Abstract
Mycoplasma hyopneumoniae, the etiology of enzootic pneumonia, is a chronic respiratory disease with a worldwide distribution. Prevention is performed through vaccination of suckling piglets, while newly introduced gilts are often boostered prior to arrival at the sow farm. The present case describes a study in a gilt rearing farm where gilts, boostered with a two-shot M. hyopneumoniae vaccine at 18-22 weeks of age after their early vaccination at 3 days of age, were serologically positive after the second vaccination. To elucidate if the seroconversion was due to booster vaccination or circulating M. hyopneumoniae infection, a comparative trial was designed. Following the second vaccination at 22 weeks of age, serological titers of gilts in the Vaccine group were increasing and all gilts became serologically positive, without any TBS detection of circulating M. hyopneumoniae. Therefore, it can be concluded that administration of a booster vaccination for M. hyopneumoniae to rearing gilts, already vaccinated in early life with a one-shot M. hyopneumoniae vaccine, induced an increase in both serological titers and the percentage of M. hyopneumoniae positive animals at the end of the rearing period.
Keywords
Mycoplasma hyopneumoniae, Seroconversion, TBS, Booster vaccination, Gilts
Abbreviations: DOI: Duration of Immunity; ELISA: Enzyme- Linked Immunosorbent Assay; IAV-S: Influenza A Virus – Swine; M. hyo: Mycoplasma hyopneumoniae; mPCR: Multiplex PCR; PCMV: Porcine Cyto Megalo Virus; PCV-2: Porcine Circo Virus – type 2; PRCV: Porcine Respiratory Corona Virus; PRRSV: Porcine Reproductive and Respiratory Syndrome Virus; S/P: Serum to Positive; SEM: Standard Error of the Mean; TBS: Trachea-bronchial Swab; USA: United States of America
Introduction
Mycoplasma hyopneumoniae (M. hyopneumoniae) is a chronic respiratory pathogen with a worldwide distribution. Enzootic pneumonia, the disease caused by M. hyopneumoniae, is considered to affect up to 70% of the swine herds in most pig producing countries. The disease is associated with high economic losses due to reduced performance, increased treatment costs and supplementary costs related to preventive vaccination [3]. To prevent spread from gilts and sows to piglets during the suckling period, intensive vaccination programs have been implemented to increase the acquired immunity to M. hyopneumoniae in the sow herd. The present case describes a study in a gilt rearing farm vaccinating the newborn gilts with a one-shot M. hyopneumoniae vaccine at day 3 of age (Stellamune One, Elanco AH) followed by a booster vaccination during the second part of the rearing period using a two-shot M. hyopneumoniae vaccine (Stellamune Mycoplasma, Elanco AH) at 18 and 22 weeks of age. Following the booster vaccination, increased serological titers are observed that might interfere with the M. hyopneumoniae serological monitoring performed at the end of the rearing period, just prior to or at gilt delivery to the multiplier sow farm location. Besides booster vaccination to M. hyopneumoniae, potential M. hyopneumoniae circulation at the end of the rearing period might also have an impact on the serological response to M. hyopneumoniae. Therefore, a comparative trial was conducted in a gilt-rearing system to determine if the serological response to M. hyopneumoniae was due to the booster vaccination on itself or the potential M. hyopneumoniae infection circulating within the gilts.
Aim of the Study
The study objective was to determine the cause of potential occurrence of seroconversion to M. hyopneumoniae following a double booster vaccination for M. hyopneumoniae with a two-shot vaccine at the age of 18 and 22 weeks.
