Abstract
The ontogeny, the basic and molecular biology and the subsets of human lymphocytes were briefed. Functional, anergic, exhausted, and senescent lymphocyte subsets were detected in severe SARS-COV-2 human infection forms. The aim of the present opinion paper tempted to reveal the comparative biology of the regulatory lymphocyte subsets RLS both in health and disease. RLS, are regulatory natural killer cells NK reg., regulatory B cell, B reg. and regulatory T cell, T reg. to visualize their roles in severe SARS-COV-2 human infections. RLS of NK, B and T cells were found almost associated with dampening the immune mediated tissue injuries following COVID-19 illness. Though it was not clear whether these regulatory lymphocytes did their dampening role in simultaneous, sequential, synergistic and/or antagonistic manner. Show case analysis was made for Nk reg and T reg in COVID-19 cases. This opinion paper suggest a clinical experimental setting in which a group of severely ill COVID-19 patients and normal controls will be blood collected and their lymphocyte separated then subjected to regulatory lymphocyte [NK reg, B reg. and T reg.], flow cytometry, single cell RNA sequencing and interactome studies in order to visualize the way they perform their immunologic roles and the possible interplay between them.
Keywords
Anergy, COVID-19, Exhausted, Lymphocyte, Regulatory, Senescent
Introduction
Regulatory Cells, regulatory immune cells, and regulatory lymphocytes are terms denoted to immunosuppressive lymphocytes RLS. RLS functioned both in the innate and adaptive immune responses and their functions mainly in down regulation of immune over-reaction and/or immune unwanted reactions that served as cellular bases for anergy, tolerance and suppression. Major regulatory subsets mapped both in human health and disease are: NK reg., B reg., and T reg. [1-15]. The present opinion tempts to reveal the actual roles played by regulatory lymphocytes in COVID-19 in a comparative attitude and show case analysis.
Ontogeny
The primordial mother cell, the heamopoietic stem cells of all of immune cells was found in fetal liver in the pregnant woman. As gestation proceeds it will migrate to fetal bone marrow and remained there in postnatal life and adulthood life. In bone marrow the haemopoietic stem cells differentiated into three progenitor cell types; the myeloid, the lymphoid and the lympho-myeloid. The lymphoid cell descend from the lymphoid progenitor cells through the action of haemopoietic cytokines. Lymphoid cells within the continuum of bone marrow microenvironments either stay therein and differentiated in to naïve B cells or trafficked and migrate to thymus. In thymic tissue niche naïve cells undergoes thymic negative and positive selection through thymic proteins and cytokines then mature to be T cells [15]. In two groups of infants, first healthy while the second was premature and stressed. Cord blood lymphocytes were separated from both groups and their phenotypes compared. The phenotyping process was done by fluorometric analysis. This analysis had shown that early third trimester cord blood lymphocytes were as 80-85% fetal T cells belongs to T4+ inducer population and 10% as T8+ suppressor/ cytokine subsets. As gestation proceeds, the T4 and T8 ratio shifts towards adults value together with increase in expression of mature antigen T12. The naïve B cells differentiated into antigens of B1, B2 and B 4 were not changed during gestation of normal healthy infants. Antenatal stress which threatens fetal survival leads to the appearance of phenotypically less mature B cells in circulation for both of the lineages than expected for the gestational age. Cells expressing very early B cell markers B2, B4 increase and exceed the numbers of the more mature B1+ cells in the cord blood most notably due to hypoxic stress during antenatal condition [1]. The fetal blood from fetuses in the second trimester of gestation were collected, lymphocyte separated and analyzed by monoclonal antibody two color immune-fluorescence technique as well as flow cytometry. Lymphocyte surface markers were evaluated. The study indicate that cells of B, T and NK lineages as well as precursor cells can be detected in fetal blood at 18 to 20 weeks of gestation. During this stage of development variable proportions of T & B cells express surface molecules such as CD 1, CD10, CD38, CD45RA indicative of precursor or naïve state; on the other hand the CD57 molecules is not detectable on membrane of NK and T cells and the RO isoform of CD45 leukocyte antigens is synthesized at low percentages of T cells. Such findings suggest that the observed phenotypic peculiarities of lymphoid cells might be due to the inductive easiness of tolerance that occur in early ontogenic stages of the immune system [2]. T, B and NK lymphocytes and their respective phenotypic subsets originate from bone marrow stem cells and their progenitor lineages. Lymphoid cell that migrate to the thymus receive signals through notch commit to the T cell lineage. The lineage development in human beings is critically dependent on IL7 for T cell, IL 4 for B cell and IL15 for NK cells. The specificity and diversity of lymphocytes are gained during the process of generation TCR in T cells and BCR in B cells. The T and B cell repertoire is determined by random variable V, diversity D and joining J somatic gene segments that recombine with an imprecise addition of nucleotides at the segment connection. This recombination is performed by an enzyme complex known as VDJ recombinase that contains the recombination activation gene RAG. RAG proteins expressed onto B & T cells. Successful recombination is determined by the expression of a functional antigen receptor which allows survival and durability of development [3].
