Introduction
Anxiety and depression increase substantially after traumatic brain injury (TBI) in humans. They are among the most common early neuropsychiatric complications of TBI [1] contributing to substantial morbidity and mortality. Owing to the current global epidemic of obesity [2], a possible role for obesity in the development of anxiety disorders has received increased attention [3]. In a recent study of TBI in humans, obesity was a significant predictor of an increased risk for the later occurrence of a composite neurodegeneration endpoint comprised of major depression and suicide, Parkinson’s disease or dementia [4]. Yet few prior studies in have explored the relationship between obesity, TBI and anxiety disorders. Here we used the Zucker fatty rat strain, a genetic model of obesity harboring homozygous mutations in the leptin receptor gene (fa/fa) [5] and Zucker lean (Fa/fa/?) rats to test a commonly-held notion that obesity is a risk factor for anxiety-like disorders. We also tested whether mild traumatic injury (induced by lateral fluid percussion-LFP) (vs. sham injury) modifies anxiety-like behavior in obese vs. lean Zucker rats.
Obesity in rats age (8-12 weeks old) was recently reported to be associated with heightened anxiety-like behavior compared to age-matched obese-resistant genetic strains [6]. Since anxiety-like behavior was not correlated with body weight (in the study) the authors concluded that acquired (vs. genetic factors) may play a less important role in the etiology of anxiety [6]. In the current study, we tested rats for anxiety-like behavior at different times in the developmental cycle to further evaluate a possible role for chronic acquired factors (related to obesity and diabetes) in anxiety-like behavior. Current drug therapy for anxiety disorders modulates serotoninergic signaling at central synapses in the cerebral cortex, hippocampus and other brain regions. We also conducted an exploratory test of whether systemic administration of a novel, putative neuroprotective synthetic peptide fragment of the human serotonin 2A receptor (SN..8) may modulate anxiety-like behavior in Zucker fatty (vs. lean) rats exposed to mild TBI (vs. sham injury).
Materials and Methods
Peptides
A linear synthetic peptide, SCLLADDN (SN..8) having a sequence identical to that of a fragment of the second extracellular loop region of the human 5-hydroxytryptamine 2A receptor was synthesized at Lifetein, Inc (Hillsborough, NJ) and had > 95% purity. A scrambled peptide containing the same amino acids as SCLLADDN had a sequence of LASNDCLD (LD.8) and a purity of 96.37%, MW 849.91. The lyophilized peptides were stored (in the presence of dessicant) at −40 degrees C prior to use. On the day of an experiment, an aliquot of lyophilized peptide was reconstituted in sterile saline at the indicated concentration. Reconstituted peptide(s) were prepared fresh before each experiment.
Animals
All procedures were conducted according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Veterans Affairs Medical Center (East Orange, New Jersey). Male ZDF (fa/fa) and lean (+/?) Zucker rats were obtained from Charles River Laboratories (Kingston, NY) at approximately 6-7 weeks of age. All rats were single housed upon arrival, with modest enrichment (a PVC tube). Rats were provided ad libitum access to food and water and maintained in a 12 h light/dark cycle with lights on at 0630. All procedures occurred during the light phase of the circadian cycle.
Injections
Peptide (SN..8 or LD.8) was dissolved in sterile saline (2 mg/kg) and SN.8 peptide vs. vehicle (sterile saline) or LD..8 peptide intraperitoneal (IP) injections were ad-ministered every other day. Peptide (SN.8) (vs. saline or LD..8) injections commenced 1-week prior to surgery/injury in ZDF rats to minimize the risk of excess acute post-injury mortality in ZDF rats thought to be related (in part) to pre-injury moderate hypertension. The peptide SN..8 was previously reported to substantially lower blood pressure in the ZDF rat [7].
Surgeries/Injury
All animals underwent a surgical procedure to attach a luer-lock connector to a craniectomy, positioned either on the left or right parietal bone plate. Rats were anesthetized with 1-4% isoflurane (5 L/min O2 for 60s) and transferred to a stereotactic instrument with 1-4% isoflurane mixed with 1-1.5 L/min O2 delivered via a nosecone to maintain anesthesia throughout the surgery. A craniectomy (2mm in diameter, centered at 3mm posterior to bregma and 3.5mm lateral to midline) was made in the skull and super glue was used to secure a luer-lock connector around the edge of the craniectomy. A plastic cylinder (cut from a 10mL syringe) was placed on the skull surrounding the luer-lock connector; this was placed to provide stability and protect the area around the craniectomy. Dental cement was used to fill the space in between the connector and the plastic cylinder. A small amount of sterile saline was deposited into the connector along with a small piece of Kimwipe to keep the dura clean of debris.
