Abstract
Objectives: Traumatic brain injury (TBI) was associated with increased plasma agonist autoantibodies targeting the serotonin 2A receptor. Repeated TBI exposure is associated with high risk for neurodegenerative and neuropsychiatric complications. Here we tested a hypothesis that repeated TBI is associated with plasma agonist autoantibodies targeting more than one kind of catecholamine G-protein coupled receptor.
Methods: Protein-A affinity chromatography was used to isolate the IgG fraction of plasma in forty-two middle-aged and older adults who had experienced one or more TBI exposures. The Ig (1/40th dilution=7.5 ug/mL) were tested for neurotoxicity in mouse neuroblastoma cells using an acute neurite retraction assay indicative of Gq11/IP3/Ca2+ and RhoA/Rho kinase signaling pathways’ activation. Three different linear synthetic peptides corresponding to the second extracellular loop of the alpha 1A, alpha 2A or serotonin 2A receptors were used as target antigen in different enzyme-linked immunoassays. The second extracellular loop receptor peptides themselves (alpha 1A, alpha 2A) or a fragment (serotonin 2A) were tested for ability to prevent Ig-induced neurite retraction.
Results: Patients who had experienced either repeated TBI (N=10) or a single TBI with a co-morbid autoimmune disease (N=5) were significantly more likely to harbor neurotoxic plasma autoantibodies targeting both alpha 1 adrenergic and serotonin 2A receptors vs. patients having only a single TBI. Ig-induced neurotoxicity was significantly prevented by co-incubation with either 850 nM prazosin (alpha 1 adrenergic receptor) and/or 500 nM M100907 (serotonin 2A receptor) antagonists. Alpha 1 adrenergic receptor and serotonin 2A receptor Ig immunoreactive level and titer were significantly increased in repeated TBI and single TBI/autoimmune patients (N=7-8) compared to age-matched TBI patients without neurotoxic plasma Ig (N=4). SN.8, a linear synthetic peptide corresponding to a conserved region of the second extracellular loop (ECL) of the serotonin 2A receptor completely prevented neurite retraction induced by repeated TBI plasma Ig. A repeated TBI patient harboring alpha adrenergic receptor AAB alone experienced prospective steep decline in cognitive function over two years.
Conclusions: Repeated TBI and TBI with associated autoimmunity harbored more than one kind of neurotoxic catecholaminergic agonist GPCR autoantibody each associated with high risk for steep rate of cognitive decline. Specific immunoassays using the second extracellular receptor loop as target antigen are needed to detect each specific different GPCR autoantibody. A fragment of the second ECL of the serotonin 2A receptor (SN.8) neutralized Ig-induced neurotoxicity in repeated TBI or TBI with associated systemic autoimmunity.
Introduction
Traumatic brain injury (TBI) contributes to substantially increased global disability including the increased risk for later occurrence of major depressive disorder, Parkinson’s disease or dementia [1]. United States military veterans of the conflicts in Afghanistan and Iraq who sustained repeated TBI exposures are also likely to suffer with chronic post- traumatic stress disorder (PTSD). Certain symptoms of chronic PTSD overlap significantly with those of TBI including mood changes (anxiety, depression, suicidal ideation), and altered cognition (attention deficits, memory impairment). A distinct subset of chronic PTSD symptoms (nightmares, panic attacks) involving hyperarousal suggest dysregulated norepineprhine and epinephrine signaling on alpha 1-adrenergic receptors expressed in the brain and sympathetic nervous systems [2].
G-protein coupled receptors are highly druggable treatment targets, and prazosin, a selective alpha1 adrenergic receptor antagonist is effective for the treatment of nightmare disorder in chronic PTSD [3]. Traumatic brain injury and its later neurodegenerative sequelae (major depression, Parkinson’s disease, dementia) was previously associated with increased plasma agonist autoantibodies targeting the serotonin 2A receptor [4,5]. Here we tested a hypothesis that repeated exposures to traumatic brain injury promotes persistent neuroinflammation leading to the development of humoral autoantibodies targeting two, related catecholaminergic GPCRs, i.e. the serotonin 2A and the alpha 1A adrenergic receptors. We used immunoassays specific for the second extracellular loop regions of the alpha1 A receptor and serotonin 2A receptors to test for co-occurrence of these catecholaminergic receptor- targeting autoantibodies (AAB) and its association with clinical outcomes in 42 middle-age and older adult TBI-sufferers.
Methods
TBI Patients
Informed consent for the local Institutional Review Board-approved studies was obtained from all participants prior to blood drawing or cognitive testing. The participants belonged to two different cohorts, A and B. Cohort A included patients age 50 years or older (N=35) among whom approximately two-thirds of patients were previously reported to harbor plasma autoantibodies targeting a second extracellular loop region of the serotonin 2A receptor [4]. Here we report additional bioassay results in thirty of thirty-five consecutively-enrolled patients from cohort A, i.e. neurite retraction in mouse neuroblastoma cells induced by plasma IgG fraction; and whether plasma IgG-induced neurite retraction was significantly reduced (70% or greater) by the presence of either a highly selective serotonin 2A receptor antagonist (M100907) or a selective alpha 1 adrenergic receptor (prazosin) antagonist. Cohort B is comprised of younger patients age 40-60 years who suffered either a single or repeated TBI exposures. In Cohort B we not only tested Ig for bio-assayable 5-HT2AR-like and alpha 1AR-like neurite retraction, but also for immunoreactivity targeting the second extracellular loop of the alpha 1AR (and/or 5HT2AR).
TBI Exposure
All participants in Cohort A experienced direct force traumatic brain injury. Cohort B participants included patients who had experienced either direct force (n=6), blast TBI (n=5) or both types of TBI (n=1). In nearly all cases, the TBI exposure was judged to have been mild.
Peptides
QN.18, PP.15 (PAPEDETICQINEEP), and RT.18 (RQPDAGAAYPQCGLNDET) are linear synthetic peptides corresponding to the second extracellular loop of the human 5HT2AR, alpha1a AR or alpha 2a AR, respectively. SN.8 (SCLLADDN) is comprised of a subregion of the human 5HT2AR involved in mediating receptor activation All peptides were synthesized at Lifetein, Inc (Hillsborough, NJ). The peptides had purity ≥ 95% and were stored under desiccated conditions at -40 degrees C prior to use. On the day of an experiment an aliquot of lyophilized peptide was dissolved in sterile, phosphate buffered saline or deionized water prior to immunoassay or bioassay.
