DOI: 10.31038/MIP.2025611
Abstract
Coronavirus disease 2019 (COVID-19) is an infectious disease that caused by SARS-CoV-2. It affects multiple systems, patients with systemic lupus erythematosus (SLE) are known to be susceptible to COVID-19. Surprisingly, there is a certain correlation and similarity between these two diseases to some extent. In this study, we performed a systematic review of relevant studies published on PubMed from December 2019 to January 2023 from the aspects of the clinical features of SLE patients with COVID-19, the immunological similarities between COVID-19 and SLE, the prevention and therapeutic approach of SLE patients with COVID-19, and the impact of the COVID-19 pandemic on SLE patients. Our findings indicate that SLE patients at higher risk of COVID-19 infection than the general population, and SLE patients treated with glucocorticoids or immunosuppressants have a higher rate of hospitalization. Consequently, the use of immunosuppressants during COVID-19 infection in SLE patients is of concern and additional treatment approaches should be explored. Moreover, SLE patients with COVID-19 also face challenges in accessing healthcare, financial hardship, and psychological distress. These issues require further attention and can be addressed by providing telemedicine, ensuring adequate supplies of medicines and promoting psychological well-beings. Together, this article summarizes the correlation and similarity between SLE and COVID-19, and provides a detailed and practical guide for the prevention, treatment and nursing of SLE patients with COVID-19. Moreover, this article also discusses the fields that require further research and provide reference for the management of other autoimmune diseases in the case of viral infection.
Keywords
Systemic lupus erythematosus, COVID-19, Autoimmune disease, Systematic review
Introduction
Since its initial outbreak in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induced coronavirus disease 2019 (COVID-19) has rapidly spread into a global pandemic, causing severe morbidity and mortality. It primarily affects the respiratory system but can also result in various extrapulmonary manifestations [1]. As of 29 January 2023, more than 753 million confirmed cases and 6.8 million deaths have been reported worldwide. It was found that COVID-19 and autoimmune diseases share many disastrous outcomes, including kidney damage, thus they interact with each other leading to more severe clinical symptoms [2,3].
Systemic Lupus Erythematosus (SLE) is a complex heterogeneous autoimmune disease caused by multiple genetic, immune, endocrine and environmental factors that can result significant morbidity and mortality. It is mainly associated with dysfunction of adaptive and innate immunity and is characterized by the production of autoantibodies, the formation of immune complex, and chronic inflammation [4]. Moreover, bacterial, viral and other opportunistic infections often lead to an increased risk of death in SLE patients [5,6]. Early reports from China, Europe, and the United States during the first wave of the pandemic suggested that the prevalence of COVID-19 in SLE patients was similar to the general population [7]. While with the deepening of research in the epidemiology of COVID-19 in SLE patients, recent evidence suggests that SLE patients may be at higher risk for severe COVID-19 infection compared with healthy controls [8]. Studies conducted in New York shows symptomatic COVID-19 prevalence of 4% in the Columbia University Lupus Cohort, which was higher than the 2% prevalence observed in the general population [9]. In a telemedicine survey of SLE patients in Italy, 12 out of 126 cases (7.5%) were confirmed or suspected cases of COVID-19, which is higher than the 2.5% incidence observed in the general population [10]. A survey in Spain reported that the incidence of confirmed and suspected COVID-19 cases among SLE patients was 7.5%, which was higher than the local population (1.9%) [11].
Lineage B coronaviruses, including SARS-CoV-2, mediate viral entry through the RBD region of the S protein through the interaction with ACE2 on cells, host proteases then cleave the S protein, releasing the spike-fusion peptide and allowing the virus to enter the cell [12]. Regarding the mechanism of higher COVID-19 incidence in SLE patients, patients with SLE tend to have higher levels of ACE2, and elevated levels of ACE2 are associated with an increased risk of developing SLE and its associated complications [13]. Oxidative stress induced by viral infection exacerbates lupus-associated DNA methylation defect, potentially leading to increased hypomethylation of ACE2 and increased viremia [14]. These findings suggest that the epigenetic dysregulation inherent in lupus may contribute to viral entry, viremia, and an exaggerated immune response to SARS-CoV-2.
In addition to the higher incidence of COVID-19 in SLE patients than that of in the general population, many investigators reported SLE patients present with mild to moderate symptoms of COVID-19. The use of immunosuppressants and steroids in SLE may be risk factors for developing severe COVID-19. Moreover, patients with other comorbidities are at increased risk of developing a severe clinical course and even mortality, thus the health management of such patients needs to be strengthened. Accordingly, the emergence and rapid global spread of the COVID-19 pandemic has raised multiple questions for rheumatologists regarding the risk of infection and the appropriate use of immunosuppressive drugs in SLE patients and other chronic Autoimmune Rheumatic Diseases (AIRD) [15].
The effect of COVID-19 on patients with SLE has been ongoing with the development of the pandemic. Understanding the relationship between SLE and COVID-19 is critical to identifying risk factors and developing strategies to reduce the risk of severe infection. On the one hand, viral infections can trigger disease flares in SLE patients, and there are concerns that COVID-19 may also contribute to disease activity in SLE patients. On the other hand, the presence of comorbidities and the use of immunosuppressive therapy may complicate the management of COVID-19 in SLE patients. The aim of this article was to provide a comprehensive overview of the current understanding of the relationship between COVID-19 and SLE, and provide insights that can guide clinical practice and highlight fields that require further research.
Methods
Search Strategy
A systematic literature search will be performed to identify studies relevant to this review. Using PubMed to retrieve relevant articles from December 2019 to February 2023. The following keywords will be used in the search: “COVID-19”, “SARS-CoV-2”, “systemic lupus erythematosus”, “autoimmune disease”, “immunomodulatory therapy”, “disease activity” and “treatment”. Our searches were limited to human studies and the language was limited to English. A two-stage search will be performed to identify relevant studies: an extensive search based on title and abstract was taken, followed by a full-text review to ensure compliance with the inclusion criteria.
Selection Criteria
The inclusion criteria for this review were as follows: studies on COVID-19 in SLE patient, including observational studies, mapping of relevant clinical data, and randomized controlled trials. The exclusion criteria for this review were as follows: studies not related to the relationship between SLE and COVID-19, studies not including SLE patients with COVID-19, and studies that were not available in English or in peer-reviewed journals, preprints, or other relevant sources.
Clinical Characteristics of COVID-19 in SLE Patients
Studies have reported that the common symptoms of COVID-19 infection in SLE patients include fever, cough, shortness of breath, anosmia and dysgeusia [11,16]. A study from New York City also noted that some patients developed gastrointestinal symptoms such as diarrhea [8]. These symptoms are not very different from those experienced by healthy people infected with COVID-19. In addition, a patient of SLE with COVID-19 could develop COVID-19–related varicelliform rashes, thrombotic events, leptomeningeal involvements and immune thrombocytopenia [17-19].