One day following implantation of the surgical hub, fluid percussion injury is achieved with a device using a computer-controlled voice-coil to deliver defined pressure waves to the dura through a water piston [8]. Water is first placed in the surgical cap to prevent any air displacing the pressure waves. Rats were anesthetized with isoflurane in an induction chamber followed by fluid percussion injury or sham. The injury procedure occurs one day after surgery to prevent the dura from drying out and thereby reducing the injury of the brain tissue. Mild to moderate severity TBI will occur at pressure amplitudes of 20-30 psi (±3 psi) and rates of rise between 10-20 milliseconds. To reduce the effects of anesthesia as much as possible, rats are connected to the device by the syringe hub and allowed to recover from anesthesia until they respond lightly to a foot pinch before injury occurs. Therefore, the rats were lightly anesthetized at the time of injury. Sham rats are treated the same way as injured rats including isoflurane anesthesia and connection of the device to the rat, but the pressure wave was not delivered. The animal is monitored for startle in response to the pressure wave and then placed on its back in a recovery cage and evaluated for apnea. Latency for the righting reflex to return is recorded and used as an index of traumatic unconsciousness.
Behavioral Tests
Open Field Test. Rats were placed in the center of a round, gray, open field apparatus (diameter of 74 cm, height of 51 cm). A light (120 W) was located 135 cm directly above the center of the apparatus which had a measured light intensity, LT300-NIST Light Meter (Extech Instruments, Knoxville, TN) of 400-500 lux. The open field apparatus was placed in a novel environment. The path of the rat in the open field was recorded, digitized, analyzed, and stored on a computer using AnyMaze version 6.21 (64-bit) (Stoelting Co., 620 Wheat Lange, Wood Dale, IL 60191 USA). For analysis, the open field was separated into two zones, the center, and the periphery. The center zone was measured to have the area of the total field (diameter of approximately 52.32 cm). Performance in the open field was scored during a 3-minute time window before returning the animal to its home cage. The apparatus was wiped with 70% ethanol solution between testing of each rat. Increased mobility in the center zone, defined as distance, speed, and time in motion, was used as an indicator of reduced anxiety, whereas staying in the peripheral zone, was used as an indicator of increased anxiety [9].
Statistical Analysis
Statistical analysis was performed using the Student’s t-test for single comparisons and one-way ANOVA for differences between more than two groups followed by post hoc Bonferroni tests. All statistical analyses were conducted using the SPSS software. A p-value <0.05 was considered significant and values are expressed as means ± SEM.
Results
Open Field Test – Bright Light
Twenty-two rats were initially tested 1.5 months post-injury (i.e. at 18=19 weeks of age). An ANOVA demonstrated a main effect of strain, P=0.037 (Figure 1). Sham-injured Zucker fatty rats spent more time in center (mean 82 ± 10 sec) compared to sham-injured Zucker lean rats (mean 55 ± 7 sec) (Figure 1A and 1B). The preliminary study of anxiety-like behavior was only powered to test for main effects. Still it suggested that Zucker fatty rats display reduced anxiety-like behavior when tested later in the developmental cycle.
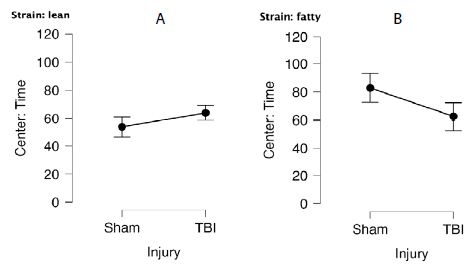
Figure 1: Center time (sec) in bright light in A) lean or B) fatty Zucker rats after sham- vs. mild TBI. A) Lean: Sham (N=7), TBI (N=6). B) Fatty: Sham (N=5), TBI (N=4).
Open Field Test – Under Dark Conditions (Mobility Test)
General mobility was assessed in the open field test performed under dark conditions. There were no significant differences in time in the center by strain (fatty vs. lean) or injury (sham vs. TBI) (Figure 2). These results suggest that the differences observed under bright light conditions were likely attributable to anxiety-like behavior.