Protein-A Affinity Chromatography
Protein-A Affinity Chromatography was carried out as previously reported [4]. The resulting IgG fraction obtained from TBI patient plasma was stored at 0-4 degrees C prior to bioassay and immunoassay.
Mouse N2A Neuroblastoma Cells
Mouse N2A Neuroblastoma Cells were obtained from the American Type Culture Collection (Rockville, MD).
N2A Neurite Retraction Bioassay for 5HT2AR-like and Alpha 1AR-like Neurotoxicity
We used a previously reported acute neurite retraction assay in cultured mouse N2A neuroblastoma cells [6] to test the IgG fraction of plasma from adult TBI patients for autoantibodies (AAB) causing significant acute neurite retraction. A selective serotonin 2AR (M100907) and alpha 1AR (prazosin) antagonists was each incubated (separately) with TBI patient IgG to test for significant inhibition (>/=70%) of the AAB-induced N2A neurite retraction.
Enzyme Linked Immunoassay
Enzyme Linked Immunoassay were performed as previously described using the QN..18 second extracellular loop of the 5HT2AR [5] as the target peptide antigen. For the alpha 1AR and alpha 2AR immunoassays, the respective specific second extracellular loop peptide for each receptor PP.15 (alpha 1AR) or RT.18 (alpha 2AR) was substituted in place of QN 18.
Rat Plasma, Protein-G Affinity Chromatography, Enzyme Linked Immunoassays
Rat Plasma, Protein-G Affinity Chromatography, Enzyme Linked Immunoassays plasma was obtained from 25-week-old male spontaneously hypertensive rats (SHR) as previously reported [7]. The IgG fraction was isolated using protein-G affinity chromatography as previously reported [8]. Enzyme linked immunoassay was performed as previously described [7] using either the 5HT2AR second extracellular loop peptide QN.18 or the alpha 1A adrenergic receptor second extracellular loop peptide PP.15 as target antigen.
Statistics
Statistical analysis was performed using unpaired Students’ t-test and Fischer’s exact test.
Results
Co-occurrence of Plasma Alpha 1 Adrenergic- and Serotonin 2A-receptor Autoantibodies in TBI
Table 1 shows the clinical characteristics in a subset of 7/30 TBI patients (from Cohort A) whose plasma IgG had the properties of both alpha1AR targeting and serotonin 2AR target AAB. All seven patients had experienced either recurrent TBI or a single TBI exposure in the setting of having an underlying autoimmune disorder. The dose-response curves of IgG-induced N2A neurite retraction in these seven patients was compared to that of twenty additional patients who had experienced a single TBI and harbored either serotonin 2AR-targeting AAB alone or a lower level of uncharacterized AAB activity (Figure 1). Mean potency (neurotoxicity) in a 1/80th dilution from repeated TBI or autoimmune + single TBI plasma IgG significantly exceeded (P < 0.05) potency in an identical concentration of IgG from single uncomplicated TBI (Figure 1).
Table 1: Clinical characteristics in a subset of older adult TBI patients (Cohort A) who harbored both 5HT2AR-like and Alpha 1 AR- like bioactive Ig or Alpha 1 AR-like Ig alone.
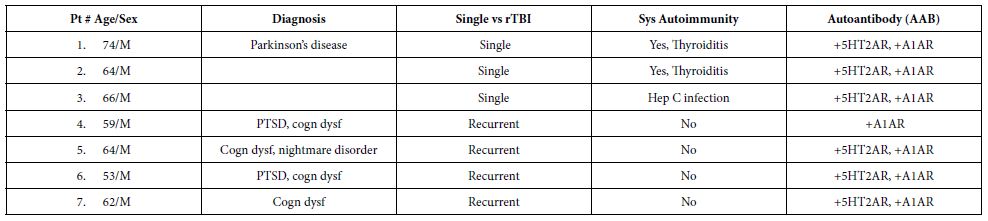
rTBI: Repeated Traumatic Brain Injury; Sys: Systemic; PTSD: Post Traumatic Stress Disorder; cognitive dysfunction.
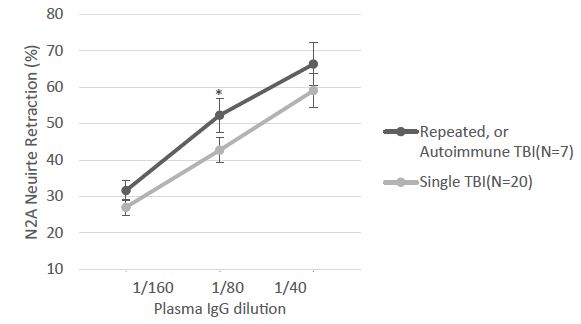
Figure 1: The indicated dilution of protein-A eluate fraction of plasma was incubated with mouse neuroblastoma cells and acute neurite retraction was determined as previously reported [ ]. Results are the mean ± SE of two or more measurements. *P<0.05: mean N2A neurite retraction in Ig from seven repeated or autoimmune TBI plasma significantly exceeded level in Ig from twenty patients who suffered a single TBI, not complicated by an autoimmune disorder.
Younger TBI patients many of whom are US military combat veterans having served in Afghanistan or Iraq they had a high rate of repeated TBI exposure and also suffer from post-traumatic stress disorder, whose symptoms overlap with those of TBI (e.g. anxiety and depression). The clinical characteristics in a subset of younger TBI patients (Cohort B) is shown in Table 2 with comparison to Cohort A. Cohort B patients had significantly lower mean age compared to Cohort B patients (Table 2). Six of twelve Cohort B patients tested had (N2A neurite retraction bioassay) evidence for both plasma 5HT2AR and alpha 1AR-like AAB (Table 2) including four patients who experienced repeat mild TBI and two patients who had a single TBI and a coexisting autoimmune disorder (not show in Table 2). The prevalence of chronic PTSD was quite high in Cohort B (75%) but it did not differ significantly from the PTSD prevalence in Cohort A (42%).