According to a nationwide study in Denmark, SLE patients are three times more likely to be hospitalized after contracting COVID-19 than the general population [20]. Two statistical studies conducted in France and the United States have demonstrated that the hospitalization rates of COVID-19 infected SLE patients were 82% and 59%, respectively, and more than half of them required oxygen therapy due to respiratory failure [8,21]. SLE patients with COVID-19 generally have mild or moderate disease, but some do require hospitalization and admission to the Intensive Care Unit (ICU). Moreover, a study from Brazil has revealed that patients with SLE had adverse outcomes twice as often as the general population without comorbidities, but had comparable risks to those with comorbidities [22]. Studies also shown that the poor outcome of COVID-19 infection in SLE patients may be attributed to the underlying features of SLE and the use of immunosuppressive medications. A cohort study by Solé et al. revealed that hypocomplementemia is a risk factor for severity, while the presence of anti-SSA/Ro52 antibodies may increase the susceptibility of SLE patients to COVID-19 [23]. Sakthiswary et al. collected case reports and found that lupus nephritis were patients more likely to experience severe to critical illness [24].
Due to their unique characteristics, both pediatric SLE and pregnant women with SLE have received extensive attention. The clinical presentation described in pediatric SLE population with COVID-19 might be similar to those in adults, characterized by increased work of breathing and low oxygen saturation [25]. In addition, case reports suggested that pediatric SLE is associated with reduced rates of hospitalization while pregnant women with SLE may experience mild symptoms such as difficulty breathing and joint pain after the infection [26-28]. However, evidence to date suggests no increased risk of severe disease in pregnant women and a low risk of vertical transmission or fetal distress [29-31].
Overall, the available evidence suggests that COVID-19 in SLE patients can exhibit a range of clinical features, with disease severity ranging from mild to severe. Given the high rate of hospitalization and disease severity among SLE patients after contracting COVID-19, better health management of this patient population is needed.
Shared Immunological Features of COVID-19 and SLE
Accumulating evidence suggests that COVID-19 and SLE share some common immunological features, which may contribute to the increased susceptibility of SLE patients to severe COVID-19. The similar immune disorders involved in SLE and COVID-19 patients were described below (Figure 1).
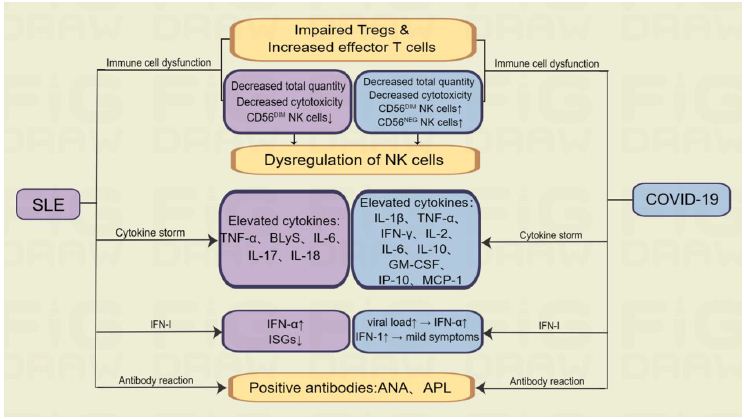
Figure 1: Shared immunological features of COVID-19 and SLE.
Table 1: Characteristics and outcomes of studies included in treatment and prevention.
|
Author |
Study details |
Conclusions |
| Martin Kolb et al., 2021 |
European Respiratory Society COVID-19 treatment guideline |
Strong recommendations for oxygen therapy, anticoagulation and the careful use of corticosteroids. |
|
RECOVERY Collaborative Group, et al., 2021 |
Controlled, open-label trial of COVID-19 patients from England |
2104 patients in the study were treated with dexamethasone and 4321 patients received usual care. In hospitalized COVID-19 patients, treatment with dexamethasone resulted in reduced 28-day mortality in patients receiving invasive mechanical ventilation or oxygen alone, but not in those not receiving respiratory support. |
|
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, et al., 2020 |
Meta-analysis of 7 randomized clinical trials totaling 1703 patients with COVID-19 |
Systemic corticosteroid treatment was related to lower 28-day all-cause mortality compared to conventional treatment or placebo. |
| Libster R, et al., 2021 |
Randomized, double-blind, placebo-controlled study (n=160) |
COVID-19 progression is reduced by early administration of high-titer anti-SARS-CoV-2 plasma to mildly infected elderly patients. |
| Mason A, et al., 2021 | Review |
Prospective data collection and registration studies on the impact of vaccination on disease control in SLE, the prevalence of COVID-19 in SLE patients, and the severity of COVID-19 disease course would be beneficial. |
| Ugarte-Gil MF, et al., 2022 |
Multivariable ordinal logistic regression mode |
Demographic factors, comorbidities, glucocorticoid use, and untreated or active SLE are significant determinants of more severe COVID-19 outcomes in patients with SLE. |
| Gendebien Z, et al., 2021 | Systematic analysis |
The dose of glucocorticoids was positively associated with hospitalization risk in SLE patients. |
| Tang W, et al., 2021 | Review |
SLE patients have a poorer immune response to SARS-CoV-2 vaccination than healthy controls, but the benefits far outweigh the risks in patients with SLE. |
| Furer V, et al., 2021 |
A multicentre study, patients with AIIRD (n=686) and general population (n=121) |
The BNTb262 vaccine was found to be immunogenic in the majority of patients with AIIRD and had an acceptable safety profile. BNTb262-induced immunogenicity was significantly reduced by treatment with glucocorticoids, rituximab, MMF and abatacept. |
| Boekel L, et al. 2021 |
Patients with rheumatic diseases (n=3682) and healthy controls (n=1147) |
The differences in seroconversion rates and antibody titers were similar between autoimmune diseases and the two main vaccine types, suggesting that immunosuppressive drug treatment, not underlying autoimmune disease, is the main factor affecting immunogenicity of the vaccine. |
| Izmirly PM, et al., 2022 |
Patients with SLE (n=90) and healthy controls (n=20) |
In a multi-ethnic/multi-racial study of SLE patients, 29% had a poor response to COVID-19 vaccine that was associated with use of immunosuppressant therapy. |
| So H, et al., 2022 |
Single-center, prospective, observational study (n=65) |
COVID-19 vaccines elicited satisfactory but impaired humoral responses in SLE patients versus controls, depending on immunosuppression and type of vaccination received. |
| Mehta P, et al., 2022 | Review |
Lupus patients are at a higher risk than the general population of becoming infected with COVID-19, resulting in hospitalization, serious illness, and death. COVID-19 vaccination is relatively safe for lupus patients, with a minimal risk of serious flare-ups. |
| Bartels LE, et al., 2021 |
Patients with RA or SLE (n=285) |
The Pfizer-BioNTech BNT162b2 COVID-19 vaccine is reactogenic in patients with SLE and RA. Compared to healthy controls, reactogenicity was more frequent but not more severe in these patients. |
| Cherian S, et al., 2021 |
Follow-up visit of patients with RA (n=724) |
Vaccine adverse events were no more common in AIIRDs than in non-AIIRD rheumatic and musculoskeletal conditions, and no serious side effects were observed in the overall population. |
| Esquivel-Valerio JA, et al., 2021 |
Patients with autoimmune rheumatic diseases (n=225) |
The safety of various COVID-19 vaccines in patients with AIIRD has been supported by a number of results. |
| Moyon Q, et al., 2022 | A prospective study (n=126) |
Impaired BNT162b2 mRNA antibody response is independently associated with MMF, MTX and poor baseline humoral immune status, particularly low frequencies of naive B cells. |
| Ferri C, et al., 2021 |
An observational multicenter study (n=478) |
Early, post-vaccination serum NAb determination may allow the identification of three main groups of patients with ASD: responders, suboptimal responders, non-responders. Patients at high risk of developing COVID-19 are those with weak/no seroconversion, who are presumed to be immune to SARS-CoV-2 infection. |
| Felten R, et al., 2021 | Patients with SLE (n=696) |
COVID-19 vaccination appears to be well tolerated in SLE patients with minimal or no risk of relapse, even after mRNA vaccination. |
Immune Cell Dysfunction
Under normal circumstances, immune cells do not recognize self-antigens, but in patients with autoimmune diseases such as SLE, dysregulation of immune tolerance mechanisms or inflammatory signaling can activate auto-reactive immune cells, leading to immune malfunctions. Adaptive immune activation in patients with COVID-19 is manifested by dysregulation of T-cell numbers and function, as well as the production of antibodies, several of which are also seen in SLE patients. These are described in more detail below.