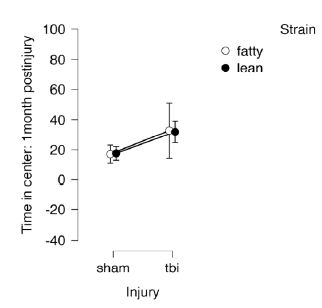
Figure 2: Open field test under dark conditions, 1 month post-injury in Zucker lean and fatty rats. Results are mean ± SEM. Lean: Sham (N=7), TBI (N=6). Fatty: Sham (N=5), TBI (N=4).
Next, in a different cohort of Zucker fatty (N=22) and lean (N=12) rats, the open field test (under bright light) was performed before injury (11-12 weeks of age), 1-week after injury (15-16 weeks of age) and 1-month after injury (18-19 weeks of age). In a repeated measures ANOVA of center time at (preinjury, 1week and 1 month post-injury), there was a significant main effect of strain (F(1,26)=5.639, p=0.025) and a significant interaction effect of (time x strain) F(2,52)=8.248, p<0.001 (Figure 3). The post-hoc tests showed significant differences between fatty, preinjury vs. fatty, 1week post-injury (Pbonf=0.002) and between fatty, 1week post-injury vs. lean, 1week post-injury (Pbonf=0.023) (Figure 3). The fatty rats spent significantly more time in the center zone after injury compared to preinjury, and spent significantly more time in the center zone after injury than leans rats (Figure 3). There was no significant (strain x injury) interaction.
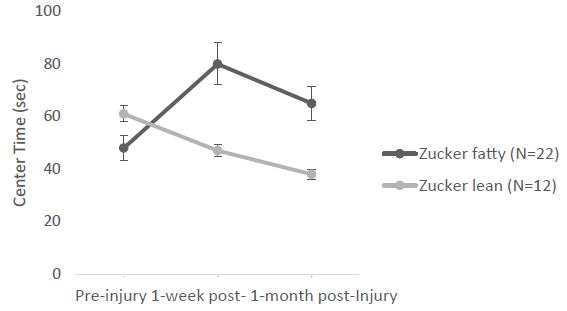
Figure 3: Center time: significant (strain x time) interaction. Each point is mean ± SEM
Mean difference (fatty vs. lean Zucker rats) in center time (Figure 4) underwent a ‘reversal’ after 11-12 weeks and before 15-16 weeks of age. At the earlier timepoint fatty rats had higher anxiety and later underwent a shift to reduced anxiety-like behavior (vs. lean rats). Zucker fatty rats acquired body weight significantly more rapidly than lean rats between 7-12.5 weeks of age (Figure 5), however injury (sham or TBI) which occurred at 13 weeks of age (Figure 6, arrowhead) contributed to a temporary ‘fall off’ in the normal trajectory of weight gain in Zucker fatty rats (Figure 5). Severe hyperglycemia in Zucker fatty rats (16 weeks and older) may have also contributed to a slowing in the relative trajectory of weight gain in fatty vs. lean (normoglycemic) Zucker rats (Figure 5).
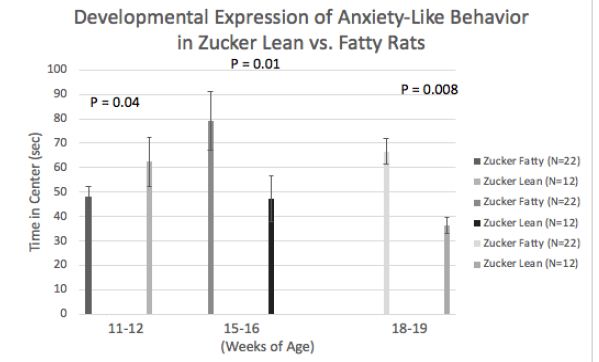
Figure 4: Developmental ‘onset’ of reduced anxiety-like behavior in fatty vs. lean Zucker rats. Data are mean ± SEM.
