Abstract
Pharmaceutical oral dosage forms intended to children have to be administered under liquid dosage forms. These oral liquid formulations contained into multidose containers require the addition of antimicrobial agents to avoid the growth of microorganisms. Moreover, the oral administration of poorly water soluble drugs requires the use of excipients (organic solvent, cyclodextrins, surfactants, polymers) able to improve their water solubility. 2-pyrrolidone (Soluphor® P) a co-solvent usually used to dissolve drugs for the manufacture of parenteral dosage forms and suggested also for other administration routes, showed antimicrobial activity. To study the extent of its preservative efficacy, we determined the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) first, for bacteria (Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Enterococcus faecalis, Staphylococcus epidermidis, Staphylococcus capitis) and for bacterial spores (Bacillus cereus, Bacillus sphaericus, Bacillus subtilis), then for yeast and mould (Candida albicans, Aspergillus niger). The results showed that 2-pyrrolidone has a bactericidal and fungistatic efficacy. Challenge tests were realized on oral aqueous paediatric formulations of vitamin D3 containing 2-pyrrolidone as a co-solvent and all known micro-organisms in such formulations were studied. The preservative efficacy of 2-pyrrolidone was observed at a dosage level of 75 mg/mL.
Keywords
preservative efficacy, antimicrobial, bactericidal, sporistatic, paediatric dosage forms, 2-pyrrolidone, challenge test, micellar solution, Vitamin E TPGS®.
1. Introduction
Beside the issues of immaturity of enzymes or reduced organ functions, encountered by the neonates and infants, which may determine the pharmacokinetics of the administered drugs, children [1] show difficulties in swallowing solid dosageforms for oral use. Fot this reason, for this patient group, small sized particulates or liquid dosage forms are preferred to classic tablets or capsules [2]. The main problems occurring using liquids are (i) the palatability of the solution [3,4], especially when the taste sensation differs interindividually, (ii) the poor water solubility of many drugs, (iii) the shelf stability of the final liquid dosage forms, often prone to degradation reactions (oxidation, reduction, epimerization, hydrolysis, …) and (iv) the microbiological safety of the product, namely its protection from microbial contaminations occurring from consumer use [5] Amongst the different techniques used to increase the water-solubility of drugs [6], the ones using co-solvents and surfactants are the easiest to use industrially. Unfortunately, for the formulations and developments of paediatric medicines [7,8] the use of co-solvents (e.g. ethanol, glycerol) is not recommended at high levels due to their neurological and laxative effects. Furthermore, the addition of surfactants into multidose dosage forms, necessitating the need of preservatives, can lead to the formation of complex of molecules with these compounds and also to a decrease of their activity [9–11]. Amongst solubilising excipients used in oral and injectable formulations, 2-pyrrolidone is a pharmaceutical FDA-approved solvent found in many marketed forms of poorly soluble drugs. Nevertheless, even if 2-pyrrolidone is recommended by suppliers to be used to construct oral dosage forms, only few studies have been conducted on this excipient. Beside the well-known solubilizing property on this excipient, we found also an antibacterial activity. To validate this new concept in the paediatric field, we used an antirachitic lipophilic model drug such as vitamin D3 and its antioxidizing agent, propyl gallate and sodium fluoride as hydrophilic drug. To prove the extent of this new antimicrobial activity, we determined the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) first for gram-positive bacteria (Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus capitis) gram-negative bacteria (Pseudomonas aeruginosa, Escherichia coli) spore-forming gram-positive rods (Bacillus cereus, Bacillus sphaericus, Bacillus subtilis) and then for yeast (Candida albicans) and fungus (Aspergillus niger). Then challenge tests were realized according to European Pharmacopeia 9.0 (EP) criteria on oral aqueous paediatric formulations containing 2-pyrrolidone as a co-solvent of vitamin D3. The results presented below suggest that 2-pyrrolidone could find very interesting applications as a potent excipient with antimicrobial activity into liquid dosage forms.
2. Methods and Materials
2.1. Solubility determinations
The phase-solubility method developed by Higuchi and Connors [12] was used to determine the solubility for vitamin D3 (cholecalciferol, Fluka, Saint Quentin Fallavier, France). Sample vials were prepared in triplicate by adding an excess amount of vitamin D3 to vials containing either 100 g of 2-pyrrolidone aqueous solutions at various concentrations (w/w: 1, 5, 10, 20, 30, 40, 50%); or 100 g of 2-pyrrolidone aqueous solutions at 40% (w/w) containing also 1.50 or 3.0% (w/w) of d-α-tocopheryl polyethylene glycol 1000 succinate (Vitamin E TPGS® NF Grade, Eastman, Anglesey, United Kingdom). The precipitates were removed through 0.45-µm TEFE syringe filters (Millipore, Molsheim, France). The vials were then closed withTEFE-lined rubber-stoppers and were rotated at 40 rpm using an end-over-end mechanical rotator (Vankel, VK 750D) at 25°C for 30 minutes. The drug solubility in aqueous solutions at various 2-pyrrolidone/Vitamin E TPGS® ratio was determined using a high performance liquid chromatography (HPLC) assay as stated below.
2.2. Preparation of micellar solutions
The micellar solutions were prepared according to a standard method employing a room temperature homogeneization [13]. The three tested compositions of micellar solutions are presented in
(Table 1). Briefly, d-α-tocopheryl polyethylene glycol 1000 succinate and 2-pyrrolidone were heated at 40°C and then the mixture was cooled at 20°C. The vitamin D3 and propyl gallate (Fluka, Saint Quentin Fallavier, France) were dissolved and homogeneized into this solution. This solution was added and homogenized with an aqueous solution constituted of a mixture of sodium fluoride, saccharin sodium, aroma of red fruits without vanillin and liquid maltitol (Lycasin® 80/55-sirup, Roquette, Lestrem, France).
