Abstract
Bioisosterism in rational drug design approach specifically the divalent atom and group in their modification and optimization to improve the pharmacokinetic and pharmacodynamics properties of compound have been studied.
Keywords
Bioisosterism, Divalent
Introduction
Bioisosterism is a strategy of Medicinal Chemistry for the rational design of new drugs, applied with a lead compound (LC) as a special process of molecular modification [1]. The LC should be of a completely well-known chemical structure and possess an equally well-known mechanism of action, if possible at the level of topographic interaction with the receptor, including knowledge of all of its pharmacophoric group. Furthermore, the pathways of metabolic inactivation [2], as well as the main determining structural factors of the physicochemical properties which regulate the bioavailability, and its side effects, whether directly or not, should be known so as to allow for a broad prediction.
There are two kinds of divalent isosteres. They are:
One that involves the swapping of an atom that is involved in the double bond such as C=C, C=N, C=O, and C=S.
The other types are those when substituting atoms of different kinds result in the alteration of a single bond. such as seen here:
C-C-C, C-NH-C, C-O-C and C-S-C.
In the structural activity relationship study of many different active pharmacological agents, both types of isosteres substitution have been used widely.
Divalent Replacement Involving Double Bonds
As mention above this group involves such replacements as C=C, C=N, C=O, and C=S. tautomerization of these groups is facilitated by the presence of a heterocyclic system in the lead compounds while studying therapeutic agents and the absence of moving proton migrating in the ring system. Replacing C=S with C=O in the aldose reductase inhibitor Tolerstat, which is being studied for its activity in diabetic neuropathy had produced oxo-Tolerstat which have both in- Vivo and in-vitro [3].
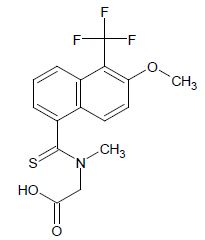
{[6-methoxy 5(trifluoromethyl) naphthalene-1-carbothioyl] (methyl) amino} acetic acid.
Aldose reductase inhibitory activity of Tolrestat and Oxo- Tolrestat.
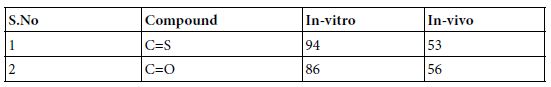
Inhibition of enzyme activity in partially purified bovine lens preparation. 2, Inhibition of galactitol accumulation in the sciatic nerves of rats fed 20% galactose for 4 days. This class of divalent isosteres can also be used with purine nucleoside analogs that are tested for in- Vivo antiviral activity against the Semliki Forest virus [4]. The percentage of mice surviving for 3 weeks for different bioisosteric analogs compared to the absence of control group mice survivors. A weakly active compound has resulted when a sulfur replacement at C-8 is done with oxygen or selenium.
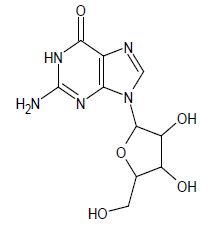
2-amino-9-[3,4-dihydroxy-5-(hydroxymethyl) oxolan-2-yl]-1,9-dihydro-6H-purin-6-one (Table 1).
Table 1: Guanosine bioisosteric analogues activity against semliki Forest Virus.
|
S.no |
C-8 | %Total survivor |
| 1 | C=S |
83 (10/12) |
|
2 |
C=O | 67 (8/12) |
| 3 | C=Se |
58 (7/12) |
Divalent Replacement Involving Single Bonds
These Bioisosterism are a group of an atom which are attached to different substituent attachment of two different substituents to the Bioisosterism the polar and chemical difference is less noticeable. The biological activity of these divalent bioisosteres hold could be linked to the bond angle. In the example below the relationship between the anti-allergic activity and bond angle of different divalent bioisosteres is shown [5].
