DOI: 10.31038/CST.2023832
Abstract
Brown and beige adipocytes dissipate energy in a non-shivering thermogenesis manner, exerting beneficial impact on metabolic homeostasis. RGCC (protein regulator of cell cycle) is BAT-enriched protein, while its role in thermogenic adipocytes remains unknown. RGCC is upregulated by acute cold challenge or β3 agonist in BAT and iWAT. Lack of RGCC constrains expression of a set of thermogenic genes in brown and beige adipocytes. Conversely, ectopic expression of RGCC drives a full of program of thermogenesis and promotes browning. Pgc1α knockdown obviously prevents expression of RGCC-elicited thermogenic genes. Together, these findings uncover that physiological role for RGCC-mediated activation of the thermogenic program in adipocytes.
Keywords
RGCC, Pgc1α, Brown adipocytes, Beige adipocytes, Thermogenesis
Introduction
Brown and beige adipocytes have long been well recognized as an organ specialized for energy expenditure by dissipating energy as heat in a process called non-shivering thermogenesis [1]. Beige adipocytes, resided in white adipose tissue (WAT), are induced by chronic cold exposure, exercise, and treatment with other external cues [2]. These adipocytes have a multilocular lipid droplet morphology, specifically expressing a group of thermogenic genes, involving in uncoupling protein 1(Ucp1)-dependent or Ucp1-independent pathway, which contributes to generate heat [3]. Both brown adipose tissue (BAT) and beige adipocytes are found in rodents and humans [4-6]. Recent studies further demonstrate that the BAT-positive group were younger and showed lower metabolism-related parameters such as the body mass index (BMI), body fat mass, glycated hemoglobin (HbA1c), glucose, total cholesterol and the LDL-cholesterol [4,7]. These findings lead to the proposal that increasing BAT mass/ activity or beige adipocytes transformation might be a promising therapeutic strategy for metabolic disease. RGCC (protein regulator of cell cycle), also known as RGC-32 (response gene to complement 32 protein), is an ancient and conserved intracellular proteins existed in all eukaryotes. It was found to function as a role in the cell cycle, cell differentiation, fibrosis and cell metabolism in various physiological and pathological states [8-16]. The previous data revealed that RGCC is a unique protein expressed in brown adipocytes, and regulates adipogenesis in the Pdgrfa +/ Thy1– (LP) cells sorted from E14.5 embryos to determine adipocyte fate. Adipocytes, derived from multipotent mesenchymal stem cells, goes through two phases of adipogenesis. The first phase, known as determination, converts the pluripotent stem cell into the adipocyte lineage which lost the potential to differentiate into other cell type. In the second phase, the preadipocytes give rise to mature adipocytes, which is called terminal differentiation [17]. However, the role of RGCC in the terminal differentiation stage of brown adipocytes, especially in the regulation of thermogenesis, had not yet been studied.
In this study, we found that RGCC expression in BAT and iWAT was strongly induced by β3-adrenergic signaling. Depletion of RGCC in brown and beige adipocytes led to defect in maintain of thermogenesis. Consistently, RGCC overexpression strongly promotes expression of BAT-selective gene. Mechanistically, RGCC drives a full program of thermogenesis in part through Pgc1α. Our studies identified RGCC as a major regulator for thermogenesis of brown and beige adipocytes, and may provide a potential therapeutic target for obesity and metabolic diseases.
Results
RGCC is BAT-enriched Protein and Triggered by Cold Exposure
BAT-enriched regulators have the potential function in adaptive thermogenesis. To identify the presumed activators, we analyzed a previously published RNA-Seq datasets of mouse tissues, and found that the RGCC gene was highly expressed in adipose tissues [18]. Similarly, real time-quantitative PCR (RT-PCR) analysis confirmed that RGCC was mostly expressed in the brown fat and white fat of adult mice (Figure 1A). While the protein levels of RGCC were strikingly higher in BAT, and were extremely lower in iWAT and eWAT compared to BAT (Figure 1A). Cold exposure or noradrenergic cascade activate thermogenesis in BAT and recruit beige adipocytes in WAT. RGCC mRNA level and protein level in BAT were evidently provoked upon mice were subjected to acute cold exposure (Figure 1B). Chronic activation of β3-signaling by Cl316,243 agonist certainly induced RGCC expression in BAT and iWAT (Figure 1C and 1D). Moreover, the RGCC messenger levels were progressively inducted during adipocyte differentiation and peaked at late stage in immortalized brown adipocytes and C3H10T1/2 cells (a beige-like adipocyte model) (Figure 1E and 1F). These data suggested RGCC may be participated in maintaining thermogenesis of brown adipocytes and browning process of white adipocytes.