Molecular Biology of Lymphocytes
In an experimental setting for a human lymphocyte cell line that was irradiated with 12C ion beams at; 0, 0.1, 0.5 and 2.0 Gy. The transcriptional profiles were evaluated by human gene expression microarray method at 24 hr. post-irradiation. In accordance with microarray assays, there are 1113 genes were up regulated and 833 genes were down regulated in human lymphocyte irradiated with 0.1 Gy 12C ion beams compared to the control group. 1095 genes were up regulated and 1220 down regulated in cells irradiated with 0.5 Gy 12C ion beams and 1055 genes were up regulated and 1356 genes were down regulated with 2.0 Gy. A total sum of 504 genes were differentially expressed in all irradiated groups of which 88 were up regulated and 416 were down regulated. Most of these altered genes were related to; cell cycle, apoptosis, signal transduction, DNA transcription, repair and replication. Thus, the differentially expressed genes at 24 hr post- irradiation increased as irradiation dose increased, up regulated genes gradually decreased and down regulated genes increased. 12C ion beams irradiation could express a number of genes in dose dependent manner which might initiate failure of multiple biological functions of the cell [4].
Lymphocyte Functional Phenotypes and Subsets
Lymphocytes are white blood cells that forms parts of the systemic and mucosal immune system with uniform appearance and varied functions. Bone marrow derived cells, the B cells are involved in the innate immune function of antigen presenting cells, adaptive antibody and IL10 responses. T cells functions in; cell mediated immune response, TH1, TH2, Th17 cytokine production as well as delayed type IV hypersensitivity reactions. TH17 take part in the immune cross- road functions both for innate and adaptive responses. Regulatory T cells the T regs. are commited in down regulation of over-immune and/or unwanted immune reactions. Natural killer cell the NK interplay direct cell mediated cytotoxicity of virus and tumor cells. NK includes innate and adaptive phenotypes [LaRosa and Orange 2008, National human genome institute 2022 [3,5].
Regulatory Lymphocyte Subsets
The B, T and NK lymphocytes are expressing regulatory phenotypes as; B reg., T reg. and NK reg. All of which are involved in down regulation of immune over and/or unwanted reaction noted through the cellular events of immune responses. Such down regulation processes almost mediated by various cytokines through; signal transduction, cell-cell communication and cross-talk [3,7,12].