T cell dysfunction, including impaired Tregs and increased effector T cells, has been observed in both SLE and COVID-19, which may be responsible for prolonged infection and severe symptoms [32]. Th17 cells are members of the CD4+ T lymphocytes that produce pro-inflammatory cytokines, including IL-17 and IL-22. Studies have shown that increased amounts of Th17 cells and IL-17 cytokines in the blood of people with SLE are inversely correlated with the severity of this immune disease and its clinical outcomes [33]. In addition, elevated levels of IL-17A also play a role in COVID-19 patients, with elevated levels of IL-17A often predictive of more severe lung tissue damage. These results suggest immune cell dysfunction in both SLE and COVID-19 [34]. In conclusion, SLE and COVID-19 have almost the same dysfunction in immune cell function.
As one of the key elements of the innate immune system, NK cells exhibit cytotoxic activity and also play a critical role in the early response to viruses. Moreover, it acts as a proper coordinator between the adaptive and innate immune systems. NK cells were also affected in both COVID-19 and SLE patients, with decreased cytotoxicity and increased levels of CD56DIM/CD16NEG NK cells in COVID-19 patients and decreased levels of CD56DIM subpopulations in SLE patients [35,36]. The total quantity of NK cells in SLE patients is reduced, and the existing NK cells are less cytotoxic than normal NK cells [37,38]. A similar phenomenon has been demonstrated in COVID-19, where patients had reduced total NK and CD8+ T cells and exhibited an functionally exhausted phenotype [39-41]. The expression of CXCR3, a marker of CD56BRIGHT NK cells, is increased in COVID-19 [42]. In addition, a number of other innate immune cell disorders may also play an important role in COVID-19, including a decrease in eosinophils and an increase in DC and activated neutrophils in bronchoalveolar lavage fluid [12]. The present findings indicate dysregulation of immune cells, specifically natural killer cells, in COVID-19 and SLE patients. Alterations in the cells’ frequency and phenotypic ratio appear to be associated with distinct inflammatory pathways, revealing both similarities and dissimilarities in the immune mechanisms between the two diseases.
Cytokine Storm
Cytokine storm is an overproduction of cytokines with inflammatory activity caused by inflammation and is present in many infectious and non-infectious diseases. Both SLE and COVID-19 can trigger a cytokine storm, which is characterized by the excessive production of inflammatory cytokines that leads to widespread inflammation. This phenomenon typically originates locally and can spread rapidly throughout the body through the systemic circulation, which is a common feature of many infectious and non-infectious diseases [43]. In SLE, some cytokines are elevated, such as B-lymphocyte stimulator (BLyS), TNF-α, type I interferon (IFN-I), IL-17, IL-6 and IL-18, while the role of cytokine storms in the pathogenesis of SLE is much more limited than that of COVID-19 [44]. Cytokine storm is considered to be an important part of the pathogenesis of COVID-19 and is associated with severe symptoms such as acute respiratory distress syndrome (ARDS) [45]. Elevated inflammatory cytokines in COVID-19 patients include TNF-α, IFN-γ, IL-1β, IL-2, IL-6, IL-10, inducible protein 10 (IP-10), granulocyte macrophage-colony stimulating factor (GM-CSF), and monocyte chemoattractant protein-1 (MCP-1) [46,47]. In summary, although the specific cytokines involved in the inflammatory cascade are different, both SLE and COVID-19 can cause inflammatory cytokine storms.
Type-I Interferon
IFN-I has received extensive attention as one of the cytokines involved in cytokine storm. It is widely accepted that the IFN-I response plays a critical role in the development of both rheumatoid and viral infections. Upon infection with SARS-CoV-2, the immune system produces IFN-I [48]. SLE patients have been found to have a dysregulated IFN-I response, characterized by elevated IFN-α and decreased expression of IFN-stimulated genes (ISGs) [49]. In a COVID-19 cohort study, levels of IFN-α and ISGs were found to be positively correlated with both viral load and disease severity [50]. This suggests that high viral load may drive the production of these cytokines during severe infection. However, it should be noted that IFN-I do not appear to directly control viral replication or reduce viral load [51]. Individuals who are asymptomatic and negative for antibodies may exhibit a strong IFN-I response, suggesting intrinsic resistance to severe COVID-19 [52]. Studies have shown that IFN-I production is reduced in COVID-19 patients [53]. In an international cohort, loss-of-function variants in IFN-I signaling were found in 3% of life-threatening COVID-19 patients, and plasmacytoid DCs were unable to produce IFN-I in response to SARS-CoV-2 [54]. Therefore, it can be speculated that due to more efficient viral clearance, high titers of IFN-I in SLE patients may prevent the development of COVID-19 in these patients [55]. Although IFN-1 plays an important role in both SLE and COVID19, the relationship between the titer level of IFN-1 and the severity of the two diseases as well as the mechanism of IFN-1 action need to be further investigated.
Antibody Reaction
Anti-dsDNA autoantibodies are considered to be a diagnostic marker for SLE and can lead to systemic deposition of immune complexes, especially in the joints, vascular, and renal system [56,57]. Approximately 34.5% of patients with severe COVID-19 infection have been reported to have elevated levels of autoantibodies, such as Antinuclear Antibodies (ANA) [58]. Anti-phospholipid antibodies (APL), including Lupus Anticoagulant (LA), anti-cardiolipin (aCL), and anti-β2 glycoprotein (β2GPI), which are common in SLE, have also been studied in patients with COVID-19 and may be associated with thrombotic events [59-62].
Overall, these differences in immune responses may help to explain why SLE patients are at increased risk for severe COVID-19 and why the management of COVID-19 in SLE patients may require a different approach than the general population. Further research is needed to fully understand the interplay between these two conditions and their impact on the immune system.