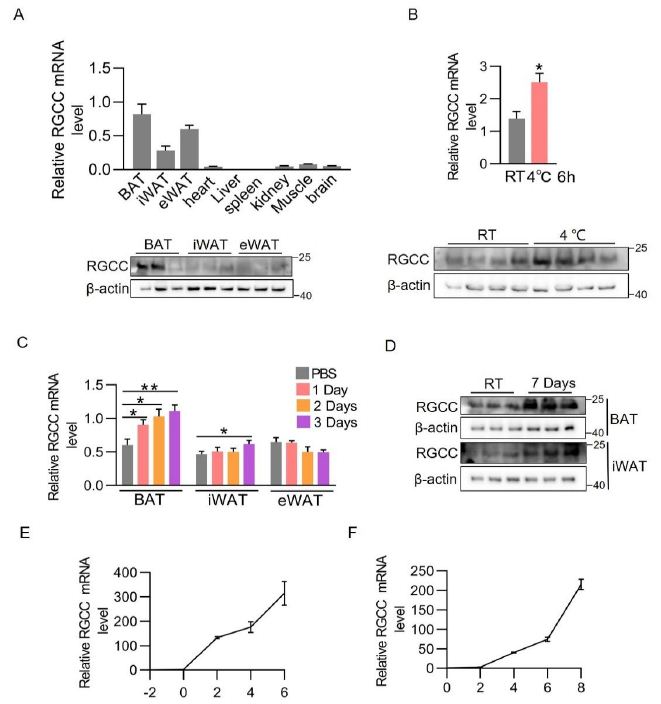
Figure 1: RGCC is a BAT-enriched protein and induced by cold challenge.
(A) RGCC mRNA levels in several tissues (top) (n=5 each group) and RGCC protein levels in adipose tissues of 8-week-old C57BL/6J mice (bottom).
(B) RGCC mRNA level (top) and protein expression (bottom) in BAT after cold challenge for 6 h (n=4 each group).
(C-D) RGCC mRNA levels (C) and protein levels (D) in adiposes of mice. C57BL/6J mice were intraperitoneally (i.p.) injected Cl316,243 or phosphate-buffered saline at a dose 0.5 ug /g body weight for 1, 2, or 3 days.
(E-F) qPCR analysis of RGCC mRNA expression in differentiating immortalized brown adipocytes (E) and C3H10T1/2 cells (F) (n=3 independent cultures).
Data are expressed as mean ± SEM of biological independent samples. Two-tailed unpaired Student’s t-test was performed. *P<0.05.
RGCC Perturbation in Brown Adipocytes Impairs Thermogenesis
To investigate whether RGCC is responsible for function of brown adipocytes, we knocked down RGCC with lentiviral short hairpin RNAs (shRNAs) in immortalized brown preadipocytes, which then were induced to differentiation. The previous study indicated that RGCC depletion by 80% in the Pdgrfa +/ Thy1– cells isolated from E14.5 embryos obviously hinders adipogenesis [19]. While we knocked down RGCC by 50-60% from preadipocytes which would not affect adipocyte differentiation as indicated by picture during differentiation course and similar expression levels of common fat marker genes ap2 and Pparγ (Figure 2A, 2B, 2D and 2E). RGCC-knockdown adipocytes were accumulated more enlarged lipid droplets and triglyceride (TG) content (Figure 2B and 2C), indicating weaker energy metabolism. Knockdown of Rgcc reduced a broad of BAT-selective gene expression, including Ucp1, Cox7a1, Cpt1b, which was further confirmed by western blot analysis (Figure 2D and 2E). Importantly, the effect of Rgcc knockdown is functionally relevant, as basal and uncoupled oxygen consumption rate was greatly reduced (Figure 2F). FCCP-induced maximal respiration was also lower than controls (Figure 2F). Collectively, these data demonstrates that RGCC is required for thermogenesis in brown adipocytes.