Table 2: Clinical characteristics in nested cohort of twelve younger TBI patients (Cohort B): comparison to subset of Cohort A patients harboring 5HT2A and/or alpha 1A receptor AAB.
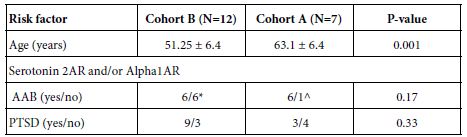
*Four patients had repeated TBI exposures, two patients had single TBI and an autoimmune disorder; ^ six patients harboring both 5HT2A and alpha1- receptor autoantibodies, one patient with only alpha1- receptor autoantibodies. AAB- autoantibodies, PTSD- post traumatic stress disorder. Alpha 1AR- alpha 1 adrenergic receptor.
When the results from Cohort A (N=30) and B (N=12) were combined, a striking association was evident between the presence of 5HT2AR and/or alpha 1AR-targeting bioactive Ig (vs. 5HT2AR Ig alone vs. neither AAB) and either recurrent TBI (7/12 vs 0/15 vs. 0/15; P=0.0016) or single TBI patients having a comorbid systemic autoimmune disorder (P=0.003) (Table 3). These data suggest that repeated TBI exposure carries an equivalent high risk as systemic autoimmunity for development of agonist autoantibodies targeting both 5HT2A and alpha 1A receptors (Table 4).
Table 3: Risk factors associated with combined presence of 5HT2AR and/or Alpha 1AR agonist Ig neurotoxic bioactivity in mouse N2A neuroblastoma cells.
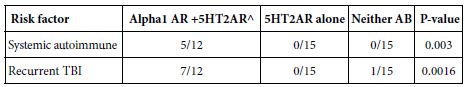
^ N=1 patient with recurrent TBI had alpha 1 AR AAB alone
Table 4: PTSD symptoms in Cohort B TBI patients: relation to AAB status
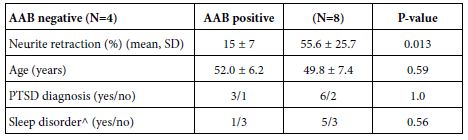
^excluding obstructive sleep apnea
Agonist autoantibodies targeting each kind of receptor have been previously reported to be associated with an increased risk for dementia [9,10]. Neurodegenerative disease (dementia, cognitive dysfunction and/or Parkinson’s disease) was highly prevalent (5/7) among older Cohort A patients harboring both 5-HT2AR and/or alpha 1AR AAB (Table 1). In two such patients with recurrent TBI and chronic PTSD (Pt 4 and 6; Table 1) a 7.5 mg/mL (50 nanomolar) concentration of the plasma Ig caused potent 70-75% neurite retraction after 5 mins exposure in N2A cells (Figure 2A). Neurite retraction was substantially prevented by co-incubation with an 850 nanomolar concentration of prazosin (Figure 2A). Plasma Ig in two patients having Parkinsons’ disease (PD) and/or dementia (Pt 1 and 4; Table 1) mediated dose-dependent potent N2A neurotoxicity (i.e. neurite retraction) (Figure 2B). One of the patients (Patient 4, Table 1) who was observed prospectively (over two years) progressed from near normal baseline cognition to clinical dementia, in serial St. Louis University Mental Status testing (Figure 2c). The Patient 4 plasma alpha 1AR-targeting AAB was not detected in an enzyme-linked immunoassay specific for the second extracellular loop of the 5HT2AR (data not shown in Figure 2).
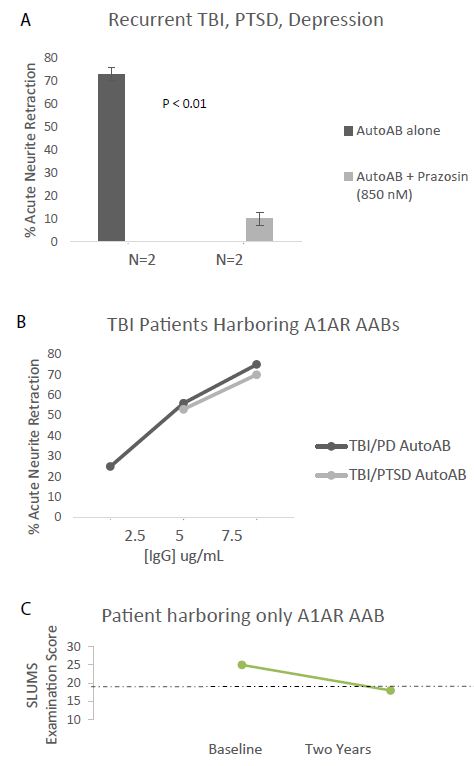
Figure 2: A) Protein A- eluate (7.5 mg/mL) from two patients suffering with recurrent TBI, PTSD (Pts 4&6, Table 1) induced acute neurite retraction in N2A mouse neuroblastoma cells. The Ig- induced neurotoxicity (neurite retraction) was substantially prevented by co-incubating cells together with 850 nM concentration of the alpha 1 AR antagonist prazosin. B) Dose-response curves of neurotoxicity in the protein-A eluate fraction of plasma from TBI/PTSD (Pt 4) or TBI/Parkinson disease (Pt 1, Table 1). C) Pt 4 (TBI/chronic PTSD) harboring alpha1AR agonist Ig tested negative in 5HT2AR enzyme linked immunoassay, but experienced substantial 2-year decline in St. Louis University Mental Status (SLUMS) examination score. Dashed line indicates the cutoff score below which the score is indicative of dementia.
Taken together, these data suggested that younger recurrent TBI patients (including those in Cohort B) may be at substantially increased risk for the future development of clinically significant decline in cognitive function. An immunoassay specific for 5HT2AR Ig alone may not suffice to identify all such high- risk TBI patients.