Table 1. Compositions of the micellar solutions.
|
Compounds |
Composition (g) |
||
|
Formulation A |
Formulation B |
Formulation C |
|
|
Vitamin D3 |
– |
0.0080 |
0.0080 |
|
Sodium fluoride |
0.2200 |
0.2200 |
0.2200 |
|
Propyl gallate |
– |
0.0200 |
0.200 |
|
Vitmain E TPGS® |
– |
1.5000 |
1.5000 |
|
Saccharin sodium |
0.5000 |
0.5000 |
0.5000 |
|
Aroma without vanillin |
0.4000 |
0.4000 |
0.4000 |
|
2-pyrrolidone |
40.00 |
40.00 |
40.00 |
|
Lycasin® 80/55-sirup |
40.00 |
40.00 |
40.00 |
|
Purified water |
QSP 100.00 |
QSP 100.00 |
QSP 100.00 |
2.3. Granulometry analyses
The average size and distribution of surfactant micelles were determined by light scattering (DLS) using a Zeta Sizer Nano Zs (Malvern) with a He-Ne laser light (λ= 633 nm) and a detection angle of 90°. The viscosities of the micellar solutions were comprised between 0.65 and 0.90 cP.
The measurements were carried out at 25°C. The final sample of micellar solution was diluted in water (MilliQ) to bring the concentration to 0.1mL/1mL. Measurements were made in conventional cuvettes, eliminating the possibility of sample cross-contamination. The intensity average diameter was computed from the intensity autocorrelation data using the cumulate analysis method. Micelle size measurements were performed 5 times for each sample.
2.4. Quantitations of vitamin D3 and sodium fluoride
2.4.1. Quantitation of vitamin D3
2.4.1.1. Equipment
Vitamin D3 was quantified by HPLC. The chromatographic system consisted of a model LC-126 programmable pump, a model LC-507 automatic injector, and a model LC-166 variable wavelength UV – Vis detector. All items were purchased from Beckman System Gold (Villepinte, France). HPLC analysis was carried out at a temperature of 20 ± 2°C using a Macherey-Nagel C8 5 μm (125 x 4.0 mm I.D.) reversed-phase column with spherical particles having pores diameter of 10 nm. Chromatographic data were analysed by Karat Software Version 32.0.
2.4.1.2. Chromatographic conditions
The mobile phase consisted of 100 volumes of a 0.11% (v/v) solution of phosphoric acid (Merck, Darmstadt, Germany) and 900 volumes of acetonitrile (Sigma-Aldrich, Saint Quentin Fallavier, France). The flow rate was 1.4 ml/min and detection wavelength was set at 268 nm. Aliquot of 20 µl were injected onto reversed-phase Lichrospher C8 column. All the solvents used in chromatography were of HPLC grade and deionized water used for the preparations of the micellar solutions and for HPLC was obtained from a synergy UV water purification system (Millipore, Molsheim, France). All other chemicals were of analytical reagent grade and used as received.
2.4.1.3. Preparation of standard solutions
Vitamin D3 (≥99.0%) was used for preparing standard solutions in Methanol. The concentrations of these solutions were approximately 8.0 mg per 100 mL; they were stored at +4°C in the dark. Serial dilutions for the analytical HPLC were made in methanol/water (70/30, v/v) to obtain concentrations ranging from 11.50 to 26.83 µg/mL. All procedures were performed in a darkened container. In addition, glassware used in this assay was rinsed with acetone before use. The samples of vitamin D3 in 2-pyrrolidone (solubility determinations) and in micellar solutions (see below) were diluted with a mixture of methanol/water (70/30, v/v) before to be analysed.
2.4.1.4. Method validation
The method was validated for parameters such as linearity, precision, accuracy, and stability following the analytical validation guidelines defined by Caporal et al. and Chaminade et al. [14–17] three different blank samples were processed and injected into the HPLC system to investigate whether the components of the micellar solutions interfere with analytes. Three calibration curves were constructed according to section 2.4.1.3 and analyzed on 3 consecutive days.
The accuracy and precision were evaluated using samples at five concentration levels. Three replicate samples at each concentration were analyzed in a sequence to evaluate within-day variation. For evaluation of the between-day variation, three replicate samples at each concentration were analyzed on 3 different days. The accuracy was expressed by [(mean observed concentration)/(spiked concentration)] × 100% and the precision by relative standard deviation. The recoveries of vitamin D3 were evaluated by comparing the mean peak area of samples to the mean peak area of pure standards of equivalent concentrations.
2.4.2. Quantitation of sodium fluoride
2.4.2.1. Equipment
An ion analyzer (CyberScan ion 510 Meter, Eutech Instruments, Nijkerk, Netherlands) was used to measure the fluoride concentrations using a fluoride ion-specific combination electrode (Fischer Bioblock Scientific, Illkirch, France). The instrument was calibrated to measure fluoride concentrations between 0.02 ppm and 10 ppm.
2.4.2.2. Preparation of standard solutions and method validation
The fluoride concentrations in micellar solutions were determined after dilution. A set of fluoride standard solutions (ranging between 0.025 and 3.200 μg F/mL) was prepared, using serial dilutions from 100 μg F/mL NaF stock solution (Fischer Bioblock Scientific, Illkirch, France). The multivoltage potentials were converted to μg F /g using a standard curve with a coefficient correlation of r ≥0.99. The mean repeatability of the readings, based on duplicate samples, was 92.3%. The total amount of fluoride (mg) in the micellar preparations was calculated from the amount of fluoride in the sample and the total volume of the sample after homogeneization.