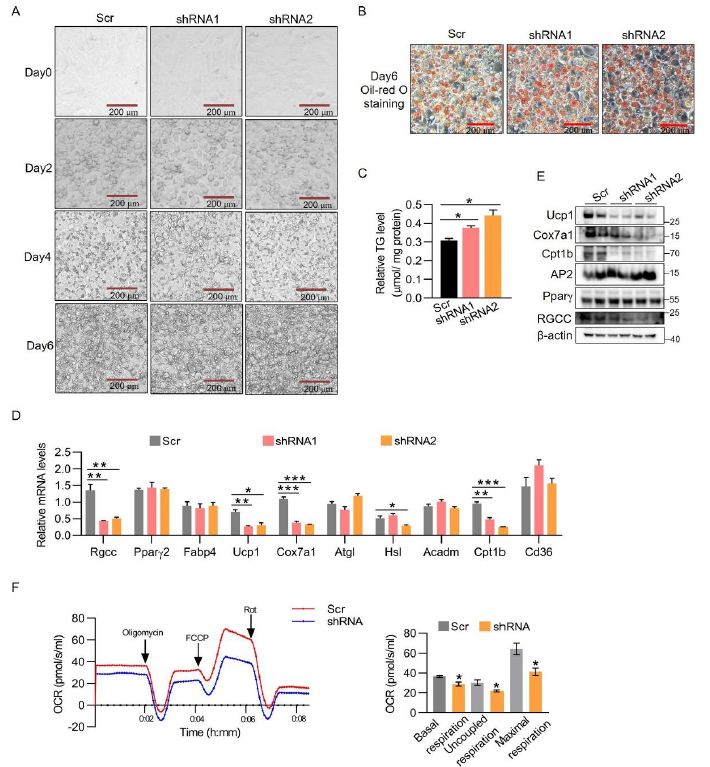
Figure 2: RGCC modulates the thermogenesis gene program in brown adipocytes.
(A) Representative images of brown adipocytes during differentiation. The immortalized brown preadipocytes were infected with shRGCC or scramble knockdown lentiviruses and differentiated. Scale bar is 200 μm.
(B) Representative Oil-red staining of mature adipocyte generated as in (A). Scale bar is 200 μm.
(C) TG contents in RGCC knockdown cells generated as in (A) (n=3 each group).
(D-E) Gene mRNA levels (D) and protein levels (E) in RGCC knockdown cells from (A) (n=3 each group).
(F) Oxygen consumption rate (OCR) in brown adipocytes generated as in (A) by Oroboros O2K. Oligomycin (Oligo), FCCP and Rotenone (Rot) were added at the time points indicated by the arrows (n=3 independent cultures). Basal respiration, uncoupled respiration, and maximal respiration were showed in the right panel.
Data are expressed as mean ± SEM of biological independent samples. Two-tailed unpaired Student’s t-test was performed. *P<0.05, **P<0.01, ***P<0.001.
RGCC Depletion Restrains Browning of White Adipocytes
We observed that the RGCC protein is triggered in iWAT when subjected to activation of chronic β3-adregenic signaling, denoting it may be involved in white fat browning (Figure 1C and 1D). C3H10T1/2 derived from mesenchymal stem cells were utilized to induce beige adipocytes. Similar to what is observed in brown adipocytes, RGCC knockdown (nearly 50-60%) had no effect on adipogenesis, but clearly increased larger lipid droplets and more intracellular triglyceride TG content (Figure 3A and 3C). At a molecular level, diminishing RGCC expression in beige adipocytes led to systematic decreased expression of adipocyte genes including Ucp1, Cox7a1 and Cpt1b, which basically phenocopied the knockdown brown adipocytes (Figure 3C and 3D). Moreover, Rgcc disruption constrained the basal and FCCP-stimulated OCR of adipocytes (Figure 3E). Together, RGCC deficiency in beige adipocytes inhibits the expression of BAT-specific genes and β-oxidation genes, hinders the function of beige adipocytes.