Materials and Methods
Gilts from the same farm of origin were placed in two different rearing compartments for the comparative trial. The gilts were followed up throughout the entire rearing period through blood serum sampling and collection of trachea-bronchial swab (TBS) samples [7] combined with mPCR at 18-22-26 and 29 weeks of age. The blood samples were analyzed using the IDEXX ELISA Mhyo Ab kit for the presence of antibodies to M. hyopneumoniae. Results were reported as S/P ratios and were categorized into positive (S/P > 0.40) or negative (S/P ≤ 0.40) for further statistical analysis. The TBS samples were analyzed using a mPCR detecting multiple respiratory pathogens including M. hyopneumoniae as previously described [9]. Other respiratory pathogens examined were Porcine Reproductive and Respiratory Syndrome Virus (PRRSV), Influenza A Virus – Swine (IAV-S), Porcine Circo Virus – type 2 (PCV-2), Porcine Cyto Megalo Virus (PCMV), Porcine Respiratory Corona Virus (PRCV), and Mycoplasma hyorhinis. Results were reported as positive or negative for the presence of M. hyopneumoniae genetic material in the samples. The rearing gilts in the Control group remained exceptionally unvaccinated at 18 and 22 weeks of age to evaluate the serological kinetics at the end of the rearing period without a booster vaccination for M. hyopneumoniae. The rearing gilts in the Vaccine group received their standard booster vaccination at 18 and 22 weeks of age and were treated orally with tiamulin for 7 days after their second vaccination to omit potential spread of M. hyopneumoniae that might interfere with seroconversion to M. hyopneumoniae. During each visit related to the collection of the different samples, clinical observation towards the presence of signs of respiratory disease were performed and documented. All information on vaccination, sampling and antimicrobial treatment is given in Table 1.
Table 1: Overview of all interventions related to vaccination, blood and treacheobronchial swab (TBS) sampling, and antimicrobial treatment in the rearing gilts of the unvaccinated Control group and the vaccinated Vaccine group.
|
Group |
||||||||
| Control |
Vaccine |
|||||||
|
Week |
Vacc* | Blood | TBS** | Ab*** | Vacc* | Blood | TBS** |
Ab*** |
|
18 |
– | X | X | – | X | X | X | – |
| 22 | – | X | X | – | X | X | X |
7 d |
|
26 |
– | X | X | – | – | X | X | – |
| 29 | – | X | X | – | – | X | X |
– |
*Vacc, vaccination with an M. hyopneumoniae two-shot vaccine (Stellamune Mycoplasma; Elanco AH).
**TBS, trachea-bronchial swab sampling.
***Ab, antimicrobial treatment with tiamulin for 7 d following the 2nd vaccine administration.
Results
Serological Results
The S/P ratio of the gilts in the Control group was 0.39 ± 0.07 (18 wks; minimum 0.28, maximum 1.05) and significantly increased (P < 0.05) to 0.54 ± 0.08 (22 wks; minimum 0.34, maximum 1.17) and a maximum of 0.68 ± 0.09 (26 wks; minimum 0.40, maximum 1.26; P < 0.01). In the Vaccine group, the S/P ratio of the gilts was 0.20 ± 0.05 (18 wks; minimum 0.00, maximum 0.75) and significantly increased (P < 0.001) to 1.34 (22 wks; minimum 0.77, maximum 2.07) and a maximum of 1.41 (26 wks; minimum 0.92, maximum 1.30) (Figure1).
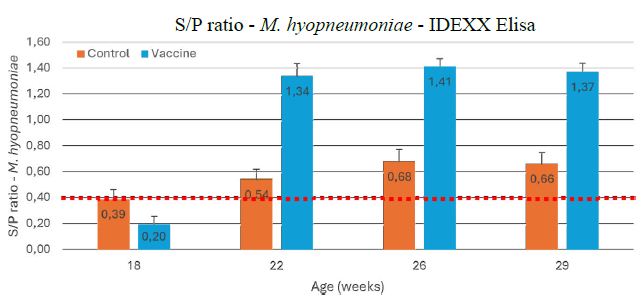
Figure 1: Serological results (expressed S/P ratio ± SEM) of M. hyopneumoniae IDEXX ELISA at 18-22-26 and 29 weeks of age in rearing gilts in the unvaccinated Control (orange) and double vaccinated Vaccine (blue) group. The dashed red line is the ELISA S/P cut-off value of 0.40 between negative and positive serological results.