2.5. Determination of the minimum inhibitory concentration (MIC) and of the minimum bactericidal concentration (MBC) of 2-pyrrolidone
2.5.1. Bacterial, yeast and mould strains culture conditions
Bacterial strains Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 8739); yeast strain Candida albicans (ATCC 10231) and mould strain Aspergillus niger (ATCC 16404) all recommended by the EP have been used. Furthermore, wild bacterial strains such as Enterococcus faecalis, Staphylococcus epidermidis, Staphylococcus capitis provided from our laboratory and spores from Bacillus subtilis, Bacillus cereus and Bacillus sphaericus have been also tested. Before use, the bacteria were kept frozen at -80°C in brain-heart infusion broth (bioMérieux, Marcy l’Etoile, France) supplemented with 20% glycerol.
Bacterial cultures were grown on trypticase soy agar (TSA) (bioMérieux, Marcy l’Etoile, France) at 37°C. Yeast and mould cultures were grown on Sabouraud agar medium (SAB) (bioMérieux, Marcy l’Etoile, France) at 30°C. The inoculum was prepared by suspending overnight cultures at 37°C in Mueller-Hinton (bioMérieux, Marcy l’Etoile, France) broth and adjusting to an OD620 of 0.15–0.16 corresponding to 1–3×108 colony-forming units (CFU)/mL. C. albicans (ATCC 10231) (6.7×107 CFU/tablet) and A. niger (ATCC 16404) (5.4×106 CFU/tablet) were provided by AES laboratoire (Combourg, France). MIC for C. albicans and A. niger has been realised in Sabouraud medium broth (bioMérieux, Marcy l’Etoile, France).
B. sphaericus and B. cereus spores were isolated from growth culture on specific medium (meat extract 2.4g; peptone 2.4g; NaCl 1.2g; MnSO4.4 H2O 0.03g; KH2PO4 0.25g; agar 13g; water 1000 mL) while B. subtilis spores (106 spores/tablet) was purchased from AES laboratoire, Combourg, France. Briefly, B. sphaericus and B. cereus spores were obtained after 48h culture at 37°C on a slant of specific medium. Specific medium was flooded with 2 mL of sterile water and shaked 15 s. The water was transferred in a sterile tube and heated 15 min at 80°C. Serial 10-1 dilutions of the spore solutions were enumerated by the plating method on TSA medium. MIC determination has been done in Mueller-Hinton broth (bioMérieux, Marcy l’Etoile, France).
2.5.2. Preparation of the samples
The experiments for the determinations of MIC and MBC have been carried out under aseptic conditions and precautions have been taken against microbial contamination. Nevertheless, experimental conditions were such that they do not affect any micro-organisms which were to be revealed in the test. The working conditions in which the tests were performed were monitored regularly by appropriate sampling of the working area and by carrying out appropriate controls (such as those indicated in those indicated in the appropriate European Community Diretives and associated guidance documents on GMP).
2-pyrrolidone and water were sterilized by filtration (0.45 μm pore size filters, Dutscher, Brumath, France) before use. First of all, a solution containing 800 mg/mL was prepared by adding 2.75 mL of deionized water to 7.25 mL of 2-pyrrolidone.
In a first step, a series of test tubes containing different concentrations in descending order of 2-pyrrolidone into Mueller-Hinton broth were inoculated with a same amount of bacteria or spores (106 CFU/ml) or into Sabouraud broth for yeast and mould strains. A broth not containing antimicrobial agent was inoculated as a control for organism viability. After 18-24 h of incubation at 37°C, the test tube of the highest concentration in which no microbial growth was observed gives the MIC value (mg/mL). In a second step, 100 μl of samples were taken from each test tube in which no growth was observed. Serial 10-1 dilutions of the samples were enumerated by the plating method on Mueller-Hinton agar or Sabouraud agar. After 24h of incubation at 37°C, or 48h at 30°C for C. albicans and A. niger the numbers of surviving micro-organisms were counted and were compared to the standard ranges (106 CFU/mL). MBC value (mg/mL) corresponds to the concentration of the sample tube in which surviving micro-organisms were equivalent to 0.01%. The ratio MBC/MIC allows determining the antibacterial activity as follows:
MBC/MIC < or = 4 characterize a bactericidal, fungistatic or sporicidal activity
MBC/MIC > 4 characterizes a bacteriostatic, fungistatic or sporostatic activity.
2.6. Test for efficacy of antimicrobial preservation
The official EP Test for the efficacy of antimicrobial preservation is usually applied to preserved oral liquid formulations. Challenge testing was performed using micro-organisms selected from those listed in section 5.1.3. of the EP (S. aureus (ATCC 6538)), P. aeruginosa (ATCC 9027), E. coli (ATCC 8739), C. albicans (ATCC 10231), and A. niger (ATCC 16404)). Containers of the product to be examined were inoculated with a suspension of one of the test organism to give an inoculum at 106 CFU/g.