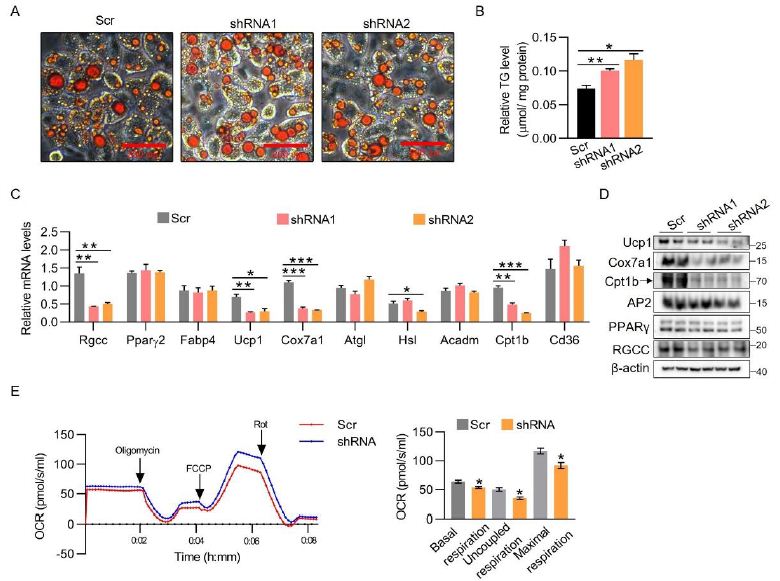
Figure 3: RGCC depletion constrains BAT-selective gene expression in beige cells.
(A) C3H10T1/2 preadipocytes were infected with RGCC knockdown lentiviruses and then subjected to the adipogenic differentiation process. Oil-red staining was performed on day 8.
(B) TG contents were performed in C3H10T1/2 adipocytes generated as in (A) (n=3 each group).
(C-D) mRNA levels (C) and protein levels (D) were analyzed in C3H10T1/2 adipocytes generated as in (A) (n=3 each group).
(E) OCR analysis in C3H10T1/2 adipocytes generated as in (A). Diagram of basal respiration, uncoupled respiration and maximal respiration were showed in the right panel (n=3 each group).
Data are expressed as mean ± SEM of biological independent samples. Two-tailed unpaired Student’s t-test was performed. *P<0.05, **P<0.01, ***P<0.001.
Pgc1α Mediated RGCC-dependent Thermogenesis
To study the gain-of-function of RGCC in adipocytes, we used lentiviruses to stably overexpress RGCC during differentiation of adipocytes. Ectopic expression of RGCC did not affect common fat maker gene Pparγ and Fabp4 expression, while increased Ucp1, Cox7a1 and Cpt1b expression whether in brown adipocytes or in beige adipocytes, indicating that RGCC promotes thermogenesis of adipocytes in cell-autonomous way (Figure 4A-4D). In order to identify the driver for RGCC-mediated thermogenesis, we screened a list of known positive regulators of thermogenesis in RGCC-knockdown brown and beige cells. Figure 4E-4F implied that peroxisome proliferator-activated receptor γ coactivator 1α (Pgc1α) was the only candidate whose expression level fully responses to RGCC changes. Its protein levels were further confirmed in Rgcc-knockdown adipocytes (Figure 4E-4F). Knockdown of Pgc1α did abolish RGCC-elicited strong effect on Ucp1, Cox7a1 and Cpt1b expression, in both brown adipocytes and C3H10T1/2 adipocytes (Figure 4G-4H). Taken together, we conclude that the Pgc1α is responsible for RGCC-mediated thermogenesis.