The bioassay (N2A neurite retraction) data in all 42 Cohort A and B patients is summarized here. Twenty-one of 42 patients (50%) had IgG neurotoxicity which was significantly prevented by co-incubation of N2A cells with 200-500 nanomolar concentration of the highly selective 5HT2AR antagonist M100907 (Figure 3A). Fourteen of 42 patients (33%) had IgG neurotoxicity which was incompletely neutralized by M100907 and was not further characterized (Figure 3B). Eleven of 42 patients (26%) had IgG which was (blocked by M100907) and by co-incubation with 850 nanomolar concentration of the selective alpha 1 adrenergic receptor antagonist prazosin. Six patients (14%) demonstrated a low level of neurotoxicity in plasma which was not further characterized. One of 42 patients (2%) tested had an IgG which was solely inhibited by prazosin (alpha 1AR-like), but not by M100907 (5-HT2AR-like). The total exceeds 100% because patients (26%) harboring more than one Ig were counted twice. In an immunoassay specific for the second extracellular loop of the 5HT2AR, patients having 5HT2AR and/or alpha 1AR agonist-like neurotoxic bioactivity (N=12) had significantly higher level of 5HT2AR immunoreactivity compared to TBI patients (N=14) in whom 5HT2AR- or alpha 1 AR-like neurotoxicity could not be confirmed in bioassays (P=0.024) (Figure 3B). These data are consistent with a prior report that human pathologies’ 5-HT2AR Ig-induced neurotoxicity was highly correlated with plasma 5HT2AR immunoreactivity [5] using an enzyme linked immunoassay having QN..18, the entire second (5HT2AR) extracellular loop peptide, as target antigen.
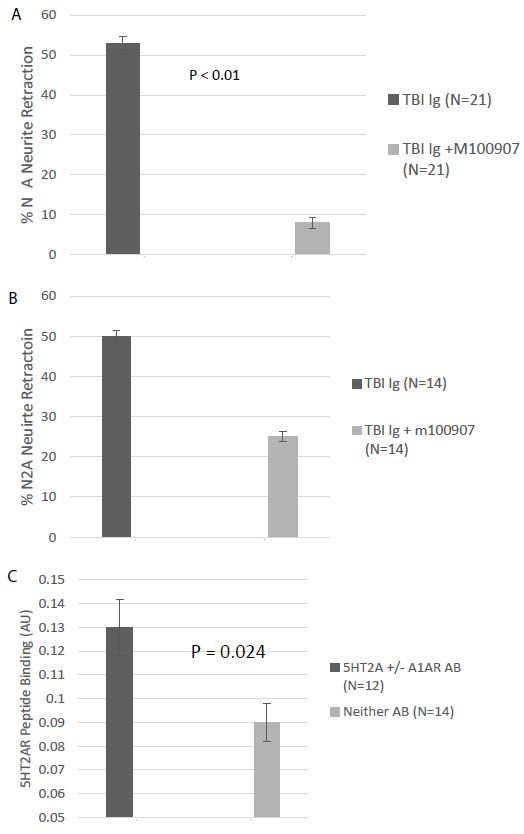
Figure 3: A) Neutralization of neurotoxicity in 21 of 42 TBI protein-A eluates by 200-500 nM concentration of the highly selective 5HT2AR antagonist M10097. B) Incomplete neutralization of neurotoxicity in 14 of 42 TBI protein A eluates by 200-500 nM concentration of M100907; C) binding to 5-HT2AR second extracellular loop peptide in protein-A eluates from TBI patients displaying (N=12) or not displaying (N=12) M100907-inhibitable neurotoxicity.
Sleep disorders including trauma nightmares are common in recurrent TBI patients suffering with chronic PTSD. Neutralization of combined (5HT2AR and alpha 1AR) plasma Ig-induced neurotoxicity in a representative patient with recurrent TBI and nightmare disorder is shown in Figure 4. Prazosin, an alpha 1 adrenergic receptor antagonist, is FDA approved to treat hypertension, and was effective in lessening the symptoms in traumatic nightmare disorder in combat veterans [3]. Here we conducted an exploratory analysis of whether alpha 1 AR agonist AAB may be associated with traumatic nightmare disorder in combat veterans suffering recurrent TBI.
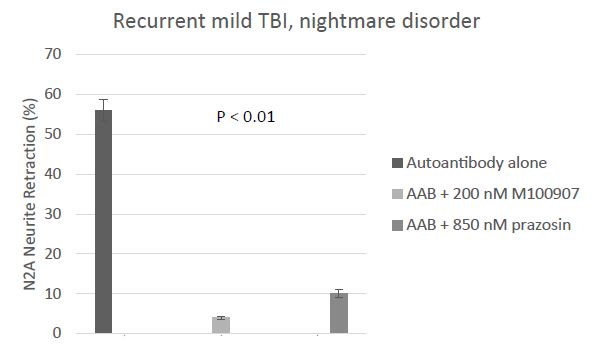
Figure 4: Ninety-three percent neutralization of neurotoxicity in the protein A eluate of plasma from recurrent TBI (Pt 5, Table 1) having traumatic nightmare disorder by 200 nanomolar concentration of the highly selective 5HT2AR antagonist M100907; eight-two percent neutralization by 850 nanomolar concentration of the specific Alpha 1AR antagonist prazosin. Results are mean ± SEM.
The prevalence of sleep-disorder (excluding obstructive sleep apnea) in Cohort B patients harboring alpha 1AR AAB vs Cohort B patients not harboring plasma alpha 1 AR AAB was increased (5/8 vs 1/4), but the sample size was too small and lacked sufficient power to detect a statistically significant difference. Of interest, Patient 4 harboring plasma alpha 1AR AAB was prescribed prazosin which provided symptomatic relief from traumatic nightmares. Yet despite regularly filling the prazosin prescription, he still experienced a substantial prospective decline in cognitive function over a two-year period. This observation may be consistent (in part) with the relatively short half-life of prazosin’s action and reports that alpha 1AR Ig mediates long-lasting receptor activation.
Mouse neuroblastoma N2A neurite retraction induced by the protein A eluate from a recurrent TBI patient harboring both alpha 1AR and 5HT2AR-targeting AAB was completely neutralized by 25 micromolar concentration of Y27632, a selective RhoA/Rho kinase inhibitor or by 50 micromolar concentration of 2-APB, an IP3R antagonist. These data are consistent with known positive coupling of TBI Ig-induced 5HT2AR and alpha 1AR signaling to Gq11/IP3R/Ca2+ and RhoA/Rho kinase signaling pathways [6].