Briefly, after micro-organism inoculation, the inoculated product (30g) was maintained at 20°C-25°C in the dark. One g aliquots of the inoculated product were removed at 0, 14 and 28 days. To each was added 9mL of a neutralizing solution (Polysorbate 80, 30g; lecithin, 3g; histidine monohydrochloride, 1g; sodium chloride, 4.4g; potassium phosphate dibasic, 3.6g; sodium phosphate dibasic, 7.2g; water,
1000 mL). After being allowed to neutralize for 0.3 h at room temperature, broths were diluted until 10-5 into the neutralizing solution and filtered through 0.45 μm filters 47 mm (Millipore, Molsheim, France). Each membrane filter was rinsed five times with 10 mL of the neutralizing solution. Finally, each membrane was plated on an appropriate agar plate. Bacterial plates were incubated at 30°C for 24h, while yeast and moulds plates were incubated 48 h at 30°C and the CFUs were counted. The control preparations were similarly sampled at 0 h to determine the viable counts of the cultures used and to confirm the ability of the unpreserved formulation to support the viability and/or the microbial growth and also the effectiveness of the neutralizing medium for the inoculum recovery.
3. Results and discussion
3.1. Solubility of vitamin D3 and its formulation
The vitamin D3 solubility in water in the presence of 2-pyrrolidone at various concentration is shown in (Figure 1). In addition, the solubility enhancement of the drug in the presence of Vitamin E TPGS® is also shown. Vitamin D3 has a solubility ranging from 0.01 μg/mL in pure water to 77 μg/mL in aqueous olutions containing 40% [w/w] of 2-pyrrolidone and 1.5 % [w/w] of Vitamin E TPGS®. The use of other excipients in the 2-pyrrolidone and Vitamin E TPGS® formulations resulted in only a slight solubility hancement (data not shown).
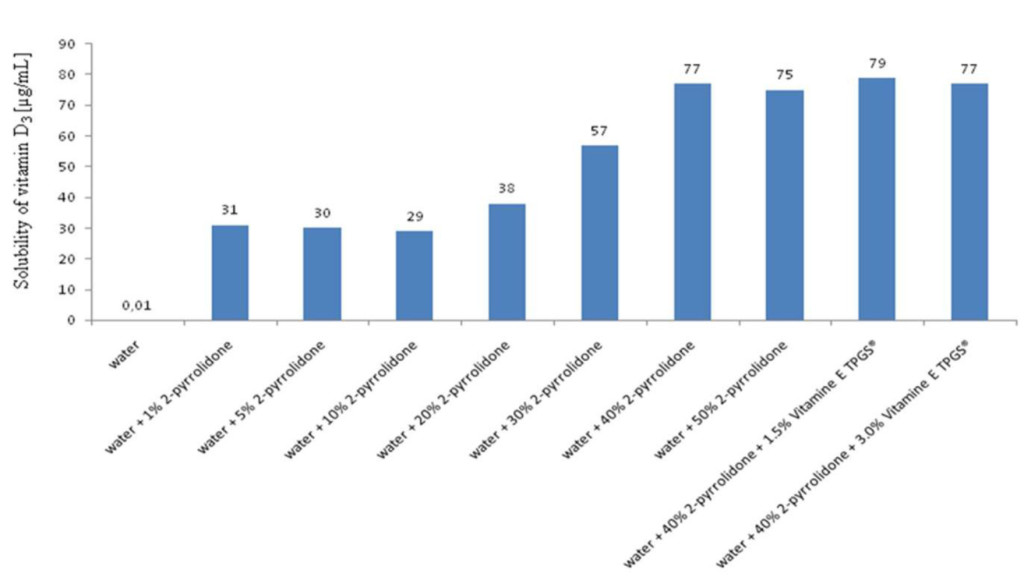
Figure 1. Solubility of vitamin D3 in various media: water, water + 2-pyrrolidone and water + 2-pyrrolidone + vitamin E-TPGS®
These results suggest that the selection of an adequate mixture of a co-solvent/surfactant can lead to a suitable formulation of a poorly soluble neutral drug candidate such as vitamin D3. As depicted on the Figure 1, the addition of Vitamin E TPGS® in the 2-pyrrolidone formulation was not efficient to improve the water solubility of vitamin D3. Nevertheless, previously, we observed that this excipient could improve the water solubility of propyl gallate, the antioxydizing agent, which is poorly soluble in a 2-pyrrolidone aqueous solution (data not shown). Therefore, in this case, the concomitant use of an excipient and a co-solvent was justified by the solubilities and the physicochemical natures of the drug, vitamin D3 and its antioxydizing agent, propyl gallate, used in the formulation (see formulation B and C below).
3.2. Granulometry analyses
DLS method has been extensively used for particle size analysis in the submicrometer range. It covers the size range of about 1 nm to 1 μm. We applied DLS method to calculate the size distribution of the surfactant micelles formed in aqueous solution with Vitamin E TPGS® micelle for three formulations (A, B, C) are presented in (Table 2). The average size for the formulations A, B and C was respectively 6.02 ± 0.4 (by number) and 11.54 nm (by intensity); 7.69 ± 0.4 (by number) and 13.20 nm (by intensity); 6.54 ± 0.4 (by number) and 13.70 nm (by intensity).
Table 2. Average size of Vitamin E TPGS micelles in formulations at 25°C.
|
Sample |
Size distribution intensity (nm)a |
Size distribution by Volume (nm)a |
Size distribution by Number (nm)a |
|
Formulation A |
11.54±0.5 |
7.97±0.3 |
6.02±0.4 |
|
Formulation B |
13.20±0.7 |
8.20±0.4 |
7.69±0.4 |
|
Formulation C |
13.70±0.9 |
9.18±0.3 |
6.54±0.4 |
|
aMean of 5 values ± SD |
|||
(Figure 2) shows the typical graphs for granulometry analyses for formulation B (formulation C shows a similar graph). This graph shows the size distribution by intensity of micelles formed with 1.5% [w/w] Vitamin E TPGS® in the final formulation.