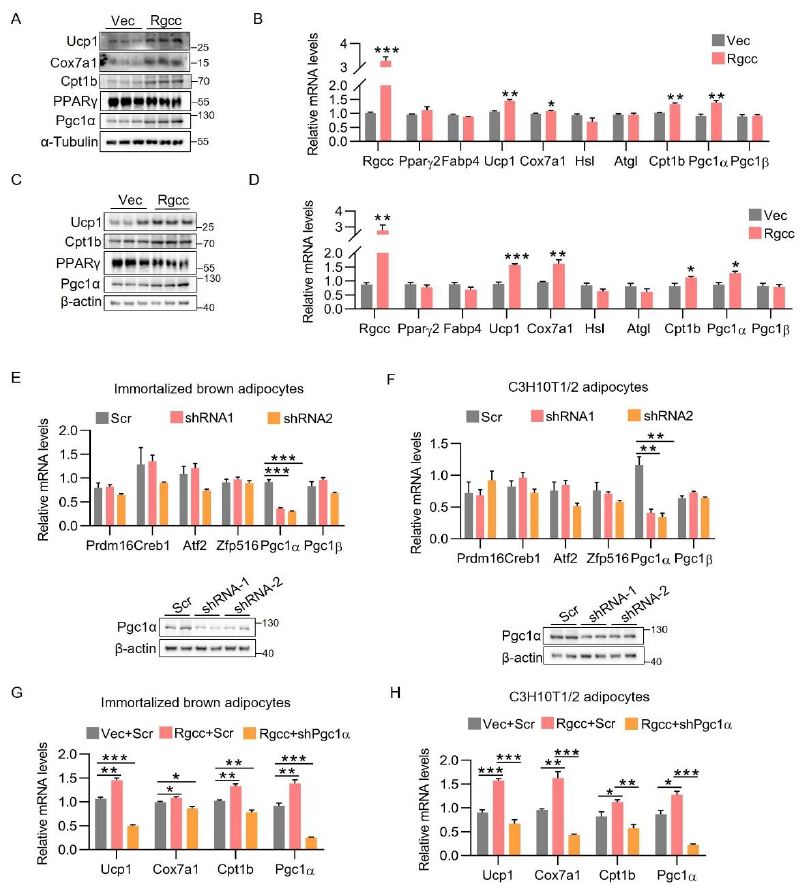
Figure 4: Pgc1α mediates RGCC-dependent thermogenesis.
(A-B) protein levels (A) and mRNA levels (B) expression in mature brown adipocytes. Preadipocytes were infected with RGCC overexpression lentiviruses on day 2 and harvested on day6 (n=3 each group).
(C-D) Protein levels (C) and mRNA levels (D) levels analysis in C3H10T1/2 adipocytes. C3H10T1/2 preadipocytes were infected with lentiviruses expressing RGCC on day 4 and harvested on day 8 (n=3 each group).
(E-F) RT-PCR analysis in mature brown adipocytes (E) and C3H10T1/2 adipocytes (F). Immortalized brown preadipocyte or C3H10T1/2 preadipocytes were infected with shRGCC lentiviruses, differentiated, and harvested for analysis (n=3 each group). Pgc1α protein levels were separately showed in the bottom panel.
(G) Relative mRNA levels in brown adipocytes. lentiviral Pgc1α shRNA was infected with Rgcc-overexpressed cells on differentiation day 4 (n=3 each group).
(H) Relative mRNA levels in C3H10T1/2 adipocytes. Lentiviral Pgc1α shRNA was infected with Rgcc-overexpressed cells on differentiation day 5 (n=3 each group).
Data are expressed as mean ± SEM of biological independent samples. Two-tailed unpaired Student’s t-test was performed. *P<0.05, **P<0.01, ***P<0.001.
Discussion
Here, we found RGCC is induced upon β3-adregenic signaling and modulates the adaptive thermogenesis gene expression. Knockdown of RGCC in cultured brown and beige adipocytes evidently weaken the expression of a broad panel of thermogenic and fatty acid oxidation genes in cell-autonomous way. Consistently, RGCC overexpression strengthens expression of thermogenic marker genes and β-oxidation-related genes. Pgc1α expression is a potential key mechanism for RGCC-mediated thermogenesis. Our results demonstrate that RGCC is crucial for maintaining thermogenesis in brown and beige adipocytes. Previous studies reported that RGCC deficiency had no effect on 3T3-L1 differentiation, but modestly boosted Lipe and Pgc1α expression, which is contrary to our results. It may be because brown, beige and white adipocytes originate from different adipocyte lineage, involving divergent regulation mechanisms. Several regulators have been revealed the inconsistent regulation function in different adipocytes. In 3T3-L1 adipocytes, nutlin-3a-mediated activation of p53 or p53 overexpression suppresses adipogenesis [20]. In C3H10T1/2 cells and human adipose-derived stem cells, p53 knockdown enhances differentiation [21]. While in the skeletal muscle myogenic cell line-C2, as the brown preadipocyte, p53 abrogation substantially impaired differentiation [21]. RGCC-/- mice exhibited a lean phenotype and improved systemic inflammation, further alleviative dyslipidemia and insulin resistance upon HFD. It should not exclude the contribution of RGCC expressed in nonadipose cells on metabolism using while-body Rgcc KO mice. RGCC-mediated thermogenesis and energy homeostasis in vivo, especially in BAT and iWAT, need to be investigated in next study.Pgc1α is induced early in brown fat differentiation and is preferentially expressed in mature brown adipocytes compared to white adipocytes. Moreover, Pgc1α is highly induced by cold exposure and involved in the adaptive thermogenic program in BAT, including fatty-acid oxidation, mitochondrial biogenesis to increase thermogenic genes and promotes browning [22,23]. Obviously, Pgc1α is not a direct target of RGCC. How RGCC regulates Pgc1α mRNA level needs to be studied in future. In conclusion, our results have revealed that RGCC play a fundamental role in regulating thermogenic gene expression in brown adipocytes and beige adipocytes, which makes RGCC a potential drug target in the therapeutics of obesity.