Treatment of COVID-19 in Patients with SLE
General Treatment of COVID-19 Infection
Since the emergence of COVID-19 in Wuhan, China in December 2019, numerous treatments have been explored. To date, several therapeutic strategies have been implemented clinically and received positive feedback. The common treatments include: a) oxygen therapy, which improves the patient’s dyspnea [63]; b) symptomatic treatment, including the use of drugs such as acetaminophen or ibuprofen that relieve fever and pain; c) antiviral medication; such as molnupiravir which is an oral active RdRp inhibitor with anti-RNA polymerase activity that is being investigated for the treatment of COVID-19 patients [64,65], and remdesivir which is superior to placebo but still need various therapeutic approaches to improve its efficacy in COVID-19 patients [66]. d) corticosteroids, corticosteroids may reduce inflammation and improve respiration, especially in patients with severe disease [67]. However, it also suppresses the immune response, which may impede viral clearance [67]. Thus, the use of corticosteroids has been controversial, and ongoing clinical trials address this issue [68,69]. e) immunoglobulin therapy, which provides immediate antiviral humoral immunity [70]; f) convalescent plasma and monoclonal antibodies, which are based on immune-mediated viral clearance [71]. Convalescent plasma therapy is a promising treatment option for COVID-19 [72]. A series of cases in China demonstrates that infusions of convalescent plasma from COVID-19 worked better. The U.S. Food and Drug Administration (FDA) has also approved the use of recovery plasma as an emergency treatment for individuals with COVID-19 [73]. Only two adverse events were found in a randomized trial of 52 patients who received recovery plasma therapy [74]. Another study found that recovery plasma transfusions were safe in hospitalized COVID-19 patients [75]. These two reports confirm the safety of plasma transfusions during the recovery period. However, there are no standardization or evidence-based reasons for donor selection, recipient transfusion indications, or convalescent plasma quality control due to a lack of understanding of the exact mechanism and precise therapeutics of convalescent plasma [67]. g) monoclonal antibodies, which are based on immune-mediated viral clearance [71]. Several neutralizing SARS-CoV-2 monoclonal antibodies are currently being evaluated in clinical trials [76]. These antibodies target specific regions of the viral spike, mainly of the IgG1 subtype, and are characterized by a long half-life [76]. This suggests that these antibodies can be administered in a single infusion. However, the bioavailability of antibodies to tissues and organs affected by COVID-19 remains unknown [77]. The above described general treatment options can also be applied to patients with SLE; however, given the specificity of SLE disease and treatment, certain drugs should be used with caution during treatment.
Role of Immunosuppressive Therapy in the Outcomes of COVID-19 in SLE Patients
Immunotherapy is considered a pivotal component of the therapeutic regimen for COVID-19 infection, while careful dosing is required in patients with autoimmune disorders such as SLE when receiving immunosuppressive drugs. Numerous studies have shown that immunosuppressive drugs increase the risk of hospitalization in SLE patients. Both the Mason’s and Ugarte-Gil’s studies confirmed that SLE patients treated with corticosteroids and rituximab may experience more severe clinical symptoms after COVID-19 infection. It has also been observed that untreated and active SLE patients experience more severe COVID-19 outcomes [20,78]. Furthermore, glucocorticoid dose was positively associated with a higher risk of hospitalization in SLE patients [79].
Recently, immunosuppressants used in SLE patients have been found to increase the risk of COVID-19 by reducing COVID-19 vaccine response, but rarely develop into severe disease [80,81]. Another study found that immunosuppressants reduce the reactivity of COVID-19 vaccine by reducing the level of antibodies in humoral immunity and the number of CD8T cells in adaptive immunity in SLE patients, which further revealed the possible mechanism of immune response that immunosuppressants can increase the risk of COVID-19 infection in SLE patients [23].
Overall, the role of immunosuppressive therapy on COVID-19 outcomes in SLE patients remains unclear and require further investigation. The decision to continue or modify immunosuppressive therapy in SLE patients with COVID-19 should be individualized based on the patient’s disease activity, comorbidities, and severity of COVID-19 infection. Further research is needed to fully understand the relationship between COVID-19 and SLE, especially the long-term effects of COVID-19 on the activity and outcome of SLE.
Prevention of COVID-19 in Patients with SLE
SLE patients need to be educated about the risks of COVID-19, the importance of social distancing, and wearing a mask to reduce the risk of infection. Therefore, SLE patients should avoid unnecessary hospital visits to reduce their risk of exposure to COVID-19. Teleconsultations through telemedicine can help SLE patients receive medical care and advice without leaving their homes [10,82,83]. And SLE patients who are taking immunosuppressive therapy may require medication adjustments to balance the risk of COVID-19 infection and SLE activity.
Increase the Levels of Vitamin D in SLE Patients
SLE patients are often accompanied by varying degrees of vitamin D deficiency, so an appropriate increase in vitamin D level may be beneficial to improve the immune response level of SLE patients, thus reducing the risk of COVID-19 infection [84]. In addition, vitamin D can inhibit the release of cytokines in inflammation to reduce the risk of inflammation caused by the cytokine storm of COVID-19 [85,86], and stimulate the production of neurotrophic factors, e.g. Nerve Growth Factor (NGF) to prevent nerve sensory loss in COVID-19 [87]. The antiviral and antibacterial effects of vitamin D have been verified in a variety of other viral infections [88,89], which is beneficial for COVID-19 intervention in many ways. However, the determination of vitamin D supplementation and its dosage has not been studied and confirmed.
Vaccination Against COVID-19 in Patients with SLE
Vaccination against COVID-19 is strongly recommended for SLE patients, as they are at increased risk for severe illness and complications. However, statistics show that COVID-19 vaccination may cause severe immune thrombocytopenia [90]. Therefore, in the process of using vaccination to prevent COVID-19, it is necessary to pay close attention to the detection of some normal indicators in the body, including platelets, and to discuss the benefits and disadvantages of vaccination in SLE patients [20].
According to statistics, the vaccine has a certain protective effect on SLE patients. However, the immune response in SLE patients is not adequate [91]. In many studies, seroconversion rates were significantly reduced in SLE patients after vaccination compared with healthy controls [80,91-94]. Therapies used to treat SLE, especially prednisone and immunosuppressive drugs, including GC, methotrexate, MMF, and RTX, may be the mainstay of treatment to limit the immune responses to the SARS-CoV-2 vaccine, although the response may still be sufficient to achieve seroprotection [7,20,80,91,92]. However, studies have shown that hydroxychloroquine has no significant negative impact on post-vaccine antibody responses [20]. There is evidence that vaccines are potentially less harmful in SLE patients, with SLEDAI scores, anti-DSDNA antibodies, and C3 and C4 levels remaining similar in SLE patients before and after vaccination [20,80].
Although the vaccine provides some protection for patients with SLE and prevents the development of severe COVID-19 infection, there are still some side effects. The online survey by Bartels et al. explores the safety of the vaccine, including local side effects in the form of pain and swelling as well as common systemic side effects such as fatigue and headaches [95]. Other studies from the United States, the European Union, India, and Mexico have reported similar results [80,96-100]. Vaccines can trigger and erupt autoimmunity due to the adjuvants and viral proteins used in the manufacturing process [101]. Thus, more serious side effects include SLE onset and elevated aCL and β2GPI levels [20,102].
In conclusion, although some side effects and reduced antibody production may occur in SLE patients vaccinated against COVID-19, it is unlikely to cause an outbreak and the antibody response is likely to reach protective concentrations in the serum.