As shown in Figure 2, by intensity, there is a wide distribution size for the micelles, ranging from 1.05 nm to 13.2 nm. However, micelles of dimension up to 13 nm constitute 94.8% of the total micelle population, with the maximum number of micelles observed at a diameter of 0.866 nm.
3.3. Method validation
3.3.1. Vitamin D3
Analytical separation of vitamin D3 from the excipients present in the formulations is exemplified in (Figure 3). This figure shows the method specificity as none of the added excipients interfered with vitamin D3 which retention time was 4.9 min. Calibration curves were derived from injections at 5 concentrations of vitamin D3 (11.85, 15.80, 19.75, 23.70 and 27.65 μg/mL). The following regression equation was obtained: [vitamin D3]mg/mL= 35884 (peak area)UA + 14265 with r2= 0.999 for vitamin D3. All calibration curves were found to have a good linear regression fit (r2>0.99) within the test range. The limit of detection and quantitation were respectively 0.36 ng/mL and 1.20 ng/mL for vitamin D3. The corresponding relative standard deviation (RSD) was less than 5% for this drug. The limit of detection was calculated on the basis of 3σ and the limit of the quantitation on the basis of 10σ, according to Miller JC and Miller JN [18].
The experiment reproducibility was assayed by performing six replicate injections of a standard mixture of vitamin D3 at 19.16 μg/ml, leading to calculated peak areas in the range of 0.32% and 0.93% (expressed as coefficient of variation).
3.3.2. Sodium fluoride
Using an ion analyzer, quantitation of sodium fluoride was performed. The calibration linearity was obtained over the test range of 12.0–28.2 μg/mL with coefficients of correlation being over 0.999. The mean repeatability of duplicated samples was 92.3%. For the reproducibility, three groups of six replicate injections of a standard mixture containing 19.3 or 19.8 or 19.9 ppm of sodium fluoride resulted in reproducibility of readings expressed as coefficient of variation comprised between 0.29% and 0.62 %.
3.4. Microbiological properties of 2-pyrrolidone
The observed MICs and MBCs values obtained for 2-pyrrolidone varied from one micro-organism to the other (Table 3) respectively in the range of 20 mg/L to 75 mg/L and of 75 mg/L to 200 mg/L. For MIC, the most sensitive strains were respectively S. capitis, A. niger and E. coli while Bacillus spores were the most resistant organism with MIC around 50–75 mg/L. On the other hand, the lowest MBC values were obtained for P. aeruginosa and E. coli, gram negative bacteria, while the others strains displayed higher values around 200 mg/L. 2-pyrrolidone was a bactericidal compound against gram negative bacteria and S. aureus, but was a bacteriostatic or a fungistatic compound for all the others strains tested.
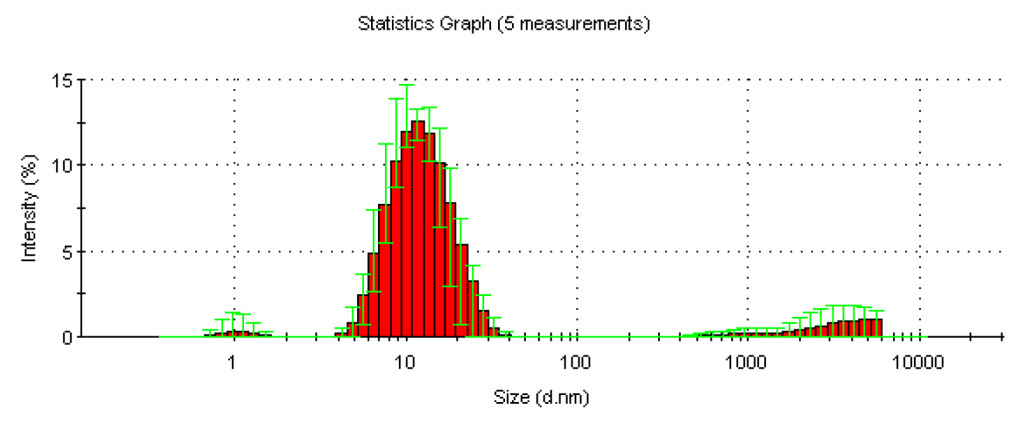
Figure 2. Statistics graph of size distribution (by intensity) of the micelles contained into the final formulation after 5 measurements.
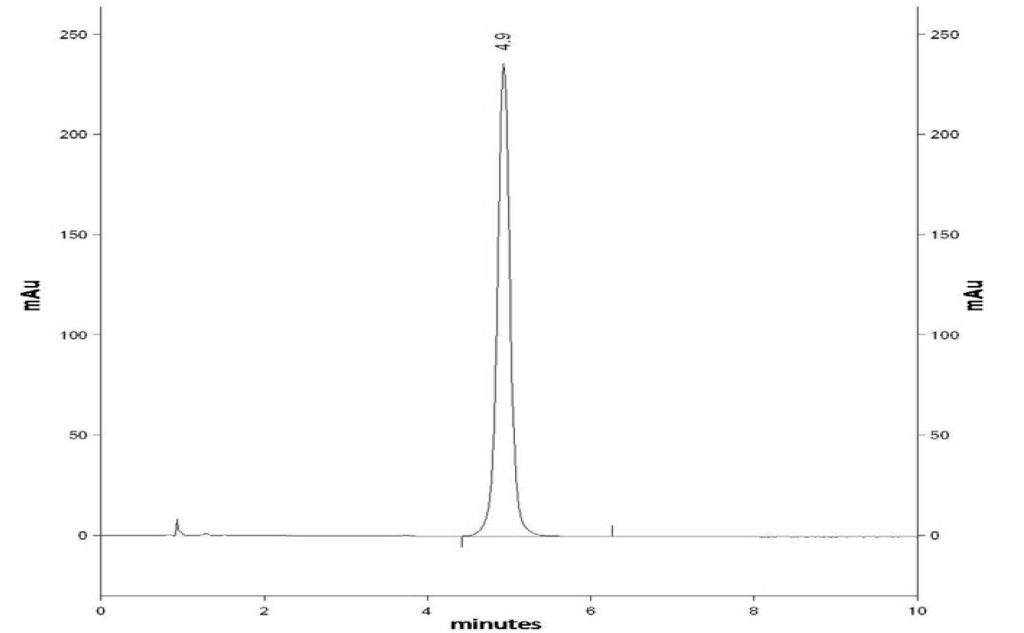
Figure 3. RP-HPLC chromatogram of a standard mixture containing Vitamin D3 (Rt= 4.9 min).