Experimental Procedures
Animals and Treatment
All mice were housed at room temperature with a 12 h light/ dark cycle and ad libitum access to food and water. All studies involving animal experimentation were approved by the University Committee on Use and Care of Animals at the Zhejiang University. For cold experiment, 8-week-old Male C57BL6/J mice were housed at 4℃ for 6h.
Cell Culture
The immortalized brown preadipocytes are cultured and differentiated to brown adipocytes as previously described [24]. Briefly, upon reaching 70% confluence, brown preadipocytes were maintained in the induction medium (DMEM containing 10% FBS, 20 nM insulin, 1 nM T3) for 2 days. Then the differentiation medium containing 10% FBS, 20 nM insulin, 1 nM T3, 0.5 mM dexamethasone, 0.5 mM isobutylmethylxanthine, 0.125 mM indomethacin were changed for another 2 days. Next cells were cultured into induction medium and changed every other day until day 6 waiting for experiments.
The mesenchymal stem cell derived C3H10T1/2 were maintained in DMEM containing 10% CS (designed day-2) for 2 days and induced to differentiate into beige adipocytes with differentiation medium (DMEM containing 10% FBS, 1 μg/ml insulin, 1 mM dexamethasone, 0.5 mM isobutylmethylxanthine, 1 μm rosiglitazone). Two days after induction, cells were switched to maintenance medium containing 10% FBS, 1 μg/ml insulin and 1μm rosiglitazone for another 2 days. Then cells were cultured in DMEM containing 10% FBS every other day until day 8.
Plasmids and Viruses
The sequences of short hairpin RNA (shRNA) were as follows: shRGCC-1: 5’-CTCGAAGACTTCATTGCCGAT-3’; shRGCC-2: 5’-GCAGCATATT
CAACAGAGAAT-3’; shPgc1α-1: 5’-GGTGGATTGAAGTGGTG-TAGC-3’; shPgc1α-2: 5’-CCTCCTCATAAAGCCAACCAA-3’. All above of shRNA oligos were respectively subcloned into lentivirus vector Psp108 (addgene). The vectors were transfected into HEK 293T cells along with packaging plasmids (Pmd2.G, psPAX2 from addgene). Full-length Rgcc cDNAs was amplified by reverse transcription and constructed into lentiviral pENTR1A (addgene) system.
Lentivirus Infection
Overexpression plasmid and packaging plasmid (pLP1, pLP2, pVSVG) were together transfected in HEK 293T cells. The viral supernatant was harvested after 48 h post-transfection. The brown and C3H10T1/2 preadipocytes were infected with lentiviruses using polybrene of 5 μg/ ml, were selected with puromycin (3 μg/ ml) and were differentiated to mature adipocytes following the standard induction protocol. Lentiviruses bearing shPgc1α infected overexpressed-RGCC adipocytes on differentiation day 2 in brown adipocytes and day 4 in C4H10T1/2 adipocytes. The mature brown adipocytes were harvested for gene and protein analysis on day 6, and mature C3H10T1/2 were harvested on day 8.
Oil-red O staining
Differentiated cells were washed with PBS twice, fixed with 4% paraformaldehyde for 10 min at room temperature, and stained with oil-red O working solution (byotime C0158M) for 30 min. Then cells were washed with PBS for several times and waited for scan using a microscope.