The Impact of the COVID-19 Epidemic on Patients with SLE
The COVID-19 epidemic has profoundly affected patients with SLE, who are at high risk for severe disease and associated complications due to immunocompromised state resulting from both the disease and immunosuppressive therapy [103]. IFN-I autoantibodies produced in SLE patients reduce the powerful antiviral effect of IFN-I and are thought to be a factor in severe COVID-19 pneumonia [104]. The pandemic has further disrupted the standard medical care provided to SLE patients, leading to delays in diagnosis, treatment, and monitoring [105,106]. During the early stages of the COVID-19 epidemic, hydroxychloroquine was widely considered to be therapeutically effective for the prevention and management of the disease. As a result, the drug has become scarce, leaving many SLE patients around the world in short supply [107-109]. In addition, social distancing measures have adversely affected the mental health and well-being of SLE patients, who may already be struggling with chronic disease-induced anxiety, depression, and sleep disorders [110,111]. Hence, it is critical to provide SLE patients with necessary assistance and resources during the ongoing pandemic, including telemedicine services, counseling for mental health, and the provision of personal protective equipment.
Discussion
The multifaceted relationship between COVID-19 and SLE is an ongoing study that includes investigations of immune interactions between SARS-CoV-2 and SLE hosts, as well as epidemiology, comorbidities, symptomatology, and the role of immunosuppressive therapies. This review provides several insights into clinical practice and future research directions.
Maintaining an SLE treatment regimen during the COVID-19 pandemic is critical to preventing disease flares and reducing the risk of SLE-related complications. Treatment approaches must be personalized, considering disease activity, comorbidities, and medication tolerance. Given that SLE patients are more susceptible to COVID-19, they must take additional steps to prevent infection, including maintaining social distancing, wearing a mask, and practicing thorough hand hygiene [112]. Prompt recognition and treatment of COVID-19 in SLE patients is critical to reducing the risk of severe illness and mortality [99]. Due to the unique nature of SLE, treatment of COVID-19 in SLE patients should be adjusted according to their disease severity, comorbidities, and medication use [78]. Furthermore, managing SLE and COVID-19 in these patients requires a multidisciplinary approach that should involve the expertise of rheumatologists, infectious disease specialists, and primary care physicians. Overall, effective management of concurrent SLE and COVID-19 demands meticulous consideration of the balance between SLE treatment and COVID-19 prevention. To ensure the best possible care for patients with these conditions, open communication between healthcare providers and patients is crucial.
Regarding clinical practice, managing SLE during the COVID-19 pandemic requires appropriate counseling and healthcare services, as well as promotion vaccination. In addition to precautions such as wearing a mask and maintaining social distancing, vaccinations are strongly recommended for people with SLE, although certain side effects may occur. Treatment of SLE patients, including glucocorticoids and immunosuppressants, may cause severe clinical symptoms and increase hospitalization rates after COVID-19 infection. Nevertheless, discontinuation of SLE-related medications during treatment of COVID-19 infection is not recommended as doing so may exacerbate SLE activity. In conclusion, the consensus guidelines from the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) recommend continuing the use of immunosuppressive drugs and minimizing corticosteroid use in SLE patients with suspected or unconfirmed COVID-19 infection.
Thus, a multidisciplinary approach should be adopted in treatment, with careful consideration of treatment options and medications. Furthermore, given the common immunological mechanisms of COVID-19 and SLE, further research is required to determine the optimal therapeutic strategies. While this article examines similarities in immune responses between SLE and COVID-19, the treatment for both diseases still primarily focuses on hormonal therapy for inflammation suppression. The dosage of hormones and other non-traditional treatment options should be evaluated on an individual basis. For SLE patients with COVID-19, polarization may occur, indicating that severe patients experience more severe symptoms, whereas mild patients have lighter lung inflammation and recover more quickly. The COVID-19 pandemic has brought many challenges, even psychological issues, to SLE patients. On the one hand, it is imperative to maintain SLE in remission to mitigate the risk of COVID-19 complications and high hospitalization rates. On the other hand, the mental health status of SLE patients during the pandemic requires further attention. Telemedicine and appropriate dissemination of health information can alleviate anxiety and depression in these patients. However, the rapid development of COVID-19 undermines the durability of the conclusions, some of which may become outdated over time. Therefore, this review has limitations.
Regarding future research directions, more high-quality studies are necessary to further explore the relationship between SLE and COVID-19, with a focus on elucidating the underlying mechanisms of the increased susceptibility to COVID-19 in SLE patients and the effect of COVID-19 on disease activity and flares in SLE patients. Longitudinal studies of large numbers of SLE patients may be required to better understand the relationship between SLE and COVID-19. Furthermore, more research is needed to evaluate the effectiveness and safety of various treatment options for COVID-19 in SLE patients, including determining the optimal timing and dosage of these treatments. In addition, it is essential to conduct further research and evaluation regarding vaccines.
Overall, the implications for clinical practice and future research directions point to the need for further investigation in this area to better understand the relationship between SLE and COVID-19 and to develop effective strategies to manage and treat these conditions simultaneously.
Authors’ Contributions
XC, CS, YG and GZ: Conceptualized, collected the data, performed the literature research and wrote the manuscript. XC, CS and YG: Prepared Figure 1 and critical revision of the manuscript. CS, BC, LX and HS: Participated in the design of the review and helped to draft the manuscript. GZ: Provided guidance and critical advice throughout the manuscript drafting, and help to revise the manuscript.
Funding
This research received no external funding.
Declarations
Ethics Approval and Consent to Participate
The manuscript does not contain clinical studies and patient data.
Consent for Publication
We confirm that this manuscript has not been published elsewhere and is also not under consideration by another journal. All authors have approved the manuscript and agree with the submission.
References
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC (2020) Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. Jama 324: 782-793. [crossref]
- Oke V, Brauner S, Larsson A, Gustafsson J, Zickert A, Gunnarsson I, Svenungsson E (2017) IFN-λ1 with Th17 axis cytokines and IFN-α define different subsets in systemic lupus erythematosus (SLE). Arthritis Research & Therapy 19: 139. [crossref]
- Zickert A, Amoudruz P, Sundström Y, Rönnelid J, Malmström V, Gunnarsson I (2015) IL-17 and IL-23 in lupus nephritis – association to histopathology and response to treatment. BMC Immunology 16: 7. [crossref]
- Fava A, Petri M (2019) Systemic lupus erythematosus: Diagnosis and clinical management. Journal of Autoimmunity 96: 1-13. [crossref]
- Doria A, Canova M, Tonon M, Zen M, Rampudda E, et al. (2008) Infections as triggers and complications of systemic lupus erythematosus. Autoimmunity Reviews 8: 24-28.