Table 3. MICs and MBCs values (mg/mL) of 2-pyrrolidone for different micro-organisms strains.
|
Strains |
MIC |
MBC |
MIC/MBC |
Activity |
|
E. coli |
30-50-30 |
75-75 |
< 4 |
bactericidal |
|
S. aureus |
40-50-50 |
100-200 |
< 4 |
bactericidal |
|
P. aeruginosa |
50-30 |
75-50 |
< 4 |
bactericidal |
|
E. faecalis |
50-50 |
200-200 |
< 4 |
bactericidal |
|
S. epidermidis |
40-40 |
200-200 |
> 4 |
bacteriostatic |
|
S. capitis |
20-20 |
75-100 |
> 4 |
bactericidal |
|
B. cereus spores |
50-75-75 |
>200->200->200 |
> 4 |
sporostatic |
|
B. sphaericus spores |
50-50-75 |
>200->200->200 |
> 4 |
sporostatic |
|
B. subtilis spores |
75-75-75 |
>200->200->200 |
> 4 |
sporostatic |
|
C. albicans |
40-40-30 |
200-200-200 |
> 4 |
fungistatic |
|
A. niger |
40-30-30 |
>200->200->200 |
> 4 |
fungistatic |
In addition to bacterial strains described in the EP, we have also used different bacterial species which can be found in oral pharmaceutical preparations.
Coagulase-negative staphylococci such as S. epidermidis and S. capitis belong to the microflora of human skin and mucous membranes; they are easily dispersed by skin scales. They are considered opportunistic pathogens and, in immunocompetent adults, are mostly associated with endocarditis, osteomyelitis, surgical site and foreign body infections [19]. S. epidermidis is one of the major causes of nosocomial infections, especially nosocomial bacteraemia. It expresses several virulence factors including those involved in the ability to adhere to and accumulate as a biofilm on a variety of surfaces, including prosthetic devices and transcutaneous catheters [20]. Enterococcus species are natural members of the human and warmblood animal intestinal flora. E. faecalis is the dominant species found in human feces [21]. Furthermore, enterococci are frequently isolated in soil, plants, vegetables, and in various foods. Because of their high concentrations in feces and their long survival period in the environment, enterococci have been proposed as water fecal contamination indicators [22]. Bacillus species are capable of undergoing sporulation, producing a dormant endospore. These spores are resistant to environmental stresses, including heat, desiccation, toxic chemicals, and extremes of ionic strength. Bacterial spores are the last organisms to remain viable during sterilization processes and environmental extremes that readily kill vegetative bacteria. For that reason, they are employed as biological indicators for monitoring the effectiveness of sterilization processes, such as vaporized hydrogen peroxide treatments [23] autoclaving, and UV irradiation [24].
3.5. Efficacy of antimicrobial preservation
EP (9th Edition, 2017. 5.1.3. Efficacy of antimicrobial preservation 577-579 and 2.6.13. Microbial examination of non-sterile products: test for specified micro-organisms) gives official quantitative methods for testing the efficacy of antimicrobial preservation in oral preparations. EP requires a log 3 reduction at 14 days in the case of bacteria and a log 1 reduction in the case of fungal count. At 28 days, EP requires that there is “no increase” in bacterial and fungal counts compared to the 14 days count.
Colony counting is not a precision method and for this reason, the criteria are given in integer logarithmic reduction values, a half log reduction value was taken to specify the requirement of “no increase”.
The EP criteria for evaluation of antimicrobial activity for oral preparations are given in terms of log reduction in the number of viable micro-organisms against the value obtained for the inoculum, and are summarised in (Table 4).
Table 4. The logarithmic reduction of bacterial cell number for 3 formulations evaluated by the official EP “test for the efficacy of antimicrobial preservation”.