Oxygen Consumption Measurement
Real-time measurements of oxygen consumption rates (OCR) were performed using a O2K (Oroboros). The mature brown and C3H10T1/2 adipocytes were washed twice by PBS, trypsinized and suspended in DMEM. The OCR were measured under basal conditions and after addition of oligomycin (2.5 μM), FCCP (1 μM), and rotenone (1 μM).
Antibodies
The following primary antibodies were used: anti-Ucp1 (Ucp11-A) from alpha diagnostic; anti-Pgc1α (66369-1-Ig), anti-Fabp4 (12802-1-AP) from Proteintech; anti-Pparγ1/2 (2443S) from Cell Signaling Technology; anti-Rgcc (A17689), anti-β-actin (AC026), anti-Cox7a1 (A21240), anti-Cpt1b (A6796), anti-β-Tubulin (AC008) from Abclonal.
Real-time qPCR
Total RNA from tissues were extracted using TRIzol (Vazyme R701) and an equal amount of RNA was reverse transcribed by HiSciptÒ QRT SuperMix with gDNA wiper (Vazyme R222). Quantitative real-time PCR was performed following the protocols of chamQ qPCR Master Mix (Vazyme Q711) with ViiA 7 Real-Time PCR system (Applied biosystems).
Western Blot
Mature adipocytes were harvested with cell lysis buffer (100 mM NaCl, 0.5% Triton-X-100, 5% glycerol, 50 mM Tris-HCl (pH 7.5), 1 mM PMSF and protease inhibitor mixture cocktail). Cell supernatants were collected by centrifugation at 16,000 g for 10 minutes at 4 ℃ and quantified for protein content using BCA kit (YEASEN, 20201ES86). The equal protein content of cells lysates was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and revealed with chemiluminescence (ECL) system.
Statistical Analysis
Data are presented as mean ± standard error of the means. Differences between two groups were estimated using the unpaired two-tailed Student’s t-test. Statistical significance was showed as *P<0.05, **P<0.01, ***P<0.001.
Acknowledgements
This research was supported by Zhejiang Provincial Natural Science Foundation of China (LQ23C070004 to Q.Z., LQ21C110001 to S.H.), China Postdoctoral Science Foundation (2020M680053 to Q.Z), the Construction Fund of Key Medical Disciplines of Hangzhou (OO20200055 to Y.G.), and the National Natural Science Foundation of China (82100904 to S.H.).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author Contributions
Q.Z. designed the project, performed most experiments, analyzed data and wrote the manuscript. Q.W., B.L., S.H., X.W., Y.Z., Y.Y., and Z.L. aided in some experiments. Y.G. supervised the study.
Data Availability
All study data, method, and results of statistical analyses are reported in this paper. We welcome any specific inquiries.
References
- T. Chouchani, S. Kajimura, Metabolic adaptation and maladaptation in adipose tissue, Nat Metab [crossref]
- Wolfrum, Z. Gerhart-Hines, Fueling the fire of adipose thermogenesis, Science [crossref]
- Ikeda, T. Yamada, UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes, Front Endocrinol (Lausanne) [crossref]
- Matsushita, T. Yoneshiro, S. Aita, T. Kameya, H. Sugie, M. Saito, Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans, International journal of obesity [crossref]
- A. Virtanen, M.E. Lidell, J. Orava, M. Heglind, R. Westergren, T. Niemi, M. Taittonen, J. Laine, N.-J. Savisto, S. Enerbäck, Functional brown adipose tissue in healthy adults, New England Journal of Medicine [crossref]
- Zhang, G. Hao, M. Shao, K. Nham, Y. An, Q. Wang, Y. Zhu, C.M. Kusminski, G. Hassan, R.K. Gupta, Q. Zhai, X. Sun, P.E. Scherer, O.K. Oz, An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents, Cell Metab [crossref]