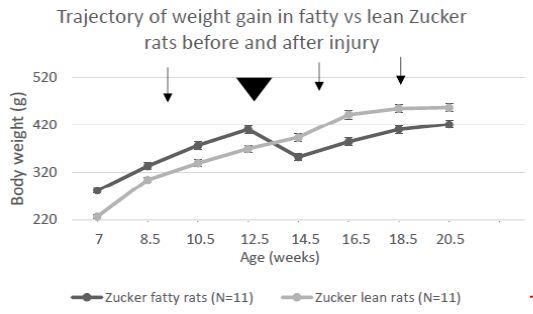
Figure 5: Change in body weight in Zucker lean and fatty rats across development and injury (arrowhead). Arrows signify pre-injury, 1-week post and 1-month post-injury timepoints. Each point is mean ± SEM.
Overall mean speed, and exploratory behavior (distance traveled) were unexpectedly significantly higher in fatty vs. lean Zucker rats (Figure 6A and 6B) and immobility time was significantly reduced in fatty vs. lean Zucker rats (Figure 6C), assessed 1-week after injury. Mean distance traveled (Figure 7A) was significantly higher in Zucker fatty (vs. lean rats), before and 1-week post injury. Total immobility time was significantly reduced, before injury, 1 week, and 1 month post-injury, in Zucker fatty vs. lean rats (Figure 7B). Taken together, center time in the open-field test under bright light conditions (a measure of anxiety-like behavior) was unexpectedly decreased in Zucker fatty (vs. lean) rats at 15-16 weeks of age and older and could not be accounted for by strain differences in general mobility, or exploratory behavior.
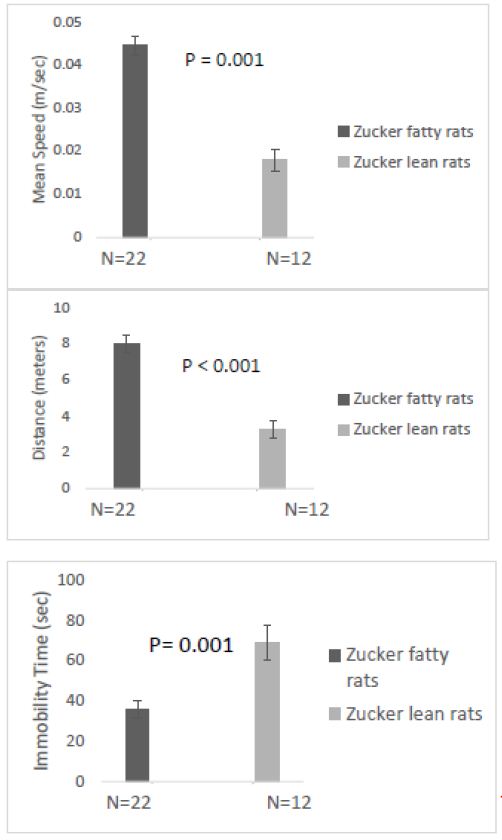
Figure 6: One week after injury: A) Mean speed and B) distance traveled were both significantly higher in fatty vs. lean Zucker rats; C) total time immobile was significantly higher in lean vs. fatty Zucker rats.
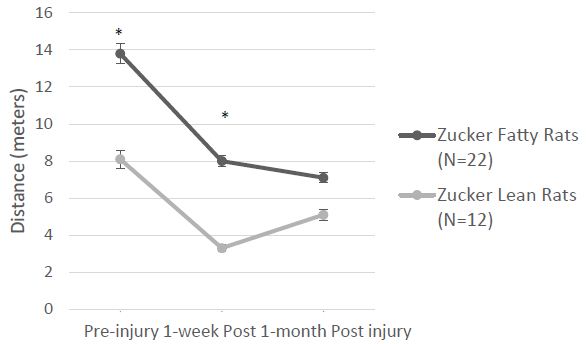
Figure 7: A) Zucker fatty (vs. lean) rats traveled significantly greater distance before and 1-week after injury B) Zucker lean (vs. fatty) rats had significantly greater immobility at all three timepoints before and after injury. * P < 0.05.
A novel small peptide medication (SN..8) comprised of a fragment of the second extracellular loop of the human 5HT2AR prevented 5HT2AR activation on mouse neuroblastoma cells in vitro. Unlike direct antagonist 5HT2AR medications which promote significant weight gain, chronic administration (for 13 weeks or longer) of SN..8 (vs. an inactive scrambled version of the peptide – LD..8) did not cause significant weight gain in Zucker rats [7]. We next tested whether systemic (intraperitoneal) administration of SN..8 (vs. LD.8) may affect anxiety-like behavior in (sham-injury vs. TBI) Zucker rats.