|
Log reduction |
||||||||
|
|
14 days |
28 days |
||||||
|
|
Expected |
Obtained |
Expected |
Obtained |
||||
|
Formulations |
|
|
|
|
|
|
|
|
|
|
A |
B |
C |
A |
B |
C |
||
|
Strains |
|
|
|
|
|
|
||
|
S.aureus |
3 |
5.57 |
5.7 |
5.32 |
3 |
5.2 |
5.13 |
6.43 |
|
P.aeruginosa |
3 |
5.43 |
5.76 |
6.06 |
3 |
5.28 |
>5.00 |
6.65 |
|
E. coli |
3 |
5.56 |
5.91 |
4.56 |
3 |
4.96 |
>5.00 |
6.08 |
|
S.albicans |
1 |
5.73 |
3.96 |
>5.00 |
1 |
>5.00 |
5.71 |
>5.00 |
|
A.niger |
1 |
>5.00 |
1.76 |
2.44 |
1 |
5.74 |
4.83 |
3.29 |
|
B.cereus |
N.C. |
<1.00 |
<1.00 |
<1.00 |
N.C. |
<1.00 |
<1.00 |
1.96 |
|
B.sphaericus |
N.C. |
<1.00 |
<1.00 |
<1.00 |
N.C. |
<1.00 |
<1.00 |
<1.00 |
|
B.subtilis |
N.C. |
<1.00 |
<1.00 |
<1.00 |
N.C. |
<1.00 |
<1.00 |
-2 |
|
N.C.: no criterion |
||||||||
According to the EP criteria for S. aureus, P. aeruginosa, E. coli, C. albicans and A. niger, 2-pyrrolidone preserved all 3 different oral preparations as well as 14 days and 28 days of microbial contaminations. The highest preservative effect was obtained with the formulation A. In this case, all the micro-organisms tested, even yeast and mould, showed a high logarithmic reduction, around log 5. Formulations B and C had a similar effect against bacterial strains tested, but were less effective against yeast and mould strains specially at 14 days.
The main difference between the 3 formulations is the presence of propyl gallate in the formula B and C. It is well known that propyl gallate, have antioxidant properties [25]. The lower efficiency of the antimicrobial activity of 2-pyrrolidone against fungus in formula B and C could be due to by the presence of propyl gallate which reduces the oxidative stress against lipid cellular membranes.
There is no criterion for bacterial spore reduction in the EP. No spore number reduction was detected within 14 days and 28 days for B. cereus spores with the formulation C in which we observed around a log 2 reduction. Such as for EP bacterial criteria at 28 days, there was “no increase” in spore count compared to the 14 days count, excluded for B. subtilis spores. In this case, a bacterial growth around log 2 was observed after 28 days contact.
Some other antimicrobial preservative compounds are used in pharmaceutical products. Parabens, especially propyl paraben, and p-hydroxybenzoic acid (pHBA) are the most frequently employed in cosmetic and pharmaceutical preparations [26]. Due to their wide antibacterial properties, low toxicity and chemical stability they have been used in food, drugs, and cosmetic for over 50 years. Parabens are antimicrobially effective in a dosage of 0.1 to 0.2%. Concentrations of use vary from product to product but seldom exceeds 1%. The main drawback is their unsatisfactory action against gram-negative bacteria, especially Pseudomonas genus which are capable of metabolising parabens as sources of nutrient materials [27]. Furthermore it has been shown that Enterobacter cloacae and nterobacter gergoviae produced an esterase involved in the hydrolysis of parabens [28]. In the same way, Van Dyk et al. [29] described that the treatment of E. coli with pHBA acid, induced an over expression of an efflux system involved in pHBA resistance. While Ramos-Gonzalez et al. [30] showed that the mechanism of pHBA tolerance in Pseudomonas putida DOT-T1E was due to an increase of the cell membrane rigidity. On the other side, it has been shown more recently, that parabens had an endocrine activity and had implications for potential risk to human health [31]. For these reasons, 2-pyrolidone could be used as new and safer antimicrobial preservative compound. The main drawbacks of this excipient is its higher MCI and MCB values compared to pHBA MCI, which is approximately 30-40 times more efficient [32]. But, unlike parabens, 2-pyrrolidone has bactericidal activity against gram negative bacteria. Moreover, 2-pyrrolidone is a cyclic amide which lacks mutagenic or genotoxic activity and its oral LD50 is very high in rats with values greater than 5000 mg/(Kg-body weight) in rats being reported [33,34].
For dissolving drugs, 2-pyrrolidone can be used in high concentrations in oral preparation (400 mg/mL). This concentration is greatly higher than the MICs measured against all micro-organims tested. For that reason we propose to use 2-pyrrolidone as the sole preservative agent in oral paediatric formulations in the absence of other preservative compound such as parabens or pHBA.
4. Conclusion
The concomitant use of 2-pyrrolidone and vitamin E TPGS® was beneficial to enhance aqueous solubility of vitamin D3 and propyl gallate, the antioxydizing agent. By comparison with conventional antimicrobial agents commonly used for oral formulations, MIC and MBC of 2-pyrrolidone were determined to be high, necessitating large amounts of this compound to provide antimicrobial efficacy for the formulation of a liquid oral drug delivery system. Therefore, the use of 2-pyrrolidone could be justified only in formulations of oral liquid drug delivery containing surfactants and it should not be used for liquid oral solutions or suspensions for which common antimicrobial agents are more suitable. To conclude, for formulations containing surfactants, 2-pyrrolidone can be promisingly considered, especially in the field of oral paediatric formulations.
References
- Tuleu C, Gangé J, Seurin S (2005) The need for paediatric formulation: oral administration of nifedipine in children, a proof of concept. J Drug Delivery Sci and Techn 15: 319–324.
- Nunn T, Williams J (2005) Formulation of medicines for children. Br J Clin Pharmacol 59: 674–676. [crossref]
- Smola M, Zhao M, Vandamme TF, Sokolowski A (2007) Design and development of oral paediatric reverse water-in-oil microemulsions as carriers for ranitidine hydrochloride, Minerva Biotechnologica 19: 7–16.