In patients suffering with Alzheimer’s or vascular dementia, alpha 1 adrenergic receptor agonist autoantibodies were previously reported to target an epitope in the second extracellular loop of the receptor [10]. We next used enzyme linked immunoassays specific for binding to the second extracellular loop of the alpha 1 AR or the 5HT2AR to test plasma Ig from repeated and/or autoimmune TBI patients.
Alpha 1AR immunoreactivity and 5HT2AR immunoreactivity were both significantly elevated (P < 0.01) in the protein-A eluates from younger Cohort B patients who suffered repeated or autoimmune TBI (N=8), compared to level in four age-matched TBI patients lacking neurotoxicity (Figure 5A and 5B). Mean binding was approx. 2-fold higher than background (0.04 Absorbance units) in plasma harboring both alpha 1AR, and 5HT2AR AAB specificities. The Ig titer was also significantly elevated in co-occurring alpha 1AR and 5HT2AR plasmas compared to plasmas from TBI patients lacking neurotoxicity (Figure 6A-6B).
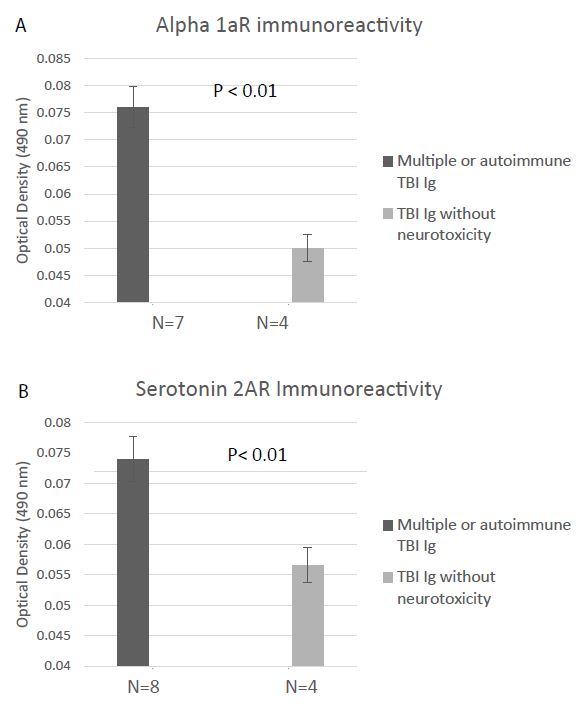
Figure 5: Binding to a linear synthetic second extracellular loop peptide of the alpha 1A adrenergic receptor(A) or of the 5HT2A receptor (B) was significantly increased in protein-A eluates (1/40th dilution) from multiple or autoimmune TBI compared to uncomplicated TBI without bioassayable neurotoxicity. Background binding = 0.04 absorbance units. Results are mean ± SEM.
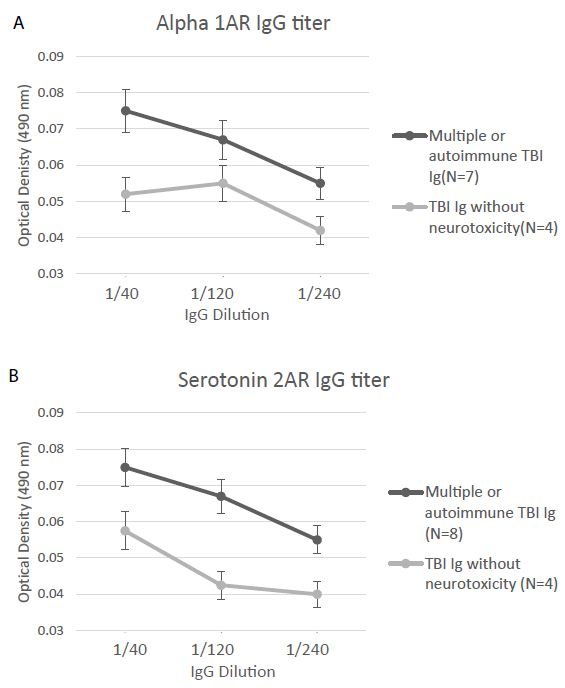
Figure 6: Titer of binding to second extracellular loop peptide of the alpha 1A adrenergic receptor (A) or the 5HT2A receptor (B) was significantly increased in the protein-A eluates from multiple or autoimmune TBI vs. uncomplicated TBI lacking bio-assayable neurotoxicity. Background = 0.04 absorbance units.
As a further test of the specificity of recurrent TBI Ig for the alpha 1 AR and 5HT2AR, we used a synthetic peptide identical to the second extracellular loop of the alpha 2A adrenergic receptor (alpha 2R) as the target antigen in an enzyme-linked immunoassay. Since the alpha 2AR couples to Gi/Go subfamily of G proteins leading to decreased intracellular cyclic AMP, its activation is not expected to cause N2A neurite retraction. Alpha 2R agonism has peripheral sympatholytic effects (decreased blood pressure) and in the CNS mediates presynaptic inhibition of neurotransmitter release (epinephrine, norepinephrine) through actions on auto-receptors. To our knowledge, there has been no reports of spontaneously-occurring alpha 2R-targeting agonist autoantibodies in human disorders.
Mean plasma Ig binding (to the A2AR second extracellular loop peptide) in all eight younger TBI patients tested (Cohort B) was minimally elevated above background (1.25-fold) even though it was significantly higher (P < 0.05) compared to binding in four Cohort B TBI patients lacking Ig neurotoxicity (Figure 7A). The titer of A2AR peptide Ig -binding was similarly low, i.e. 1-1.25-fold vs. background (0.04 AU) in multiple TBI, autoimmune TBI, and in TBI lacking neurotoxicity (Figure 7B). Only one of twelve Cohort B patients tested (a patient with potent neurotoxicity who had suffered a single TBI) harbored alpha2R immunoreactivity at a significant level, i.e. 1.5-fold above background. The clinical significance of this finding is unknown. Taken together, these data demonstrating a much lower level of binding to the second extracellular loop of the alpha 2R, an aminergic GPCR family receptor related to the 5HT2AR [11] confirms the specificity of the results in TBI Ig for the alpha 1 AR and 5HT2AR.