There were no significant differences in center time between fatty vs. lean Zucker rats subjected to (sham- vs. TBI injury) or (SN..8 vs. scrambled peptide) treatment, i.e. 2 mg/kg, IP every other day when assessed either 1 -week after injury (Figure 8) or 1-month after injury (Figure 9). In repeated measures ANOVA of center time, there was no significant (drug x injury) or (drug x strain) interaction (data not shown). Still, the sample sizes were small and had reduced power to detect possible statistically significant difference(s) in drug (SN.. 8 vs. LD.8) or injury (sham vs. TBI) effects on anxiety-like behavior.
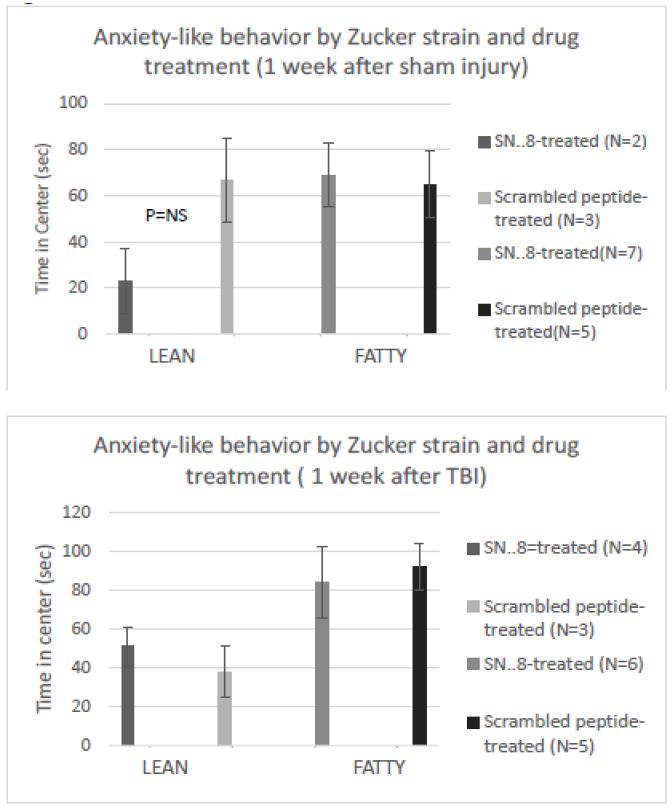
Figure 8: SN..8 (vs. scrambled peptide) treatment did not significantly modify time in center in lean or fatty rats evaluated a A) 1 week after sham injury or B) 1 week after mild TBI. Data are mean ± SEM.
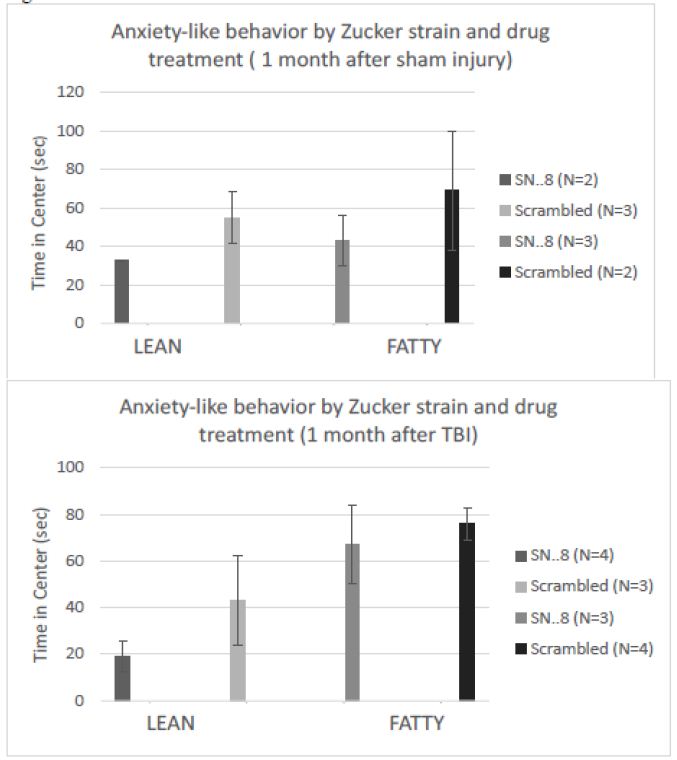
Figure 9: SN..8 (vs. scrambled peptide) treatment did not significantly modify time in center in lean or fatty rats evaluated a A) 1 month after sham injury or B) 1 month after mild TBI. Data are mean ± SEM.