The percentage of serologically positive gilts (S/P > 0.40) in the Control group was 30% (18 wks) and reached a maximum at 26 wks (80%). The percentage remained at this level during the entire further study period. In the Vaccine group, the percentage of serologically positive gilts was 20% (18 wks) and reached its maximum (100%) at 22 wks. The percentage remained at this level during the entire further study period (Figure 2).
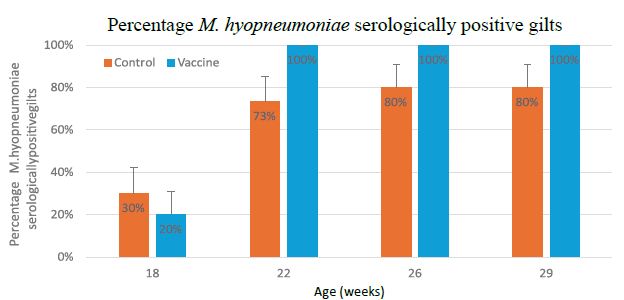
Figure 2: Serological results (expressed S/P ratio ± SEM) of M. hyopneumoniae IDEXX ELISA at 18-22-26 and 29 weeks of age in rearing gilts in the unvaccinated Control (orange) and double vaccinated Vaccine (blue) group.
Trachea-bronchial Swab Results
The TBS swabs were all negative for M. hyopneumoniae at 18- 22-26 and 29 weeks of age in both study groups, indicating that no circulating M. hyopneumoniae infection was present in the Control nor in the Vaccine group. The only other pathogen that could be detected was Porcine Cyto Megalo Virus (PCMV) at 22 weeks in the gilts of the Vaccine group and at 26 weeks in the gilts of the Control group.
Clinical Observation Results
Overall, no clinical signs of respiratory disease could be observed upon the different visits related to the collection of the samples in the comparative study.
Discussion
To date, very limited data are available regarding the effect of a late booster vaccination for M. hyopneumoniae, since most M. hyopneumoniae vaccines are administered early in life during the suckling period of the piglets (3 days to 3 weeks of age). Early vaccination has been shown to mount a sufficient immunity to protect the piglets during their productive life until slaughter [4] showing a convalescent serological immune response in response to a late infection at 77 to 105 days of age. Another study [6] demonstrated that early vaccination at 1 week of age combined with a late M. hyopneumoniae challenge at 25 weeks of age could reduce the lung lesions related to M. hyopneumoniae. Another study where early vaccinated pigs were exposed to a concurrent natural infection including M. hyopneumoniae, Influenza Virus A – Swine (IAV-S) and PRRSV at 25 weeks of age confirmed less lung lesions and better performance in the early vaccinated group [1].
A recent study comparing an early one-shot M. hyopneumoniae vaccination with an additional administration at 112 days or two additional administrations at 112 and 157 days, respectively, demonstrated a major seroconversion to M. hyopneumoniae at 194 days of age in the triple vaccinated group [10]. In contrast, in the current study, we applied a combination of a one-shot vaccination at 3 days of age with a two-shot vaccination at 18 and 22 weeks of age.
Most commercial vaccine product SPC’s indicate a duration of immunity (DOI) of 22-26 weeks after vaccination. Therefore, in the case of rearing gilts that are raised to go beyond the regular 6 months of age at slaughter, there is the need to boost immunity to M. hyopneumoniae at the end of their rearing period. This is necessary to protect the gilts from a new infection with M. hyopneumoniae and to omit massive excretion of M. hyopneumoniae from infected gilts to suckling piglets during their first lactation.
For this purpose, schedules have been developed under USA conditions to infect gilts with M. hyopneumoniae between 80 and 100 days of age to mount sufficient immunity at young age and decrease the risk of M. hyopneumoniae excretion during the first lactation [5]. However, active infection of gilts with M. hyopneumoniae through intratracheal inoculation, fogging or other means is not widely accepted or performed under European conditions [2].