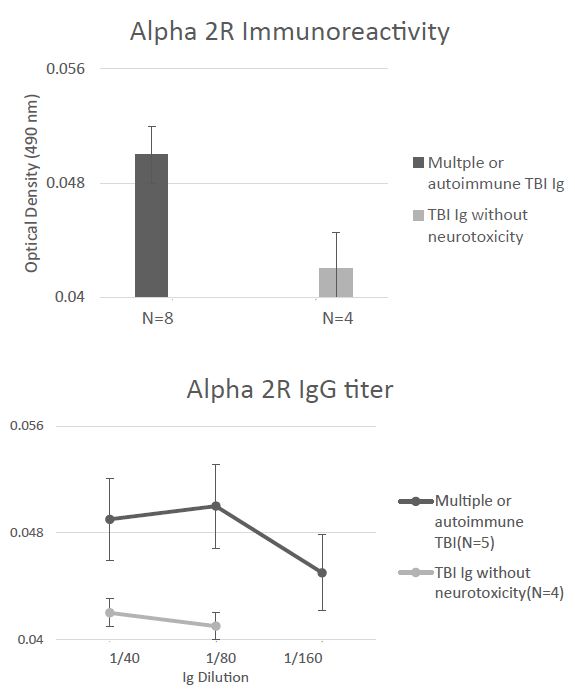
Figure 7: Absence of significant binding to a linear synthetic second extracellular loop peptide of the alpha 2A adrenergic receptor in the protein-A eluates (1/40th dilution) from multiple or autoimmune TBI compared to uncomplicated TBI lacking bio-assayable neurotoxicity. B) Non-significant, low titer of binding to alpha 2A adrenergic receptor second extracellular loop peptide in protein-A eluates from multiple or autoimmune TBI or uncomplicated TBI. Background binding = 0.04 absorbance units.
The orthosteric binding pockets of aminergic, family GPCR are conserved [9]. A novel small peptide antagonist of the 5HT2AR comprised of an epitope (SN.8) in the C-terminal region of the second ECL was previously reported to have completely neutralized the neurotoxic bioactivity in TBI and neurodegenerative disorders plasma Ig [5]. Here we tested for neutralization of N2A neurite retraction in recurrent or autoimmune TBI plasma by synthetic peptides corresponding to the entire second extracellular loop of the alpha 1A (PAPEDETICQINEEP) or the alpha 2A (RQPDAGAAYPQCGLNDET) adrenergic receptors. A twenty microgram per milliter concentration of SN.8 (SCLLADDN) completely neutralized neurotoxicity in 7.5 ug/mL concentration of autoantibodies in recurrent and single/autoimmune TBI plasma (N=4) (Figure 8A). An identical concentration of PAPEDETICQINEEP, which includes the epitope specific sequence APEDE (shown in bold text) previously reported to have blocked bioactivity in dementia plasma AAB [10] only partially prevented (52%) neurite retraction by the same four recurrent and single/autoimmune TBI plasma Igs (Figure 8B). An identical (twenty microgram per millilter) concentration of RQPDAGAAYPQCGLNDET comprising the entire second ECL of the alpha 2AR had no significant inhibitory effect on neurite retraction by two of two recurrent TBI plasma Ig tested (Figure 8C).
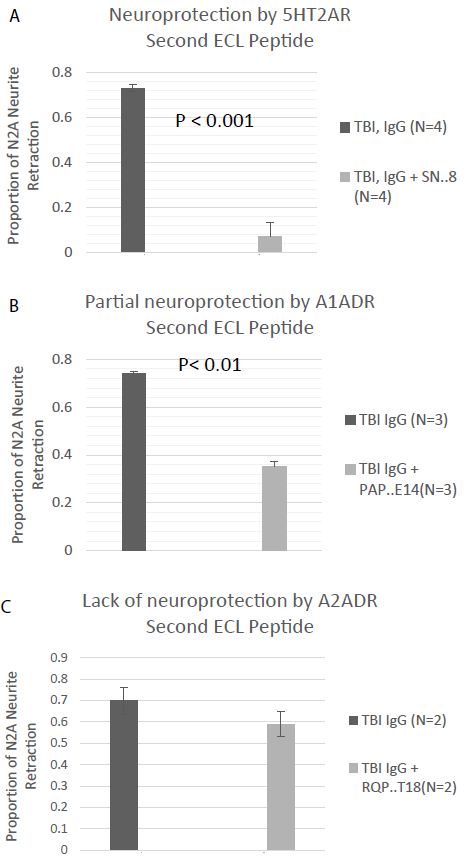
Figure 8: Neurite retraction in a 1/40th dilution of the protein-A eluate from multiple or autoimmune TBI was A) nearly completely prevented by co-incubation with a 20 microgram per milliliter concentration of the SN.8 epitope-specific 5HT2AR second extracellular loop peptide, B) partially prevented by co-incubation with a 20 ug/mL concentration of a linear synthetic second extracellular loop peptide of the alpha 1A adrenergic receptor, or C) unaffected by co-incubation with a 20 ug/mL concentration of a linear synthetic peptide of the second extracellular loop of the alpha 2A adrenergic receptor. Results are mean ± SE.
A one-fourth dilution (~1 µg/mL) of the protein-G eluate of plasma in two 25-week-old male Spontaneously Hypertensive Rats (SHR) displayed two-fold increased binding to the second extracellular loop peptides of the 5HT2AR and the alpha 1A adrenergic receptors in two separate enzyme linked immunoassays (Figure 9A). The protein G eluate (1 µg/mL) from a representative twenty-five-week old male SHR caused significant N2A neurite retraction which was completely prevented by co-incubation with a twenty microgram per milliliter concentration of the SN.8 peptide (Figure 9B). Co-incubation with an identical (20 µg/mL) concentration of the PP.15 second extracellular loop peptide from the alpha1A adrenergic receptor had much less inhibitory effect on SHR Ig-induced N2A neurite retraction (Figure 9B).
