroponics subsystem, while reintegrating these elements into the aquaculture subsystem [100]. Bag filters with backwash technology, drum filters, and sedimentation containers with meticulously engineered biofloc return flow should be considered for FLOCponics [71].
Additionally, it is essential to establish the regularity of their operation and control the water discharge rate into these filtration systems. It is important to emphasize that each of these filters can be utilized in interconnected FLOCponics systems [100]. However, in all interconnected systems, there will inevitably be a trade-off between the needs of plants and animals [36,80]. The enhancement of the technical components of FLOCponics systems should effectively mitigate or perhaps resolve these issues, mostly associated with solids management [19]. Furthermore, implementing a decoupled design would enable effective adjustments of pH levels to optimal values for each subsystem and allow for the direct addition of specific minerals to the hydroponics subsystem. Unlike commercial hydroponics that rely on completely prepared fertilizers, FLOCponics might potentially lower production costs by using only particular nutrients [46]. This is feasible because BFT effluent already contains a diverse array of nutrients. To achieve this goal, it is crucial to obtain detailed information about the quantities of nutrients present in the feed and the carbon source. Additionally, it is important to analyze the micronutrient content in the process water of the BFT system, as these micronutrients significantly impact plant physiological processes, such as photosynthesis [36,80]. An examination of the differences in the quality and diversity of micronutrients between FLOCponics systems and a properly balanced hydroponic fertilizer will shed light on the possibility of a specific nutrient deficiency. This could facilitate the creation of tailored supplementation regimens for each plant species, thereby maximizing both yield and quality of the vegetables [75,78].
Furthermore, it is essential to conduct this research at high densities to achieve greater yields. Only a limited number of animal species that can be efficiently cultivated in BFT systems, and consequently in FLOCponics systems, possess the necessary traits . However, several studies have identified additional species that may also be viable [91]. Pacific white shrimp and Nile tilapia are the most widely cultivated species using BFT. Both species are extensively farmed and make significant contributions to the global food supply. Although the limited availability of other high-value species poses a challenge for FLOCponics, focusing on established products while developing innovative technologies is advantageous [55].
Conclusions
Aquaponics involves the integrated cultivation of aquatic organisms and plants, where most nutrients required for plant growth come from aquaculture effluent. In a conventional aquaponics system, a recirculating aquaculture system is linked to a hydroponic system, allowing for the continuous exchange of water and nutrients. In conventional aquaponics, a key challenge is managing the conversion of ammonia produced during the cultivation of aquatic animals into nitrate, while simultaneously maintaining a balance between the concentrations in the aquatic animal tank and the plant growth layer. Integrating BFT into aquaponics is expected to effectively address this issue and provide innovative solutions to the challenges facing the aquaculture sector. The combined system, known as FLOCponics, merges BFT and aquaponics, and is designed to be environmentally sustainable. This integrated system can enhance economic diversity by producing value-added plant products while reducing the accumulation of nitrate and phosphorus in the BFT management system. Further research is needed to assess the environmental, social, educational, and economic impacts of implementing FLOCponics in urban settings. Such an evaluation will support the promotion of sustainable practices in aquaculture.
Author Contribution
Bilal Raza conceived designed and wrote the manuscript. M. Naeem Ramzan, and Fatima Khan assisted in drafting the manuscript. Faisal Tasleem and Arslan Emmanuel helped in review the manuscript. All authors reviewed and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gao, Y., X. Wang, J. Li, C. T. Lee, P. Y. Ong, Z. Zhang (2020) Effect of aquaculture salinity on nitrification and microbial community in moving bed bioreactors with immobilized microbial granules. Bioresource Technology 297: 122427.
- Gururani, P., P. Bhatnagar, V. Kumar, M. S. Vlaskin, A. V. Grigorenko (2022) Algal Consortiums: A Novel and Integrated Approach for Wastewater Treatment. Water 14: 3784.
- Khatoon, H., K. P. Penz, S. Banerjee, M. R. Rahman, T. M. Minhaz, Z. et al (2021) Immobilized Tetraselmis for reducing nitrogenous and phosphorous compounds from aquaculture wastewater. Bioresource Technology 338: 125529. [crossref]
- Chandramenon, P., A. Aggoun, F. Tchuenbou-Magaia (2024) Smart approaches to Aquaponics 4.0 with focus on water quality− Comprehensive review. Computers and Electronics in Agriculture, 225: 109256.