- Qin L, Qiu Z, Hsieh E, Geng T, Zhao J, et al. (2019) Association between lymphocyte subsets and cytomegalovirus infection status among patients with systemic lupus erythematosus: A pilot study. Medicine 98: e16997. [crossref]
- Mehta P, Gasparyan AY, Zimba O, Kitas GD (2022) Systemic lupus erythematosus in the light of the COVID-19 pandemic: infection, vaccination, and impact on disease management. Clinical Rheumatology 41: 2893-2910. [crossref]
- Mathian A, Mahevas M, Rohmer J, Roumier M, Cohen-Aubar F, et al. (2020) Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Annals of the Rheumatic Diseases 79: 837-839. [crossref]
- Gartshteyn Y, Askanase AD, Schmidt NM, Bernstein EJ, Khalili L, et al. (2020) COVID-19 and systemic lupus erythematosus: a case series. The Lancet Rheumatology 2: e452-e454. [crossref]
- Bozzalla Cassione E, Zanframundo G, Biglia A, Codullo V, Montecucco C, Cavagna L (2020) COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine. Annals of the Rheumatic Diseases 79: 1382-1383. [crossref]
- Espinosa G, Prieto-González S, Llevadot M, Marco-Hernández J, Martínez-Artuña A, et al. (2021) The impact of SARS-CoV-2 coronavirus infection in patients with systemic lupus erythematosus from a single center in Catalonia. Clinical Rheumatology 40: 2057-2063. [crossref]
- Rodríguez Y, Novelli L, Rojas M, De Santis M, Acosta-Ampudia Y, et al. (2020) Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. Journal of Autoimmunity 114: 102506. [crossref]
- Holtappels R, Schader SI, Oettel O, Podlech J, Seckert CK, et al. (2020) Insufficient Antigen Presentation Due to Viral Immune Evasion Explains Lethal Cytomegalovirus Organ Disease After Allogeneic Hematopoietic Cell Transplantation. Frontiers in Cellular and Infection Microbiology 10: 157. [crossref]
- Sawalha AH, Zhao M, Coit P, Lu Q (2020) Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clinical Immunology (Orlando, Fla.) 215: 108410. [crossref]
- Felsenstein S, Herbert JA, McNamara PS, Hedrich CM (2020) COVID-19: Immunology and treatment options. Clinical Immunology (Orlando, Fla.) 215: 108448. [crossref]
- Ramirez GA, Gerosa M, Beretta L, Bellocchi C, Argolini LM, et al. (2020) COVID-19 in systemic lupus erythematosus: Data from a survey on 417 patients. Seminars in Arthritis and Rheumatism 50: 1150-1157.
- Fu XL, Qian Y, Jin XH, Yu HR, Du L, et al. (2022) COVID-19 in patients with systemic lupus erythematosus: A systematic review. Lupus 31: 684-696. [crossref]
- Kondo Y, Kaneko Y, Oshige T, Fukui H, Saito S, et al. (2021) Exacerbation of immune thrombocytopenia triggered by COVID-19 in patients with systemic lupus erythematosus. Ann Rheum Dis 80: e77. [crossref]
- Slimani Y, Abbassi R, El Fatoiki FZ, Barrou L, Chiheb S (2021) Systemic lupus erythematosus and varicella-like rash following COVID-19 in a previously healthy patient. J Med Virol 93: 1184-1187. [crossref]
- Mason A, Anver H, Lwin M, Holroyd C, Faust SN, et al. (2021) Lupus, vaccinations and COVID-19: What we know now. Lupus 30: 1541-1552. [crossref]
- Fernandez-Ruiz R, Masson M, Kim MY, Myers B, Haberman RH, et al. (2020) Leveraging the United States Epicenter to Provide Insights on COVID-19 in Patients With Systemic Lupus Erythematosus. Arthritis & rheumatology (Hoboken, N.J.) 72: 1971-1980. [crossref]
- Bertoglio IM, Valim JML, Daffre D, Aikawa NE, Silva CA, et al. (2021) Poor Prognosis of COVID-19 Acute Respiratory Distress Syndrome in Lupus Erythematosus: Nationwide Cross-Sectional Population Study Of 252 119 Patients. ACR Open Rheumatology 3: 804-811. [crossref]
- Solé C, Domingo S, Vidal X, Cortés-Hernández J (2022) Humoral and cellular response in convalescent COVID-19 lupus patients. Scientific Reports 12: 13787. [crossref]
- Sakthiswary R, Chuah HY, Chiang KS, Liew YS, Muhammad Aizat NA (2021) COVID-19 in systemic lupus erythematosus: A pooled analysis and systematic review of case reports and series. Lupus 30: 1946-1954. [crossref]
- Sukhdeo S, Negroponte E, Rajasekhar H, Gaur S, Horton DB, et al. (2021) Acute respiratory distress syndrome and COVID-19 in a child with systemic lupus erythematosus. Lupus 30: 836-839. [crossref]
- Walters HM, Mian Z, Thomas L, Cerise J, Eberhard BA, et al. (2022) Seroprevalence and clinical outcomes of SARS-CoV-2 in paediatric patients with rheumatic disease. Rheumatology (Oxford, England) 61: Si112-si119. [crossref]
- Clemente D, Udaondo C, de Inocencio J, Nieto JC, Del Río PG, et al. (2021) Clinical characteristics and COVID-19 outcomes in a regional cohort of pediatric patients with rheumatic diseases. Pediatric Rheumatology Online Journal 19: 162. [crossref]
- Smeele HT, Perez-Garcia LF, Grimminck K, Schoenmakers S, Mulders AG, et al. (2021) Systemic lupus erythematosus and COVID-19 during pregnancy. Lupus 30: 1188-1191. [crossref]
- Chen L, Li Q, Zheng D, Jiang H, Wei Y, et al. (2020) Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. N Engl J Med 382: e100. [crossref]
- Schoenmakers S, Snijder P, Verdijk RM, Kuiken T, Kamphuis SSM, et al. (2021) Severe Acute Respiratory Syndrome Coronavirus 2 Placental Infection and Inflammation Leading to Fetal Distress and Neonatal Multi-Organ Failure in an Asymptomatic Woman. J Pediatric Infect Dis Soc 10: 556-561. [crossref]
- Fenizia C, Biasin M, Cetin I, Vergani P, Mileto D, et al. (2020) Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun 11: 5128.
- Frater JL, Zini G, d’Onofrio G, Rogers HJ (2020) COVID-19 and the clinical hematology laboratory. International Journal of Laboratory Hematology 42: 11-18. [crossref]
- Shan J, Jin H, Xu, Y (2020) T Cell Metabolism: A New Perspective on Th17/Treg Cell Imbalance in Systemic Lupus Erythematosus. Frontiers in Immunology 11: 1027. [crossref]
- Parackova Z, Bloomfield M, Klocperk A, Sediva A (2021) Neutrophils mediate Th17 promotion in COVID-19 patients. Journal of Leukocyte Biology 109: 73-76. [crossref]
- Leem G, Cheon S, Lee H, Choi SJ, Jeong S, et al. (2021) Abnormality in the NK-cell population is prolonged in severe COVID-19 patients. The Journal of Allergy and Clinical Immunology 148: 996-1006.e1018. [crossref]
- Liu M, Liu J, Zhang X, Xiao Y, Jiang G, et al. (2021) Activation status of CD56(dim) natural killer cells is associated with disease activity of patients with systemic lupus erythematosus. Clinical Rheumatology 40: 1103-1112. [crossref]
- Park YW, Kee SJ, Cho YN, Lee EH, Lee HY, et al. (2009) Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis and Rheumatism 60: 1753-1763. [crossref]
- Schleinitz N, Vély F, Harlé JR, Vivier E (2010) Natural killer cells in human autoimmune diseases. Immunology 131: 451-458.