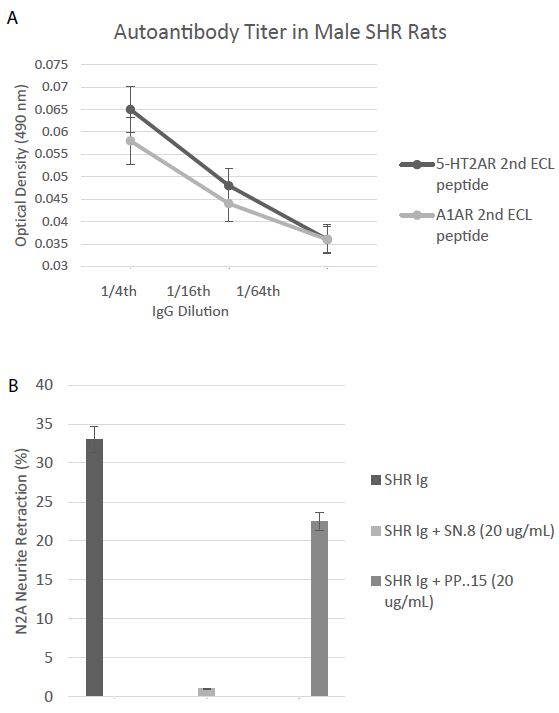
Figure 9: A) Binding to a second extracellular loop peptide of the 5HT2AR or Alpha1A AR was significantly increased in the protein-G eluate fraction of plasma in two, 25-week-old SHR rats compared to background = 0.032 AU. B) Neurite retraction in a 1/4th dilution (~1 µg/mL) of the protein-G eluate of plasma from a representative 25-week-old male SHR rat was completed prevented by co-incubation with a 20 microgram per milliliter concentration of SN..8, but was only 32% inhibited by an identical concentration of PP..15, the alpha 1A adrenergic receptor second extracellular loop peptide. Results are mean ± SEM.
Discussion
The alpha1 adrenergic receptor shares significant amino acid homology with the serotonin 2A receptor [11] in a region of the second extracellular loop involved in mediating long-lasting receptor activation. Both GPCR receptors positively couple to phospholipase C gamma/inositol triphosphate / Ca2+ release signaling pathway to promote IgG neurotoxicity in neuroblastoma cells. Repeated TBI plasma was associated with both significantly increased titer of plasma serotonin 2AR agonist IgG autoantibodies, and the appearance of additional agonist IgG having specificity for the alpha 1-adrenergic receptor based on neutralization of Ig bioactivity by a specific alpha1AR antagonist (prazosin). The striking association between multiple TBI, or single TBI/autoimmune disorder and co-occurrence of bio-assayable and immune-assayable 5HT2AR and alpha 1AR-targeting AAB suggests that specific methods for the early detection of both kinds of AAB specificities may be required to avoid missing TBI patients prone to experience rapid rate of cognitive decline. Patients with underlying systemic autoimmune disorder (e.g. autoimmune thyroid disease) who experienced only a single TBI still had the autoantibody equivalent of ‘repeated TBI exposure’ suggesting such patients may be at higher risk for future neurodegenerative complications compared to patients who experienced a single uncomplicated TBI exposure.
There are currently no medications available to slow the rate of cognitive decline in TBI patients. If future candidate drugs become available for testing, the availability of validated biomarkers (which can serve as surrogates for an increased risk of neurodegeneration) may be quite useful in identifying and monitoring high-risk TBI patients. Increased plasma 5HT2AR-immunoreactive AAB (vs lower AAB) was a significant predictor of a steep (two-year) rate of prospective cognitive decline in a cohort of older adult TBI patients from cohort A [9].
In subsets of Alzheimers’ and vascular dementia [10] autoantibodies targeted an epitope in the second ECL of the alpha 1AR (shown in bold, PAPEDETICQINEEP) located in a region N-terminal to the conserved (underlined) cysteine residue. On the other hand, 5HT2AR- targeting autoantibodies in diverse neurodegenerative pathologies were reported to target (QDDSKVFKEGSCLLADDN) a more highly conserved region of the second extracellular loop (shown in bold letters) including (underlined) amino acid residues which play a key role in mediating aminergic receptor activation [11]. For example, conserved amino acid residues leucine (L) and aspartate (D) play key roles in stabilizing hydrophobic interactions within the transmembrane core (L) and interhelical hydrogen bonding (D) important in activating GPCR signaling, respectively. Since the small hydrophobic SN.8 peptide (SCLLADDN) antagonist contains these two conserved amino acid residues it might ‘compete’ with the same amino acid residues on the native receptor for binding sites important in stabilizing and activating the receptor.
Persistent neuro-inflammation associated with repeated TBI and single/autoimmune TBI may increase the likelihood for occurrence of agonist autoantibodies directed against closely-related catecholamine receptors. Catecholamine receptors are expressed on immune cells and play a role in promoting acute and chronic inflammatory responses [12,13]. Treatment with either 5HT2AR [14] or alpha 1 AR antagonist medication [15] was previously reported to be associated with reduced mortality in severe Covid 19 infection. Plasma agonist 5HT2AR IgG autoantibodies were associated with hyperinflammation in severe Covid-19 infection and increased titer was associated with an increased risk of Covid-19 mortality [16].
Alpha 1 adrenergic receptor autoantibodies were previously reported to increase in subsets of human refractory hypertension [12], however, in the present study only two of forty-two patients (~5%) suffered with refractory hypertension. Alteration in regulatory T cells has been linked with hypertension in the genetically hypertensive male Spontaneously Hypertensive Rat (SHR) strain [17]. Male SHR rats (25-weeks-old) not subjected to TBI, spontaneously harbored both alpha 1A adrenergic and 5HT2A receptor immunoreactive autoantibodies, and the level and titer of the two kinds of plasma catecholaminergic agonist AAB were nearly indistinguishable. Acute N2A neurite retraction induced by male SHR Ig was not only prevented by the 5HT2A second extracellular loop receptor peptide SN.8 (in vitro), but systemic (in vivo) administration of SN.8 in male SHR rats was reported to mediate potent, long-lasting systolic and diastolic blood pressure-lowering [7].