- Lennard, W, S. Goddek (2019) Aquaponics: the basics. Aquaponics food production systems. 113.
- Hou, D., H. Li, S. Wang, S. Weng, J. He (2024) Nitrite nitrogen stress disrupts the intestine bacterial community by altering host-community interactions in shrimp. Science of The Total Environment 925: 171536. [crossref]
- Raza, B., Z. Zheng, J. Zhu, W. Yang (2024) A Review: Microbes and Their Effect on Growth Performance of Litopenaeus vannamei (White Leg Shrimps) during Culture in Biofloc Technology System. Microorganisms 12(5): 1013. [crossref]
- Arbour, A. J., Y. T. Chu, P. B. Brown, J. Y. Huang (2024) Life cycle assessment on marine aquaponic production of shrimp, red orache, minutina and okahajiki. Journal of Environmental Management 353: 120208.
- Gebauer, R., A. Brügmann, E. A. Folorunso, T. Goldhammer, T. Gebauer, et al (2023) Species- and diet-specific aquaculture wastewater nutrient profile: implications for aquaponics and development of sustainable aquaponics diet. Aquaculture 568: 739307.
- Flores-Aguilar, P. S., J. Sánchez-Velázquez, H., Aguirre-Becerra, G. A., Peña-Herrejón, S. A., Zamora-Castro,et al (2024) Can Aquaponics Be Utilized to Reach Zero Hunger at a Local Level? Sustainability 16 (3): 1130.
- Khanjani, M.H., Mohammadi, M. G. C. Emerenciano (2024b) Water quality in biofloc technology (BFT): an applied review for an evolving aquaculture. Aquacultural International 32: 9321-9374.
- Saseendran, S., K. Dube, M. H. Chandrakant A. B. Rani (2021) Enhanced growth response and stress mitigation of genetically improved farmed Tilapia in a biofloc integrated aquaponic system with bell pepper. Aquaculture 533: 736200.
- Khanjani, M. H., M. Alizadeh, M. Sharifinia (2020) Rearing of the Pacific white shrimp, Litopenaeus vannamei in a biofloc system: The effects of different food sources and salinity levels. Aquaculture Nutrition 26: 328-337
- Raza, B., Z. Zheng, W. Yang (2024) A Review on Biofloc System Technology, History, Types, and Future Economical Perceptions in Aquaculture. Animals 14 (10): 1489.
- Chu, Y. T., P. B. Brown (2020) Evaluation of Pacific whiteleg shrimp and three halophytic plants in marine aquaponic systems under three salinities. Sustainability 13 (1): 269.
- Pinho, S. M., J.P. de-Lima, L. H. David, M. G. Emerenciano, S. Goddek, M.et al (2022) FLOCponics: The integration of biofloc technology with plant production. Review in Aquaculture 14: 647-675
- Custódio, L., M. J. Rodrigues, C. G. Pereira, V. Castañeda-Loaiza, E. Fernandes, D. Standing, et al (2021) A review on Sarcocornia species: Ethnopharmacology, nutritional properties, phytochemistry, biological activities and propagation. Food 10 (11): 2778[crossref]
- Abdel-Rahim, M. M., Y. M. Awad, Y. A. Abdallah, S. M. Radwan (2019) Effects of four medicinal plants on the bioeconomic analysis and water-use efficiency of Nile tilapia, Oreochromis niloticus fry nursed under a small-scale aquaponics system. Aquaculture, Aquarium, Conservation & Legislation, 12 (3): 851-866.
- Pinho, S. M., L. H. C. David, S. Goddek, M. G. C. Emerenciano, M. C. Portella (2021a) Integrated production of Nile tilapia juveniles and lettuce using biofloc technology. Aquaculture International 29: 37-56.
- Pinho, S. M., J. P. Lima, L. H. David, M. G. C. Emerenciano, S. Goddek, et al ( 2021b) FLOCponics: the integration of biofloc technology with plant production. Review in Aquaculture 1-29
- Barbosa, P. T. L., J. A. Povh, K. N. N. Farias, T. V. da Silva, et al (2022) Nile tilapia production in polyculture with freshwater shrimp using an aquaponic system and biofloc technology. Aquaculture 551: 737916.