- Yoneshiro, S. Aita, M. Matsushita, Y. Okamatsu‐Ogura, T. Kameya, Y. Kawai, M. Miyagawa, M. Tsujisaki, M. Saito, Age‐related decrease in cold‐activated brown adipose tissue and accumulation of body fat in healthy humans, Obesity [crossref]
- Saigusa, I. Imoto, C. Tanikawa, M. Aoyagi, K. Ohno, Y. Nakamura, J. Inazawa, RGC32, a novel p53-inducible gene, is located on centrosomes during mitosis and results in G2/M arrest, Oncogene [crossref]
- I. Vlaicu, C. Cudrici, T. Ito, M. Fosbrink, C.A. Tegla, V. Rus, P.A. Mircea, H. Rus, Role of response gene to complement 32 in diseases, Archivum immunologiae et therapiae experimentalis [crossref]
- Badea, F. Niculescu, L. Soane, M. Fosbrink, H. Sorana, V. Rus, M.L. Shin, H. Rus, RGC-32 increases p34CDC2 kinase activity and entry of aortic smooth muscle cells into S-phase, Journal of Biological Chemistry [crossref]
- A. Tegla, C.D. Cudrici, V. Nguyen, J. Danoff, A.M. Kruszewski, D. Boodhoo, A.P. Mekala, S.I. Vlaicu, C. Chen, V. Rus, RGC-32 is a novel regulator of the T-lymphocyte cell cycle, Experimental and molecular pathology 98 [crossref]
- P. Voigt, K. Mulfaul, N.K. Mullin, M.J. Flamme-Wiese, J.C. Giacalone, E.M. Stone, B.A. Tucker, T.E. Scheetz, R.F. Mullins, Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration, Proceedings of the National Academy of Sciences [crossref]
- Guo, M. Chen, Y. Chao, C. Cai, L. Liu, L. Zhao, L. Li, Q.R. Bai, Y. Xu, W. Niu, RGCC balances self‐renewal and neuronal differentiation of neural stem cells in the developing mammalian neocortex, EMBO reports [crossref]
- G. Luzina, V. Rus, V. Lockatell, J.-P. Courneya, B.S. Hampton, R. Fishelevich, A.V. Misharin, N.W. Todd, T.C. Badea, H. Rus, Regulator of cell cycle protein (RGCC/RGC-32) protects against pulmonary fibrosis, American journal of respiratory cell and molecular biology [crossref]
- -B. Cui, J.-N. Luan, S.-Y. Chen, RGC-32 deficiency protects against hepatic steatosis by reducing lipogenesis, Journal of Biological Chemistry [crossref]
- -B. Cui, J.-N. Luan, J. Ye, S.-Y. Chen, RGC-32 deficiency protects against high fat diet-induced obesity and insulin resistance in mice, The Journal of endocrinology [crossref]
- L. Ghaben, P.E. Scherer, Adipogenesis and metabolic health, Nat Rev Mol Cell Biol [crossref]
- E. Lattin, K. Schroder, A.I. Su, J.R. Walker, J. Zhang, T. Wiltshire, K. Saijo, C.K. Glass, D.A. Hume, S. Kellie, M.J. Sweet, Expression analysis of G Protein-Coupled Receptors in mouse macrophages, Immunome Res [crossref]
- W. Fung, S. Zhou, H. Zhu, X. Wei, Z. Wu, A.R. Wu, Cell fate determining molecular switches and signaling pathways in Pax7-expressing somitic mesoderm, Cell Discov [crossref]
- K. Behera, A. Bhattacharya, M. Vasudevan, T.K. Kundu, p53 mediated regulation of coactivator associated arginine methyltransferase 1 (CARM1) expression is critical for suppression of adipogenesis, Febs j [crossref]
- Molchadsky, O. Ezra, P.G. Amendola, D. Krantz, I. Kogan-Sakin, Y. Buganim, N. Rivlin, N. Goldfinger, V. Folgiero, R. Falcioni, R. Sarig, V. Rotter, p53 is required for brown adipogenic differentiation and has a protective role against diet-induced obesity, Cell Death Differ [crossref]
- Uldry, W. Yang, J. St-Pierre, J. Lin, P. Seale, B.M. Spiegelman, Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation, Cell Metab [crossref]
- Wu, O. Boss, Targeting PGC-1 alpha to control energy homeostasis, Expert Opin Ther Targets [crossref]
- Zhao, Z. Zhang, W. Rong, W. Jin, L. Yan, W. Jin, Y. Xu, X. Cui, Q.Q. Tang, D. Pan, KMT5c modulates adipocyte thermogenesis by regulating Trp53 expression, Proc Natl Acad Sci U S A [crossref]