Alpha 1 AR and 5HT2AR are expressed in overlapping, but distinct brain regions. Decreased expression of each receptor was reported in cerebral cortex from dementia patients [18,19] perhaps consistent with shared downstream Gq11/IP3/Ca2+ signaling pathway activation (by AAB) which is toxic in neurons. There has been no prior report of a specific association between alpha 1 AR agonist autoantibodies and TBI. A much larger study is needed to test the hypothesis that alpha 1AR autoantibodies may increase in association with specific symptoms in TBI with chronic PTSD (e.g. trauma nightmares, panic disorder, anxiety).
In summary, plasma alpha 1 adrenergic and serotonin 2A receptor agonist AAB co-occur in patients who experienced repeated TBI or single TBI in the setting of systemic autoimmunity and may contribute to higher risk for dementia. A small peptide antagonist, SN.8 comprised of a highly conserved region of the serotonin 2A receptor whose amino acid residues play key roles in stabilizing aminergic GPCR activation, prevented neurotoxicity from both 5HT2AR- and alpha 1AR-targeting autoantibodies by an unknown mechanism. These observations may have particular relevance to patients expressing both kinds of autoantibodies (without a history of TBI exposure) as the results of a recent study in the Zucker rat strain (which expressed both 5HT2AR and alpha 1AR AAB) indicated significant in vivo neuroprotection (by systemically-administered SN.8) in sham-injured, but not in TBI-injured rats [20].
Acknowledgements
Supported by a grant from the New Jersey Commission of Brain Injury Research (Trenton, New Jersey) NJCBIR22 PIL022 to MBZ.
References
- Walker KR, Tesco G (2013) Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Frontiers in Aging Neuroscience 5: 29. [crossref]
- Charney DS, Woods SW, Nagy LM, Southwick SM, Krystal JH, et al. (1990) Noradrenergic function in panic disorder. J Clin Psychiatry 51: 5-11. [crossref]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, et al. (2007) A parallel group placebo- controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry 61: 928-934. [crossref]
- Zimering MB, Pulikeyil AT, Myers CE, Pang KC (2020) Serotonin 2A Receptor Autoantibodies Increase in Adult Traumatic Brain Injury in Association with Neurodegeneration. J Endocrinol Diabetes 7: 1-8. [crossref]
- Zimering MB (2019) Autoantibodies in Type-2 Diabetes having Neurovascular Complications Bind to the Second Extracellular Loop of the 5-Hydroxytryptamine 2A Receptor. Endocrinol Diabetes Metab J 3: 118. [crossref]
- Zimering MB (2017) Diabetes Autoantibodies Mediate Neural- and Endothelial Cell- Inhibitory Effects Via 5-Hydroxytryptamine- 2 Receptor Coupled to Phospholipase C/Inositol Triphosphate/Ca2+ Pathway. J Endocrinol Diabetes 4: 10. [crossref]
- Grinberg M, Zimering MB (2022) Myristolated Serotonin 2A Receptor Peptide Promotes Long-Lasting Blood Pressure-Lowering and Reno protection in Hypertensive Rat Species. Endocrinol Diabetes Metab J 6. [crossref]
- Zimering MB, Grinberg M, Burton J, Pang K (2020) Circulating Agonist Autoantibody to 5-Hydroxytryptamine 2A Receptor in Lean and Diabetic Fatty Zucker Rat Strains. Endocrinol Diabetes Metab J 4: 413. [crossref]
- Zimering MB, Grinberg M, Myers CE, Bahn G (2022) Plasma Serotonin 2A Receptor Autoantibodies Predict Rapid, Substantial Decline in Neurocognitive Performance in Older Adult Veterans with TBI. Endocrinol Diabetes Metab J 6: 614. [crossref]
- Wallukat G, Prüss H, Müller J, Schimke I (2018) Functional autoantibodies in patients with different forms of dementia. PLoS One 13: e0192778. [crossref]
- Michino M, Beuming T, Donthamsetti P, Newman AH, Javitch JA, et al. (2015) What can crystal structures of aminergic receptors tell us about designing subtype-selective ligands?. Pharmacol Rev 67: 198-213. [crossref]
- Wenzel K, Haase H, Wallukat G, Derer W, Bartel S, et al. (2008) Potential relevance of alpha(1)-adrenergic receptor autoantibodies in refractory hypertension. PLoS One 3: e3742. [crossref]
- Grisanti LA, Perez DM, Porter JE (2011) Modulation of immune cell function by α(1)-adrenergic receptor activation. Curr Top Membr 67: 113-138. [crossref]
- Zimering MB, Razzaki T, Tsang T, Shin JJ (2020) Inverse Association between Serotonin 2A Receptor Antagonist Medication Use and Mortality in Severe COVID-19 Infection. Endocrinol Diabetes Metab J 4: 1-5. [crossref]
- Rose L, Graham L, Koenecke A, Powell M, Xiong R, et al. (2021) The Association between Alpha-1 Adrenergic Receptor Antagonists and In-Hospital Mortality From COVID-19. Front Med Lausanne 8: 637-647. [crossref]
- Zimering MB (2021) Severe COVID-19 Pneumonia is Associated with Increased Plasma Immunoglobulin G Agonist Autoantibodies Targeting the 5-Hydroxytryptamine 2A Receptor. Endocrinol Diabetes Metab J 5: 1-9. [crossref]
- Katsuki M, Hirooka Y, Kishi T, Sunagawa K (2015) Decreased proportion of Foxp3+ CD4+ regulatory T cells contributes to the development of hypertension in genetically hypertensive rats. J Hypertens 33: 773-783. [crossref]
- Lai MK, Tsang SW, Alder JT, Keene J, Hope T, et al. (2005) Loss of serotonin 5-HT2A receptors in the postmortem temporal cortex correlates with rate of cognitive decline in Alzheimer’s disease. Psychopharmacology 179: 673-677. [crossref]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, et al. (2007) Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: evidence of compensatory changes. Neuroscience 146: 471-480. [crossref]
- Grinberg M, Burton J, Pang KC, Zimering MB (2023) Neuroprotective Effects of a Serotonin Receptor Peptide Following Sham vs. Mild Traumatic Brain Injury in the Zucker Rat. org 2023, 2023050004.