- Silva, H. V., M. A. Martins, S. C. M. do Espírito, V. F. do Nascimento, P. C. Rezende, et al (2022) Aquaponic production of sea asparagus and Pacific white shrimp using biofloc technology: Different irrigation regimes affect plant production of bioactive compounds and antioxidant capacity. Aquaculture Research 53 (3): 1001-1010.
- Mahari, W.A.W., K. Waiho, H. Fazhan, E. Azwar, A. C. Shu-Chien M. A.et al (2024) Emerging paradigms in sustainable shellfish aquaculture: Microalgae and biofloc technologies for wastewater treatment. Aquaculture 587: 740835.
- Shumet, A (2021) Aquaponics: A Sustainable Solution for Health, Economy, and Society—A Comprehensive Review. Aquaponics 1: 1-22.
- Shabeer, M. S., S. W. A. R. O. O. P. Nagar, and H. Y. D. E. R. A. B. A. D. Uppal.et al (2016) Isolation and characterization bacteria related to aquaponics for testing its bio potential. Tech Biotechnology thesis, National Institute of Technology, Calicut 60.
- Okomoda, V. T., S. A. Oladimeji, S. G. Solomon, S. O. Olufeagba, S. I. Ogah,et al (2023) Aquaponics production system: A review of historical perspective, opportunities, and challenges of its adoption. Food Science and Nutrition 11(3): 1157-1165. [crossref]
- Baganz, G. F. M., R. Junge, M. C. Portella, S. Goddek, K. J. Keesman, et al (2022) The aquaponic principle—It is all about coupling. Review in Aquaculture 14: 252-264.
- Abakari, G., X. Wu, X. He, L. Fan, G. Luo (2022) Bacteria in biofloc technology aquaculture systems: Roles and mediating factors. Review in Aquaculture 14: 1260-1284.
- Gao, X., H. Zhang, Y. Xu, Q. Ni, Y. Zhang, et al (2022) Effects of humic acid on the nitrogen utilization efficiency and microbial communities in aquaponic systems. Aquaculture 547: 737475
- Shreejana, K., R. Thapa, A. Lamsal, S. Ghimire, K. Kurunju, et al (2022) Aquaponics a modern approach for integrated farming and wise utilization of components for sustainability of food security: A review. Agric. Environ. Sci. 7: 121-126.
- Lee, D. H., J. Y. Kim, S. R. Lim, D. Y. Kim, J. M. Kim, et al (2019) Effect of dietary monobasic potassium phosphate levels on water quality and the growth of far eastern catfish Silurus asotus and four leafy vegetables in a hybrid biofloc technology aquaponic system. Korean J. Fish Aquat. Sci. 52: 159-172
- Aslanidou, M., A. Elvanidi, A. Mourantian, E. Levizou, E. Mente, et al (2024) Evaluation of productivity and efficiency of a large-scale coupled or decoupled aquaponic system. Scientia Horticulturae 337: 113552.
- Aslanidou, M., A. Elvanidi, A. Mourantian, E. Levizou, E. Mente, et al (2023) Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems. Horticulturae, 9 (10): 1077.
- Zhu, Z., U. Yogev, K. J. Keesman, A. Gross (2024) Promoting circular economy: Comparison of novel coupled aquaponics with anaerobic digestion and conventional aquaponic systems on nutrient dynamics and sustainability. Resources, Conservation and Recycling 208: 107716.
- Danner, R. I., U. Mankasingh, K. Anamthawat-Jonsson, R. I. Thorarinsdottir (2019) Designing aquaponic production systems towards integration into greenhouse farming. Water 11 (10): 2123.
- Monsees, H., W. Kloas and S. Wuertz (2017) Decoupled systems on trial: Eliminating bottlenecks to improve aquaponic processes. PLoS One 12 (9): e0183056. [crossref]
- Palm, H. W., U. Knaus, B. Kotzen (2024) Aquaponics nomenclature matters: It is about principles and technologies and not as much about coupling. Reviews in Aquaculture 16(1): 473-490.