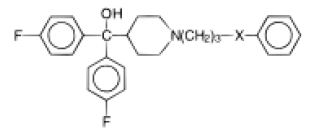
Aminophylline orally at 100 mg/kg was used as a positive control and assigned a biological response of (++); (-) not significantly different from negative control group at p<0.05 as determined by the Dunnett’s t test; (+) activity between positive and negative groups; (++) activity equivalent to positive control group; (+++) activity greater than positive control group. Passive foot anaphylaxis is an IgE mediated model used to detect compounds that have anti-allergic action. . Using oxygen as a bioisosteric linker shows a smaller bond angle and greater electronegativity proving an improved potency. Another good study on inhibitors of nuclear factors of activated T-cell (NFAT) – mediated transcription of β- galactosidase. T-cells are the main component of the immune system that gets activated on the contact with foreign matter induction interleukin-2 (IL-2) gene which are necessary autocrine growth factor for T-cells. Activated T-cell release many different bioactive molecules to initiate a series of events that leads to immune /inflammatory response [6]. The region 257- 286 base pairs upstream of the IL-2 structural gene binds to a protein, the nuclear factor of activated T cells-1 (NFAT-1), preceding to IL-2 gene transcription. NFAT-1 is conveyed in relatively few cells besides T cells and is markedly upregulated upon stimulation of the T cell receptor. This makes it an extremely specific target within activated T cells. When the cell is activated, the NFAT-1 protein binds to the DNA at its recognition site and induces the transcription of â-galactosidase. This study evaluated some of the bioisosteric analogs of quinazolinediones as an immunosuppressant agent since they can inhibit β- galactosidase (Tables 2 and 3).
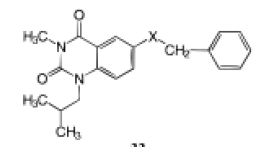
Table 2: Oral anti-allergic activity in the passive foot anaphylaxis’s Assay of analogous containing varied heteroatom.
|
S.no |
x | Electronegativity | Bond angle | Passive foot anaphylaxis’s (10 mg/kg) |
| 1 | -O- | 3.15 | 108.0 |
+++ |
|
2 |
-S- | 2.32 | 112.0 | + |
| 3 | -CH2- | 2.27 | 111.5 |
+ |
|
4 |
-NH- | 2.61 | 111.0 |
+ |
Table 3: Regulation of NFAT-1- regulated β-galactosidase activity by Quinazolinediones is shown below.
|
S.no |
X | IC50 (µM) |
| 1 | -NH- |
4.47 |
|
2 |
-CH2– | 4.03 |
| 3 | -O- |
2.5 |
Conclusion
Bioisosteres control biological activity by virtue of restrained differences in their physicochemical properties. Systematic correlation of physicochemical parameters with observed biological activity has been very effective in highlighting subtle differences within bioisosteric groups which frequently increase activity. Of significance is the ability of these bioisosteric groups to describe some of the essential requirements of the pharmacophore. This is especially important when the synthesis of a large number of drug nominees for assessment is not economically achievable. A number of less known replacements have not been reviewed because of their inability to demonstrate bioisosterism in more than a single class of agents. In this review, an attempt has been made to explain the rationale behind the use of bioisosteric replacements using examples in divalent atom and group from current literature. It is hoped that this systematic approach will facilitate the use of bioisosteric replacements in future structure activity studies.
Author Contribution
The manuscript was written through contributions by the author Hawi Matewos Daka.
Acknowledgment
Finally, I would like to thank my parents Matewos Daka and Bayush Temesgen for the emotional and financial support they have given me to write the article.
References
- Burger AA (1983) Guide to the Chemical Basis of Drug Design. NY, EUA Wiley.
- Stenlake JB (1979) Fondations of Molecular Pharmacology, Vol 2. The Chemical Basis of Drug Action, Londres, Inglaterra, Athlone Press pp: 213-290.
- Wrobel J, Millen J, Sredy J, Dietrich A, Kelly JM, et al. (1989) Orally Active Aldolase Reductase Inhibitors Derived from Bioisosteric Substitutions on Tolrestat. J Med Chem 32: 2493-2500. [crossref]
- Bonnet PA, Robins RK (1993) Modulation of Leukocyte Genetic Expression by Novel Purine Nucleoside Analogues. A New Approach to Antitumor and Antiviral Agents. J Med Chem 36: 635-653. [crossref]
- Walsh DA, Franzyshen SK, Yanni JM (1989) Synthesis and Antiallergy Activity of 4-(Diarylhydroxymethyl)-1-[3-(aryloxy)propyl] piperidines and Structurally Related Compounds. J Med Chem 32: 105-118. [crossref]
- Michne WF, Schroeder, JD, Guiles JW, Treasurywala AM, Weigelt CA, et al. (1995) Novel Inhibitors of the Nuclear Factor of Activated T Cells (NFAT)-Mediated Transcription of â-Galactosidase: Potential Immunosuppressive and Antiinflammatory Agents. J Med Chem 38: 2557-2569. [crossref]