Therefore, vaccination is the preferred method to extend and increase protection of the rearing gilts into their reproductive life on the sow farm. However, in some cases, when performing a serological evaluation of gilts upon arrival at the multiplier sow farm location, M. hyopneumoniae serologically positive titers are observed in these gilts, although they originated from an M. hyopneumoniae negative nucleus sow herd. To elucidate if booster vaccination for M. hyopneumoniae could affect the serological profile of rearing gilts, a comparative study was performed on two groups of rearing gilts in an M. hyopneumoniae negative nucleus herd. From the obtained results, booster vaccination significantly increased both the average S/P ratio to M. hyopneumoniae and the percentage of M. hyopneumoniae serologically positive animals in the vaccinated group, while no concurrent M. hyopneumoniae infection was present as shown by the M. hyopneumoniae negative mPCR TBS samples.
Conclusions
Administration of a booster vaccination for M. hyopneumoniae to rearing gilts, already vaccinated in early life with a one-shot M. hyopneumoniae vaccine, induced an increase in both serological titers and the percentage of M. hyopneumoniae positive animals at the end of the rearing period. This increase, without M. hyopneumoniae circulation in the respiratory tract, might under field circumstances give the impression of M. hyopneumoniae infection due to the high serological titers. However, the optimal technique to demonstrate active M. hyopneumoniae remains the collection of TBS samples in clinically diseased, coughing animals as previously shown [7,8].
Conflict of Interest
No conflict of interest to be reported by any of the authors.
Acknowledgements
The authors acknowledge the support of the farm staff in the follow-up of both study groups under field conditions and their assistance during sampling.
References
- Del Pozo Sacristán R, Sierens A, Marchioro SB, Vangroenweghe F, Jourquin J, Labarque J, Haesebrouck F, Maes D (2013). Efficacy of early Mycoplasma hyopneumoniae vaccination against mixed respiratory disease in older fattening Vet. Rec. [crossref]
- Garza-Moreno L, Segalés J, Pieters M, Romagosa A, Sibila M. 2018. Acclimation strategies in gilts to control Mycoplasma hyopneumoniae infection. Microbiol. 219, 23-29. [crossref]
- Maes D, Segalés J, Meyns T, Sibila M, Pieters M, Haesebrouck F (2008). Review: Control of Mycoplasma hyopneumoniae infections in Vet. Microbiol. 126, 297-309. [crossref]
- Martelli P, Terreni M, Guazezetti S, Cavirani S. 2006. Antibody response to Mycoplasma hyopneumoniae infection in vaccinated pigs with or without maternal antibodies induced by sow J. Vet. Med. B 53, 229-233. [crossref]
- Pieters M, Fano E. 2016. Mycoplasma hyopneumoniae management in Vet. Rec.[crossref]
- Reynolds SC, St Aubin LB, Sabbadini LG, Kula J, Vogelaar J, Runnels P, Peters 2009. Reduced lung lesions in pigs challenged 25 weeks after the administration of a single dose of Mycoplasma hyopneumoniae vaccine at approximately 1 week of age. Vet. J. [crossref]
- Vangroenweghe F. 2018. Early detection of Mycoplasma hyopneumoniae in pigs under field conditions. Ph.D. thesis. Ghent University, Faculty of Bioscience Engineering.
- Vangroenweghe F. 2020. Early detection of Mycoplasma hyopneumoniae in pigs under field conditions using trachea-bronchial swab Integr. J. Vet. Biosci. 4, 1-7.
- Vangroenweghe F, Thas O. 2021. Seasonal variation in prevalence of Mycoplasma hyopneumoniae and other respiratory pathogens in peri-weaned, post-weaned, and fattening pigs in Belgian and Dutch pig herds using a tracheobronchial swab sampling technique and their associations with local weather Pathogens. [crossref]
- Visscher K, Thuring V, Steenaert M, Jansen R. 2022. Repetitive MycoFlex® vaccination results in antibody seroconversion. Proceedings of 26th International Pig Veterinary Society, Rio de Janeiro, Brazil. 21-24 June 2022. p. 490.