- Goddek, S., and O. Korner (2019) A fully integrated simulation model of multi-loop aquaponics: A case study for system sizing in different environments. Syst. 171: 143-154.
- Tetreault, J., R. L. Fogle, A. Ramos, and M. B. Timmons (2023) A predictive model of nutrient recovery from ras drum-screen effluent for reuse in aquaponics. Horticulturae 9 (3): 403.
- Blanchard, C, D. E. Wells, J. M. Pickens, and D. M. Blersch ( 2020) Effect of pH on cucumber growth and nutrient availability in a decoupled aquaponic system with minimal solids removal. Horticulture 6(1): 10
- Pasch, J., B. Ratajczak, S. Appelbaum, H. W. Palm, U. Knaus (2021) Growth of basil (Ocimum basilicum) in DRF, raft, and grow pipes with effluents of African catfish (Clarias gariepinus) in decoupled aquaponics. Agri Engineering 3 (1): 92-109.
- Kotzen, B., M. G. C. Emerenciano, N. Moheimani, G. M. Burnell (2019) Aquaponics: Alternative types and approaches. Aquaponics food production systems: Combined aquaculture and hydroponic production technologies for the future 301-330
- Ghamkhar, R., C. Hartleb, F. Wu, and A. Hicks (2020) Life cycle assessment of a cold weather aquaponic food production system. Journal of Cleaner Production 244: 118767.
- Milliken S, and H. Stander (2019) Aquaponics and social enterprise. Aquaponics Food Production Systems. Springer International Publishing, Germany 607-619.
- Chen, P., G. Zhu, H. j. Kim, P. B., Brown, and J. Y. Huang (2020) Comparative life cycle assessment of aquaponics and hydroponics in the Midwestern United States. Journal of Cleaner Production 275: 122888.
- Pinheiro, I., R. F. S. Carneiro, F. Nascimento-Vieira, L. V. Gonzaga, R. Fett, et al (2020) Aquaponic production of Sarcocornia ambigua and Pacific white shrimp in biofloc system at different salinities. Aquaculture 519: 734918.
- Greenfeld, A., N. Becker, J. F. Bornman, S. Spatari, and D. L. Angel ( 2021) Monetizing environmental impact of integrated aquaponic farming compared to separate systems. Science of The Total Environment 792: 148459. [crossref]
- Robaina, L., J. Pirhonen, E. Mente, J. Sánchez, and N. Goosen (2019) Fish diets in aquaponics. Aquaponics Food Production Systems 333-352.
- Vasdravanidis, C., M. V. Alvanou, A. Lattos, D. K. Papadopoulos, L. Chatzigeorgiou, et al (2022) Aquaponics as a promising strategy to mitigate impacts of climate change on rainbow trout culture. Animals 12(19): 2523. [crossref]
- Khanjani, M. H., M. Sharifinia, M. G. C. Emerenciano (2024a) Biofloc Technology (BFT) in Aquaculture: What Goes Right, What Goes Wrong? A Scientific‐Based Snapshot. Aquaculture Nutrition 2024 (1): 7496572. [crossref]
- Martinez-Cordova, L., J. López-Elías, and M. Martinez-Porchas ( 2020) A preliminary evaluation of an integrated aquaculture-agriculture systems (tilapia and peppers) at mesocosm scale. Journal of Aquaculture and Marine Biology 9: 19-22
- Emerenciano, M. G. C., L. R. Martínez-Córdova, M. Martínez-Porchas, and A. Miranda-Baeza (2017) Biofloc Technology Technology (BFT): Tool for Water Quality Management in Aquaculture. In: Tutu H, ed. Water Quality. London: INTECH 2017: 91-109.
- Souza, J., A. Cardozo, W. J. Wasielesky, and P. C. Abreu ( 2019) Does the biofloc size matter to the nitrification process in Biofloc Technology (BFT) Systems? Aquaculture 500: 443-450.
- Pinho, S. M., D. Molinari, G. L. de-Mello, K. M. Fitzsimmons, and M. G. Emerenciano (2017) Effluent from a biofloc technology (BFT) tilapia culture on the aquaponics production of different lettuce varieties. Ecological Engineering 103: 146-153.