- Smola M, Vandamme TF (2007) Taste masking of unpleasant oral drugs. In: Boris O. Mashkevich (Ed). 2007 Chap4 in Drug Delivery Research Advances. Nova Publishers, New York, pp: 65–84. (Book Reference)
- Bloomfield SF (1990) Microbial contaminations: spoilage and hazard. In: Danyer, S, Blair R. (Eds.). Guide to micobiologigical Control in Pharmaceuticals. Chichester: Ellis Horwood, pp: 29–52. (Book Reference)
- Liu R (2000) Introduction, Chapter 1 in Water insoluble drug formulation. In: Rong Liu (Ed). Interpharm CRC Press, pp: 1–4. (Book Reference)
- Breitkreutz J (2005) Kindgerechte arzneiformen. Monattsschrift Kinderheilkunde 8: 726–34.
- Breitkreutz J, Boos J (2007) Paediatric and geriatric drug delivery. Expert Opin Drug Deliv 4: 37–45. [crossref]
- Chakravarty D, Lach JL, Blaug SM (1957) Study of complex formation between polyoxyl 40 stearate and some pharmaceuticals. Drug standard 25: 137–140.
- Sunderland VB, Watts DW (1984) Kinetics of the degradation of methyl, ethyl and n-propyl 4-hydrixybenzoate esters in aqueous solution. Int. J. Pharm 19: 1–15.
- Watrobska-Swietlikowska, D, Sznitowska M (2006) Partioning of parabens between phases of submicron emulsions stabilized with egg lecithin. Int. J. Pharm 312: 174–178.
- Higuchi T, Connors K (1965) Phase-solubility techniques. Adv. Anal. Inst 4: 117–212.
- Florence AT (1981) Drug solubilisation in surfactant systems. In: Yalkowsky SH (Ed). In Techniques of solubilisation of drugs, Marcel Dekker, Inc, New York (Book Reference)
- Caporal-Gauthier J, Nivet JM, Algranti P, Guilloteau M, Histe, M, et al. (1992a) Guide to analytical validation. Report of an SFSTP commission. I. Methodology. S.T.P. Pharma Pratiques 1992a, 2: 205–226.
- Caporal-Gauthier J, Nivet JM, Algranti P, Guilloteau M, Histe, M, et al. (1992b) Guide to analytical validation. Report of an SFSTP commission. II. Examples of applications. STP Pharma Pratiques 2: 227–239.
- Caporal J, Capuzet E (1997) Analytical validation: from protocol to report. STP. Pharma Pratiques 7: 360–363.
- Chaminade P, Feraud S, Baillet A, Ferrier D (1995) Validation of a chromatographic analysis method by HPLC. Ruggedness test and validation of the technique. S.T.P. Pharma Pratiques 5: 17- 35.
- Miller JC, Miller JN (1993) Statistics for analytic chemistry. (3rd edn). Chichester Ellis Horward.
- von Eiff C, Peters G, Heilmann C (2002) Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis 2: 677–685. [crossref]
- Gemmell CG (2001) Coagulase negative staphylococci and their role in infection. In: Sussman, M. (Ed.), Molecular Medical Microbiology. Academic Press, London, UK, pp: 889–899.
- Murray BE (1990) The life and times of the Enterococcus. Clin Microbiol Rev 3: 46–65. [crossref]
- U.S. EPA (1986) Bacteriological Ambient Water Quality Criteria for Marine and Freshwater Recreational Waters. PB86-158-045. Springfield, VA: United States Environmental Protection Agency.
- Johnston MD, Lawson S, Otter JA (2005) Evaluation of hydrogen peroxide vapour as a method for the decontamination of surfaces contaminated with Clostridium botulinum spores. J. Microbiol. Methods 60: 403–411.
- Mamane-Gravetz H, Linden KG (2004) UV disinfection of indigenous aerobic spores: implications for UV reactor validation in unfiltered waters. Water Res 38: 2898–2906.
- Pezzuto JM, Park EJ (2002) Autoxidation and antioxidants. In: Swarbrick J, Boylan JC (Eds.). Encyclopedia of Pharmaceutical Technology, volume 1. New York, Marcel Debber. Inc., 2002, 97–113.
- Soni MG, Burdock GA, Taylor SL, Greenberg NA (2001) Safety assessment of propyl paraben: a review of the published literature. Food Chem Toxicol 39: 513–532. [crossref]
- Nowak GA (1985) Microbiology, GMP, Disinfection, water treatment, preservation, 109–196 in Cosmetic Preparation Volume 1; Verlag für Chem Industrie H. ZiolkowskyKG. Augsburg, Germany.
- Valkova N, Lépine F, Labrie L, Dupont M, Beaudet R (2003) Purification and characterization of PrbA, a new esterase from Enterobacter cloacae hydrolyzing the esters of 4-hydroxybenzoic acid (parabens). J Biol Chem 278: 12779–12785. [crossref]
- Van Dyk TK, Templeton LJ, Cantera KA, Sharpe PL, Sariaslani FS (2004) Characterization of the Escherichia coli AaeAB efflux pump: a metabolic relief valve? J Bacteriol 186: 7196–7204. [crossref]
- Ramos-González MI, Godoy P, Alaminos M, Ben-Bassat A, Ramos JL (2001) Physiological characterization of Pseudomonas putida DOT-T1E tolerance to p-hydroxybenzoate. Appl Environ Microbiol 67: 4338–4341.
- Golden R, Gandy J, Vollmer G (2005) A review of the endocrine activity of parabens and implications for potential risks to human health. Crit Rev Toxicol 35: 435–458. [crossref]
- Cho JY, Moon JH, Seong KY, Park KH (1998) Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci Biotechnol Biochem 62: 2273–2276. [crossref]
- BASF AG, Abteilung. Toxikologie, unveroeffentlichte Untersuchung,(XI/407), 07.11.1961
- BASF AG, Abteilung Toxikologie; unveroeffentliche Untersuchungen (79/409), 09.04.1981