Discussion
Obesity has reached epidemic proportions in the United States and other parts of the world [2]. Anxiety and depressive disorders rank very high among conditions contributing to morbidity and mortality. Understanding biological links between obesity and anxiety or depressive disorders is of considerable public health importance. Among lifelong TBI-sufferers, obesity caused in part by a sedentary lifestyle and medications useful in treatment-refractory depression is both common and appeared to increase the risk for later occurrence of a composite neurodegenerative disease outcome including severe depression [4]. In a prior study that compared rats fed a standard diet vs. high-fat Western diet (leading to obesity), obesity was reported to increased anxiety-like behavior in the high-fat diet fed rats [10]. One proposed mechanism linking obesity to anxiety is increased inflammation [3]. In a different study that compared obese-prone vs. obese-resistant rat strains at 8-12 weeks of age, the Zucker fatty rat had lower exploratory activity, lower general mobility and decreased center time in the open field test (i.e. increased anxiety-like behavior) compared to several different obese-resistant rat strains, but not including Zucker lean rats [6]. In the present study, we compared obese and lean Zucker rats having similar overall genetic background, but differing at the leptin receptor locus (i.e. fa/fa vs. Fa/?). At 8-12 weeks of age, anxiety-like behavior was significant increased in fatty vs. lean Zucker rats perhaps consistent with prior reports of a role for inflammation and obesity [6,10]. However, when the open field test was conducted at later developmental age(s) in the present study, anxiety-like behavior was significantly reduced in fatty vs. Zucker rats. This may be indicative of development change in one or more receptors in the Zucker fatty rat brain important in mediating anxiety-like behavior.
The serotonin 2A receptor and the serotonin 1A receptor play opposing roles in the regulation of anxiety and depression [11]. Increased activity in the 5HT2AR in certain brain regions is associated with increased anxiety and depression [12] and several existing FDA-approved 5HT2AR antagonist medications are effective in treatment-resistant depression. On the other hand, sustained activity in the 5HT1A receptor underlies (in part) the anxiolytic and anti-depressant effect of SSRI medications [13].
Both 5-HT2AR and mineralocorticoid receptor (MR) are expressed in cortical brain regions and have a role in anxiety-like behavior. For example, cortical expression of 5HT2A, -B, -C receptors was identical in ‘high anxiety’ Lewis vs. ‘low anxiety’ SHR rat strains [14], however, cortical Gq11/IP accumulation (via 5HT2A, B or C receptors) was substantially higher in the ‘high anxiety’ Lewis rat [15]. Rozeboom et al. [16] reported that transgenic mice harboring chronic increased forebrain mineralocorticoid receptor (MR) expression displayed reduced anxiety-like behavior (vs. wild-type mice). Forebrain MR overexpression led to increased CA1 hippocampal expression of the 5HT1A receptor (important in mediating anxiolysis) and reduced hippocampal expression of the glucocorticoid receptor (important in mediating the stress response). Zucker fatty (vs. lean) rat were reported to have two-fold higher level of plasma aldosterone [17] (at 25 weeks of age) owing in part to obesity-associated hypertension, and to activation of the renin-angiotensin-aldosterone as a result of severe insulin resistance, hyperglycemia and insulin deficiency [18]. Taken together, morbid obesity- and diabetes-associated hormonal changes may cause increased brain MR activity (in the Zucker fatty rat) resulting in increased hippocampal expression of the 5HT1A receptor and decreased glucocorticoid expression – both mediating anxiolysis. Future study can directly test for changes in the hippocampal expression level of the 5HT1A and glucocorticoid receptor in Zucker fatty vs. lean rat brain, and its developmental onset and possible modulation by sham-injury vs. TBI.