- Panigrahi, A., C. Saranya, K. Ambiganandam, M. Sundaram, M. R. Sivakumar, et al (2020) Evaluation of biofloc generation protocols to adopt high density nursery rearing of Penaeus vannamei for better growth performances, protective responses and immuno modulation in biofloc based technology. Aquaculture 522: 735095.
- Walker, D. A. U., S. M. C. Morales, and M. G. C. Emerenciano (2020) Biofloc technology: principles focused on potential species and the case study of Chilean river shrimp Cryphiops caementarius. Review in Aquaculture 12 (1): 1759-1782.
- Soares, J., M. A. Martins, L. Castilho‐Barros, C. M. do Espírito Santo, F. do Nascimento Vieira, et al (2022) Reducing the feed input per unit of plant area as a means to improve the efficiency of sea asparagus and Pacific white shrimp biofloc technology‐based aquaponics. Aquaculture Research 53 (18): 6536-6544.
- Emerenciano, M. G. C., K. Fitzsimmons, A. Rombenso, G. Martins, R. Lazzari, et al (2021) Biofoc technology (BFT) in tilapia culture biology and aquaculture of tilapia, edn. CRC Press/Taylor & Fran‑cis Group Boca Raton 258-293.
- Boyd, C.E., L. R. D’Abramo, B.D. Glencross, D. C. Huyben, L. M. Juarez, et al ( 2020) Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. Journal of the World Aquaculture Society 51 (3): 578-633.
- Hwang, J. A., J. S. Park, H. S., Jeong, H. Kim, and S. Y. Oh ( 2023) Productivity of Fish and Crop Growth and Characteristics of Bacterial Communities in the FLOCponics System. Fishes 8 (8): 422.
- Deswati, D., L. P. Isara, and H. Pardi (2021) Hydroton-biofloc-based aquaponics (hydrotonfocponics): towards good water quality and macro-micronutrient. Aquar. Conserv. Legis. 14(5): 3127-3144.
- Pinho, S. M., J. P. Lima, L. H. David, M. S. Oliveira, S. Goddek, et al (2021) Decoupled FLOCponics systems as an alternative approach to reduce the protein level of tilapia juveniles’ diet in integrated Agri-aquaculture production. Aquaculture 543: 736932.
- Rono, K., G. Matolla, J. O. Manyala, and F. O. Masese (2024) Economic Viability of Agricultural Carbon Sources on Asian Rice (Oryza sativa Lejeunia) and Nile Tilapia (Oreochromis niloticus) Production in a Flocponic System. Journal of Aquatic and Terrestrial Ecosystems, 2(2), 31-45.
- Mahkeswaran, R., A. K. Ng, C. Toh, B. Toh (2024) Multi-loop Aquaponics Systems: A Review and Proposed Multi-loop Agrogeological Aquaponics System. Journal of Advanced Agricultural Technologies 11(1).
- Park, J., J. A. Hwang, J. Choe, D. Lee, and H. Kim (2024) Enhancing Indoor Culture of Weather Loach (Misgurnus anguillicaudatus) and Caipira Lettuce (Lactuca sativa) in a Decoupled FLOCponics System. Fishes 9 (5): 150.
- Oladimeji, A. S., S. O. Olufeagba, V. O. Ayuba, S. G. Sololmon, V. T. Okomoda (2020) Effects of different growth media on water quality and plant yield in a catfish-pumpkin aquaponics system. Journal of King Saud University-Science 32 (1): 60-66.
- Bordignon, F., E. Sturaro, A. Trocino, M. Birolo, G. Xiccato, et al (2022) Comparative life cycle assessment of rainbow trout (Oncorhynchus mykiss) farming at two stocking densities in a low-tech aquaponic system. Aquaculture 556: 738264.
- Luo, X. L., J. J. Yan, R. Abdessan, X. X. Zhang, et al ( 2025) Effect of dietary iron sources on Cyprinus carpio var. specularis and Lactuca sativa var. ramosa hort in aquaponic system. Aquaculture 595: 741479.
- Fischer, H., N. Romano, J. Jones, J. Howe, N. Renukdas, et al (2021) Comparing water quality/bacterial composition and productivity of largemouth bass Micropterus salmoides juveniles in a recirculating aquaculture system versus aquaponics as well as plant growth/mineral composition with or without media. Aquaculture 538: 736554.