- Wang F, Nie J, Wang H, Zhao Q, Xiong Y, et al. (2020) Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. The Journal of Infectious Diseases 221: 1762-1769. [crossref]
- Li D, Chen Y, Liu H, Jia Y, Li F, et al. (2020) Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study. Signal Transduction and Targeted Therapy 5: 62. [crossref]
- Zheng M, Gao Y, Wang G, Song G, Liu S, et al. (2020) Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cellular & Molecular Immunology 17: 533-535. [crossref]
- Leng Z, Zhu R, Hou W, Feng Y, Yang Y, et al. (2020) Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging and Disease 11: 216-228. [crossref]
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, et al. (2012) Into the eye of the cytokine storm. Microbiology and molecular biology reviews: MMBR 76: 16-32. [crossref]
- Yap DY, Lai KN (2013) The role of cytokines in the pathogenesis of systemic lupus erythematosus – from bench to bedside. Nephrology (Carlton, Vic.) 18: 243-255. [crossref]
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 395: 497-506. [crossref]
- Hu B, Huang S, Yin L (2021) The cytokine storm and COVID-19. Journal of Medical Virology 93: 250-256. [crossref]
- Jiang Y, Rubin L, Peng T, Liu L, Xing X, et al. (2022) Cytokine storm in COVID-19: from viral infection to immune responses, diagnosis and therapy. International Journal of Biological Sciences 18: 459-472. [crossref]
- Lee JH, Kanwar B, Khattak A, Balentine J, Nguyen NH, et al. (2022) COVID-19 Molecular Pathophysiology: Acetylation of Repurposing Drugs. International Journal of Molecular Sciences 2022: 23. [crossref]
- Yao Y, Higgs BW, Richman L, White B, Jallal B (2010) Use of type I interferon-inducible mRNAs as pharmacodynamic markers and potential diagnostic markers in trials with sifalimumab, an anti-IFNα antibody, in systemic lupus erythematosus. Arthritis Research & Therapy 2010: 12. [crossref]
- Park A, Iwasaki A (2020) Type I and Type III Interferons – Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host & Microbe 27: 870-878. [crossref]
- Lucas C, Wong P, Klein J, Castro TBR, Silva J, et al. (2020) Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584: 463-469.
- Arkin LM, Moon JJ, Tran JM, Asgari S, O’Farrelly C, et al. (2021) From Your Nose to Your Toes: A Review of Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic‒Associated Pernio. The Journal of Investigative Dermatology 141: 2791-2796. [crossref]
- Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, et al. (2020) Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science (New York, N.Y.) 2020: 370. [crossref]
- Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science (New York, N.Y.) 2020: 370. [crossref]
- Hejazian SS, Hejazian SM, Farnood F, Abedi Azar S (2022) Dysregulation of immunity in COVID-19 and SLE. Inflammopharmacology 30: 1517-1531. [crossref]
- Rekvig OP (2015) Anti-dsDNA antibodies as a classification criterion and a diagnostic marker for systemic lupus erythematosus: critical remarks. Clinical and Experimental Immunology 179: 5-10. [crossref]
- Spihlman AP, Gadi N, Wu SC, Moulton VR (2020) COVID-19 and Systemic Lupus Erythematosus: Focus on Immune Response and Therapeutics. Frontiers in Immunology 11: 589474. [crossref]
- Vlachoyiannopoulos PG, Magira E, Alexopoulos H, Jahaj E, Theophilopoulou K, et al. (2020) Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Annals of the Rheumatic Diseases 79: 1661-1663. [crossref]
- Zuo Y, Estes SK, Ali RA, Gandhi AA, Yalavarthi S, et al. (2020) Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Science Translational Medicine 2020: 12. [crossref]
- Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, et al. (2020) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Medicine 46: 1089-1098. [crossref]
- Harzallah I, Debliquis A, Drénou B (2020) Lupus anticoagulant is frequent in patients with Covid-19: Response to reply. Journal of Thrombosis And Haemostasis: JTH 18: E3-e4. [crossref]
- Zhang Y, Xiao M, Zhang S, Xia P, Cao W, et al. (2020) Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. The New England Journal of Medicine 382: e38. [crossref]
- Kolb M Dinh-Xuan AT, Brochard L (2021) Guideline-directed management of COVID-19: Do’s and Don’ts. The European Respiratory Journal 2021: 57. [crossref]
- Pourkarim F, Pourtaghi-Anvarian S, Rezaee H (2022) Molnupiravir: A new candidate for COVID-19 treatment. Pharmacology Research & Perspectives 2022: 10. [crossref]
- Imran M, Kumar Arora M, Asdaq SMB, Khan SA, Alaqel SI, et al. (2021) Discovery, Development, and Patent Trends on Molnupiravir: A Prospective Oral Treatment for COVID-19. Molecules (Basel, Switzerland) 2021: 26. [crossref]
- Rosenberg K (2021) Remdesivir in The Treatment of COVID-19. The American Journal of Nursing 121: 55.
- Rehman SU, Yoo HH (2021) COVID-19 challenges and its therapeutics. Biomed Pharmacother 142: 112015. [crossref]
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. (2021) Dexamethasone in Hospitalized Patients with Covid-19. The New England Journal of Medicine 384: 693-704.
- Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. (2020) Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA324: 1330-1341. [crossref]
- van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, Derde L, Leavis H, et al. (2021) A guide to immunotherapy for COVID-19. Nature Medicine 28: 39-50. [crossref]
- Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, et al. (2021) Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. The New England Journal of Medicine 384: 610-618. [crossref]
- Kaku Y, Kuwata T, Zahid HM, Hashiguchi T, Noda T, et al. (2021) Resistance of SARS-CoV-2 variants to neutralization by antibodies induced in convalescent patients with COVID-19. Cell Rep 36: 109385. [crossref]
- Ruggiero HA, Pérez Isquierdo F, Milani HA, Barri A, Val A, et al. (1986) [Treatment of Argentine hemorrhagic fever with convalescent’s plasma. 4433 cases]. Presse Med 15: 2239-2242. [crossref]
- Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, et al. (2021) Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev 5: Cd013600. [crossref]
- Li L, Zhang W, Hu Y, Tong X, Zheng S, et al. (2021) Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA 324: 460-470. [crossref]
- Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, Fotiou D, Migkou M, et al. (2021) Emerging treatment strategies for COVID-19 infection. Clin Exp Med 21: 167-179. [crossref]
- Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, et al. (2021) Clinical Characteristics of Refractory Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis 73: e4208-e4213. [crossref]
- Ugarte-Gil MF, Alarcón GS, Izadi Z, Duarte-García A, Reátegui-Sokolova C, et al. (2022) Characteristics associated with poor COVID-19 outcomes in individuals with systemic lupus erythematosus: data from the COVID-19 Global Rheumatology Alliance. Annals of The Rheumatic Diseases 81: 970-978. [crossref]
- Gendebien Z, von Frenckell C, Ribbens C, André B, Thys M, et al. (2021) Systematic analysis of COVID-19 infection and symptoms in a systemic lupus erythematosus population: correlation with disease characteristics, hydroxychloroquine use and immunosuppressive treatments. Annals of The Rheumatic Diseases 80: e94. [crossref]