A limitation of the present study is that subgroups of rats treated with SN..8 (vs. LD..8) and subjected to sham-injury vs. TBI may have been too small in their number(s) to detect significant differences in anxiety-like behavior. Zucker diabetic fatty rats (ZDF) spontaneously harbor 5-HT2AR agonist autoantibodies causing Gq11/IP3 accumulation [19] and the ZDF Ig mediated neurotoxicity in vitro was nearly completely prevented by incubation (of neuroblastoma cells) with SN..8 [19]. If the circulating 5-HT2AR agonist Ig were able to access (anxiogenic) cortical 5HT2A receptors, e.g. following mild TBI and disruption of the blood brain barrier, SN..8 might prevent Ig-induced 5HT2AR activation. A larger study is needed to test whether preventing cortical 5HT2AR activation by Ig, with SN..8 (vs. LD..8) may have an anxiolytic effect in lean vs. fatty Zucker rats.
Acknowledgements
This work was supported in part by a grant CBIR 22 PIL022 from the New Jersey Commission on Brain Injury Research (Trenton, NJ) to MBZ, and by grants from the Technology Transfer Program/BLRD, Office of Research and Development, Department of Veterans Affairs (Washington, DC) to MBZ. The opinions expressed herein are solely those of the authors and do not reflect the official position of the US Government.
References
- Howlett JR, Nelson LD, Stein MB (2022) Mental health consequences of traumatic brain injury. Biol Psychiatry 91: 413-422. [crossref]
- Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, et al. (2019) The obesity transition: stages of the global epidemic. The lancet. Diabetes & endocrinology 7: 231-240. [crossref]
- Castanon, Nathalie, Julie Lasselin, Lucile Capuron (2014) Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Frontiers in endocrinology 5: 74. [crossref]
- Zimering MB, Patel D, Bahn G (2019) Type 2 Diabetes Predicts Increased Risk of Neurodegenerative Complications in Veterans Suffering Traumatic Brain Injury. J Endocrinol Diabetes 6: 137. [crossref]
- Kurtz TW, Morris RC, Pershadsingh HA (1989) The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension 3: 896-901. [crossref]
- Vogel H, Kraemer M, Rabasa C, Askevik K, Adan RAH, et al. (2017) Genetic predisposition to obesity affects behavioural traits including food reward and anxiety-like behaviour in rats. Behav Brain Res 328: 95-104. [crossref]
- Zimering MB (2021) A serotonin 2A receptor decoy peptide potently lowers blood pressure in male Zucker diabetic fatty rats. Endo Diab Metab J 5: 1-13. [crossref]
- Wahab RA, Neuberger EJ, Lyeth BG, Santhakumar V, Pfister BJ (2015) Fluid percussion injury device for the precise control of injury parameters. J Neurosci Methods 248: 16-26. [crossref]
- Walsh RN, Cummins RA (1976) The open-field test: A critical review. Psychological Bulletin 83: 482-504. [crossref]
- André C, Dinel AL, Ferreira G, Layé S, Castanon N (2014) Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun 41: 10-21. [crossref]
- Kandel Eric R, James H Schwartz, Thomas M Jessell, Steven Siegelbaum, A James Hudspeth, et al. (2000) Principles of neural science 4.
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F (2004) The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci 29: 252-265. [crossref]
- Gordon JA, Hen R (2004) The serotonergic system and anxiety. Neuromolecular Med 5: 27-40. [crossref]
- Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F (1998) Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience 82: 147-159. [crossref]
- Gauffre JC, Aguerre S, Mormède P, Chaouloff F (1997) Cortical [3H] ketanserin binding and 5-HT2A receptor-mediated inositol phosphate production in the spontaneously hypertensive rat and Lewis rat strains. Neurosci Lett 236: 112-116. [crossref]
- Rozeboom AM, Akil H, Seasholtz AF (2007) Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci USA 104: 4688-4893. [crossref]
- Fredersdorf S, Endemann DH, Luchner A, Heitzmann D, Ulucan C, et al. (2009) Increased aldosterone levels in a model of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 117: 15-20. [crossref]
- Miller Judith A (1999) “Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus.” Journal of the American Society of Nephrology 10: 1778-1785. [crossref]
- Zimering MB, Grinberg M, Burton J, Pang K (2020) Circulating Agonist Autoantibody to 5-Hydroxytryptamine 2A Receptor in Lean and Diabetic Fatty Zucker Rat Strains. Endocrinol Diabetes Metab J 4: 413. [crossref]