- Fotedar, R (2016) Water quality, growth and stress responses of juvenile barramundi (Lates calcarifer Bloch), reared at four different densities in integrated recirculating aquaculture systems. Aquaculture 458: 113-120.
- Pinho, S. M., L. H. C. David, S. Goddek, M. G. C. Emerenciano, M. C. Portella (2021) Integrated production of Nile tilapia juveniles and lettuce using biofloc technology. Aquaculture International 29 (1): 37-56.
- Gao, X., Y. Xu, J. Shan, J. Jiang, H. Zhang,et al (2024) Effects of different stocking density start-up conditions on water nitrogen and phosphorus use efficiency, production, and microbial composition in aquaponics systems. Aquaculture 585: 740696.
- Zappernick, N., K. V. Nedunuri, K. R. Islam, S. Khanal, T. Worley,et al (2022) Techno-economic analysis of a recirculating tilapia-lettuce aquaponics system. Journal of Cleaner Production 365, 132753.
- Knaus, U., and H. W. Palm (2017) Effects of the fish species choice on vegetables in aquaponics under spring-summer conditions in northern Germany (Mecklenburg Western Pomerania) Aquaculture 473: 62e73.
- Nadia, Z. M., A. R. Akhi, P. Roy, F. B. Farhad, M. M. Hossain, et al ( 2023) Yielding of aquaponics using probiotics to grow tomatoes with tilapia. Aquaculture Reports 33: 101799.
- Alarcón-Silvas, S. G., J. A. León-Cañedo, J. F. Fierro-Sañudo, J. Ramírez-Rochín, M. G. Fregoso-López, et al (2021) Water quality, water usage, nutrient use efficiency and growth of shrimp Litopenaeus vannamei in an integrated aquaponic system with basil Ocimum basilicum. Aquaculture, 543: 737023.
- Li, T., B. Zhang, C. Zhu, J. Su, J. Li, et al (2021) Effects of an ex situ shrimp-rice aquaponic system on the water quality of aquaculture ponds in the Pearl River estuary, China. Aquaculture 545: 737179.
- Sousa, R., L. Bragança, M. V. Da Silva, and R. S. Oliveira (2024) Challenges and Solutions for Sustainable Food Systems: The Potential of Home Hydroponics. Sustainability 16: 817.
- Muñoz-Euán, N., L. Mendoza-Espinosa. And J. G. Correa-Reyes (2024) The Use of Aquaponic Systems to Achieve the Sustainable Development Objectives of the 2030 Agenda: A Systematic Review. 123-147.
- Khater, E. S., A. Bahnasawy, H. Mosa, W. Abbas, and O. Morsy (2024) Nutrient supply systems and their effect on the performance of the Nile Tilapia (Oreochromis niloticus) and Lettuce (Lactuca sativa) plant integration system. Scientific Reports 14 (1): 4229. [crossref]
- Piñero, M. C., J. Collado-González, G. Otálora, J. López-Marín, F. M. Del Amor (2024) Plant Growth-Promoting Rhizobacteria as Tools to Improve the Growth of Kohlrabi (Brassica oleracea var. gongylodes) Plants in an Aquaponics System. Plants 13 (5): 595. [crossref]
- Munekata, P.E., Pateiro, M., Domínguez, R., Pollonio, M.A., Sepúlveda, N.,et al (2021) Beta vulgaris as a natural nitrate source for meat products: A review. Foods 10(9): 2094. [crossref]
- Romano, N., S. Islam, and H. Fischer (2023) Ebb and flow versus constant water level on the sweet banana chili pepper (Capsicum annuum) production in an aquaponic system. Aquacultural Engineering 102: 102340.
- Castilla-Gavilán, M., M. Muñoz-Martínez, E. Zuasti, J. Canoura-Baldonado, R. Mondoñedo, R., and I. Hachero-Cruzado (2024) Yield, nutrients uptake and lipid profile of the halophyte Salicornia ramosissima cultivated in two different integrated multi-trophic aquaculture systems (IMTA) Aquaculture 583: 740547.