- Izmirly PM, Kim MY, Samanovic M, Fernandez-Ruiz R, Ohana S, et al. (2022) Evaluation of Immune Response and Disease Status in Systemic Lupus Erythematosus Patients Following SARS-CoV-2 Vaccination. Arthritis & Rheumatology (Hoboken, N.J.) 74: 284-294. [crossref]
- Petri M, Joyce D, Haag K, Fava A, Goldman DW, et al. (2023) Effect of Systemic Lupus Erythematosus and Immunosuppressive Agents on COVID-19 Vaccination Antibody Response. Arthritis Care & Research 2023. [crossref]
- Perniola S, Alivernini S, Varriano V, Paglionico A, Tanti G, et al. (2021) Telemedicine will not keep us apart in COVID-19 pandemic. Annals of The Rheumatic Diseases 80: e48. [crossref]
- López-Medina C, Escudero A, Collantes-Estevez E (2021) COVID-19 pandemic: an opportunity to assess the utility of telemedicine in patients with rheumatic diseases. Annals of The Rheumatic Diseases 80: e50. [crossref]
- Ao T, Kikuta J, Ishii M (2021) The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 11. [crossref]
- Khoo AL, Chai LY, Koenen HJ, Oosting M, Steinmeyer A, et al. (2011) Vitamin D(3) down-regulates proinflammatory cytokine response to Mycobacterium tuberculosis through pattern recognition receptors while inducing protective cathelicidin production. Cytokine 55: 294-300. [crossref]
- Vabret N, Britto GJ, Gruber C, Hegde S, Kim J, et al. (2020) Immunology of COVID-19: Current State of the Science. Immunity 52: 910-941. [crossref]
- Xu Y, Baylink DJ, Chen CS, Reeves ME, Xiao J, et al. (2020) The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. Journal of Translational Medicine 18: 322. [crossref]
- Teymoori-Ra M, Shokri F, Salimi V, Marashi SM (2019) The interplay between vitamin D and viral infections. Reviews In Medical Virology 29: e2032. [crossref]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, et al. (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science (New York, N.Y.) 311: 1770-1773. [crossref]
- Shah SRA, Dolkar S, Mathew J, Vishnu P (2021) COVID-19 vaccination associated severe immune thrombocytopenia. Experimental Hematology & Oncology 10: 42. [crossref]
- Tang W, Gartshteyn Y, Ricker E, Inzerillo S, Murray S, et al. (2021) The Use of COVID-19 Vaccines in Patients with SLE. Current Rheumatology Reports 23: 79. [crossref]
- Furer V, Eviatar T, Zisman D, Peleg H, Paran D, et al. (2021) Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Annals of The Rheumatic Diseases 80: 1330-1338. [crossref]
- Boekel L, Steenhuis M, Hooijberg F, Besten YR, van Kempen ZLE, et al. (2021) Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. The lancet. Rheumatology 3: e778-e788. [crossref]
- So H, Li T, Chan V, Tam LS, Chan PK (2022) Immunogenicity and safety of inactivated and mRNA COVID-19 vaccines in patients with systemic lupus erythematosus. Therapeutic Advances In Musculoskeletal Disease 14: 1759720×221089586. [crossref]
- Bartels LE, Ammitzbøll C, Andersen JB, Vils SR, Mistegaard CE, et al. (2021) Local and systemic reactogenicity of COVID-19 vaccine BNT162b2 in patients with systemic lupus erythematosus and rheumatoid arthritis. Rheumatology International 41: 1925-1931. [crossref]
- Cherian S, Paul A, Ahmed S, Alias B, Manoj M, et al. (2021) Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post-vaccination cross-sectional survey. Rheumatology International 41: 1441-1445. [crossref]
- Esquivel-Valerio JA, Skinner-Taylor CM, Moreno-Arquieta IA, Cardenas-de la Garza JA, Garcia-Arellano G, et al. (2021) Adverse events of six COVID-19 vaccines in patients with autoimmune rheumatic diseases: a cross-sectional study. Rheumatology International 41: 2105-2108. [crossref]
- Moyon Q, Sterlin D, Miyara M, Anna F, Mathian A, et al. (2022) BNT162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Annals of The Rheumatic Diseases 81: 575-583. [crossref]
- Ferri C, Ursini F, Gragnani L, Raimondo V, Giuggioli D, et al. (2021) Impaired immunogenicity to COVID-19 vaccines in autoimmune systemic diseases. High prevalence of non-response in different patients’ subgroups. Journal of Autoimmunity 125: 102744. [crossref]
- Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, et al. (2021) Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Annals of The Rheumatic Diseases 80: 1100-1101. [crossref]
- van der Laan JW, Gould S, Tanir JY (2015) Safety of vaccine adjuvants: focus on autoimmunity. Vaccine 33: 1507-1514. [crossref]
- Felten R, Kawka L, Dubois M, Ugarte-Gil MF, Fuentes-Silva Y, et al. (2021) Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. The lancet. Rheumatology 3: e613-e615. [crossref]
- Bournia VK, Fragoulis GE, Mitrou P, Mathioudakis K, Tsolakidis A, et al. (2022) Different Covid-19 Outcomes Among Systemic Rheumatic Diseases: A Nation-wide Cohort Study. Rheumatology (Oxford, England) 2022. [crossref]
- Mathian A, Breillat P, Dorgham K, Bastard P, Charre C, et al. (2022) Lower disease activity but higher risk of severe COVID-19 and herpes zoster in patients with systemic lupus erythematosus with pre-existing autoantibodies neutralising IFN-α. Annals of The Rheumatic Diseases 81: 1695-1703. [crossref]
- Chen C, Yao B, Yan M, Su KE, Wang H, et al. (2020) The Plight of Patients with Lupus Nephritis during the Outbreak of COVID-19 in Wuhan, China. The Journal of Rheumatology 47: 1452. [crossref]
- Rathi M, Singh P, Bi HP, Shivanna A, Kavadichanda C, et al. (2021) Impact of the COVID-19 pandemic on patients with systemic lupus erythematosus: Observations from an Indian inception cohort. Lupus 30: 158-164. [crossref]
- Kim AHJ, Sparks JA, Liew JW, Putman MS, Berenbaum F, et al. (2020) A Rush to Judgment? Rapid Reporting and Dissemination of Results and Its Consequences Regarding the Use of Hydroxychloroquine for COVID-19. Annals of Internal Medicine 172: 819-821. [crossref]
- Yazdany J, Kim AHJ (2020) Use of Hydroxychloroquine and Chloroquine During the COVID-19 Pandemic: What Every Clinician Should Know. Annals of Internal Medicine 172: 754-755. [crossref]
- Das RR, Jaiswal N, Dev N, Jaiswal N, Naik SS, et al. (2020) Efficacy and Safety of Anti-malarial Drugs (Chloroquine and Hydroxy-Chloroquine) in Treatment of COVID-19 Infection: A Systematic Review and Meta-Analysis. Frontiers In Medicine 7: 482. [crossref]
- Wang Y, Di Y, Ye J, Wei W (2021) Study on the public psychological states and its related factors during the outbreak of coronavirus disease 2019 (COVID-19) in some regions of China. Psychology, Health & Medicine 26: 13-22. [crossref]
- Wańkowicz P, Szylińska A, Rotter I (2020) Evaluation of Mental Health Factors among People with Systemic Lupus Erythematosus during the SARS-CoV-2 Pandemic. Journal of Clinical Medicine 9: 2872. [crossref]
- Teslya A, Pham TM, Godijk NG, Kretzschmar ME, Bootsma MCJ, et al. (2020) Impact of self-imposed prevention measures and short-term government-imposed social distancing on mitigating and delaying a COVID-19 epidemic: A modelling study. Plos Medicine 17: e1003166. [crossref]