- Knaus, U., D. H. D. Hübner, C. Küchenmeister, S. Appelbaum, W. Iten, et al (2024) Aquaponic growth of basil (Ocimum basilicum) with African catfish (Clarias gariepinus) in standard substrate combined with a Humicacid Fiber-Substrate (HFS) Scientific Reports 14 (1): 17725.
- Vasdravanidis, C., M. V. Alvanou, A. Lattos, D. K. Papadopoulos, L. Chatzigeorgiou, M. Ravani, et al (2022) Aquaponics as a promising strategy to mitigate impacts of climate change on rainbow trout culture. Animals 12(19): 2523.
- Pinho, S. M., and G. C. Emerenciano (2021) Sensorial attributes and growth performance of whiteleg shrimp (Litopenaeus vannamei) cultured in biofloc technology with varying water salinity and dietary protein content. Aquaculture 540: 736727.
- Doncato, B., and C. S. B. Costa (2021) Micronutrient supplementation needs for halophytes in saline aquaponics with BFT system water. Aquaculture 531: 735815
- Pickens, J. M., J. J. Danaher, J. L. Sibley, J. A. Chappell, and T. R. Hanson ( 2020) Integrating greenhouse cherry tomato production with biofloc tilapia production. Horticulture 6(3): 44.
- Castro-Castellón, A. E., G. Castro-Mejía, J. Castro-Mejía, and A. M. Martínez-Meingüer (2020) Culture of Melanochromis and Lycopersicon esculentum var. Cerasifonne in a combined system of Biofloc and aquaponics. Int. J. Fish. Aquat. Stud. 8 (2): 186-192.
- Walker, D. A. U., S. M. C. Morales, and M. G. C. Emerenciano (2020) Biofloc technology: principles focused on potential species and the case study of Chilean river shrimp Cryphiops caementarius. Review in Aquaculture 12 (3): 1759-1782.
- Poli, M. A., E. C. Legarda, M. A. de Lorenzo, I. Pinheiro, M. A. Martins, W. Q.et al (2019) Integrated multitrophic aquaculture applied to shrimp rearing in a biofloc system. Aquaculture 511: 734274
- Fimbres‐Acedo, Y. E., R. Servín‐Villegas, R. Garza‐Torres, M. Endo, K. M. Fitzsimmons, et al (2020) Hydroponic horticulture using residual waters from Oreochromis niloticus aquaculture with biofloc technology in photoautotrophic conditions with Chlorella Aquaculture Research 51 (10): 4340-4360.
- Rocha, A. F., F. M. L. Biazzetti, M.R. Stech, R. P. Silva (2017) Lettuce production in aquaponic and biofloc systems with silver catfish (Rhamdia quelen.) Inst. De. Pesca. 44: 64-73.
- Pinheiro, I., R. Arantes, C. M. E. Santo, F. N. Vieira, K. R. Lapa, et al (2017) Production of the halophyte Sarcocornia ambigua and Pacific white shrimp in an aquaponic system with biofloc technology. Ecological Engineering 100: 261-267.
- David, L. H. C., S. M. Pinho, and F. Garcia (2018) Improving the sustainability of tilapia cage farming in Brazil: An emergy approach. Journal of Cleaner Production 201: 1012-1018.
- Maucieri, C., A. A. Forchino, C. Nicoletto, R. Junge, R. Pastres, P.et al (2018) Life cycle assessment of a micro aquaponic system for educational purposes built using recovered material. Journal of Cleaner Production 172: 3119-3127.
- Francisco, E. C., T. A. Freato, A. L. Piolli, M. E. S. D. Poz (2024) Analysis of the Aquaponic System Sustainability via System Dynamics Modelling-FEW Nexus Approach. Circular Economy and Sustainability 1-16.
- Junge, R., T. G. Bulc, D. Anseeuw, Y. H. Yavuzcan, S. Milliken ( 2019 ) Aquaponics as an Educational Tool. In: Goddek S, Joyce A, Kotzen B, Burnell G, eds. Aquaponics Food Production Systems. Springer International Publishing 2019: 561-595.
- David, L. H., S. M. Pinho, F. Agostinho, J. M. Kimpara, K. J. Keesman, et al (2020) Emergy synthesis for aquaculture: A review on its constraints and potential. Review in Aquaculture 13 (2): 1119-1138.