Regulatory Natural Killer Cells
Cellular Immuno-Biology
A large lymphocyte with finely granular cytoplasm commit in antiviral and anti-cancerous cells. These cells are known as Natural Killer Cells NK cell that belongs to innate immunity with an adaptive immune potentials. From the structural point of view these cells are devoid from both TCR an BCR. But they have; cytotoxicity receptor family (NKp30, NKp44, NKp16, NKp80), type C lectin domain containing receptors (NKG2D), the CD2 superfamily receptor and IgG receptor (CD16). The legend of some of these receptors up regulated by stress and infection like vial haemagglutinins for NKp46, Cd46 for CD2 and IgG for CD16 [LaRosa and Orange [3]. NK cells have distinct subsets with desperate function, location and developmental origin. Peripheral blood NK cells can be of two functional subsets are known based on expression of CD56 and CD16. the first subset isCD56 dim CD 16+ form 90% of the total blood NK effective in killing target cells and secret low cytokines. While, the second is CD56 bright CD16- constitute 10% of the total blood NK but are enriched in secondary lymphoid tissues. The effector functions of the NK are cytotoxicity and cytokine production. These functions are separate in NK subpopulations. The bright and the dim subsets were differing in; (i) the expression of inhibitory and activating receptors (ii) adhesion molecules and (iii) chemockine receptors (Leunemann et al.) [6]. The subsets, Table 1 and immune regulatory subsets of NK cells are; Bright [Gross et al. [7] and CD73 + [8].
Table 1: Natural Killer Cell subsets*
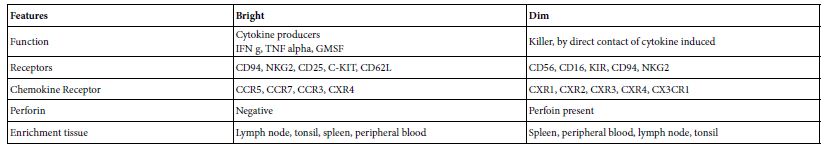
*Adapted from Lunemann et al. [6]
NK Immune Functions
NK have an array of immune functions as; Controlling T cell responses and maintaining homeostasis [Gross et al. [7], Orchestrate immune responses, linking innate and adaptive immune responses and regulating, T, B and DC [9].
Regulation by Natural Killer Cell, A Mechanistic View
NK cells performed their immune regulatory function using either of the following mechanisms; (i) direct contact cytotoxicity of myeloid cells, (ii) polarization of TH1, shaping and dampening through cell mediated cytotoxicity and (iii) antibody dependent blocked of Qa 1 NKG2A interaction resulted in potent NK dependent elimination of T cells [7,12].
Regulatory NK in Human Diseases
The immune regulatory actions of NK cells are operable in a number of human diseases; CD72 NK mediated pathology in tumor micro-environment [8], autoimmune and inflammatory diseases [6] autoimmune liver diseases [Jian and Wang [9] and multiple seclerosis [6,7].
NK in COVID-19
SARS-COV-2 clearance rate, antibody response and disease progression in COVID-19 correlate with NK pathophysiological status and NK dysfunction is linked to disease susceptibility. Thus NK may act as a key element in the switch events from effective to harmful immune responses in COVID-19 illness. NK depletion and dysfunction correlated with severity and anti-fibrotic activity [10]. There were significant reduction in number and function of NK attributed to their exhaustion [11].
Regulatory NK in COVID-19
A bidirectional of cell-cell cross-talk of NK with, neutrophil, DC, and monocyte/ macrophages have shown elimination of neutrophil and DC in a separate experimental settings [12]. High NK count, low T cell CD8+ and regulatory CD56 bright CD16- dim in severe COVID-19 patients [13] as shown in the show case analysis with a notable NK subsets of NK reg. in COVID-19 disease (Table 2).
Table 2: Show case Analysis of natural killer cells in COVID-19
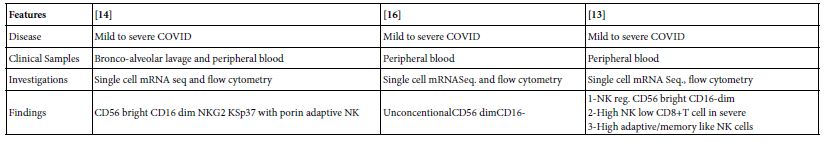
Regulatory B Lymphocyte
Cell Immunobiology
B lymphocytes are bone marrow derived lymphocytes. They developed in bone marrow and matured in the peripheral lymphoid tissues starting with pro B then Pre B, immature B and mature B. The immune potentials of B lymphocytes are multiple. As antibody producing, antigen presenting and cytokine producing as well as immune regulating [3,17].
The Immune Functions of B lymphocytes
Naive B lymphocytes on activation through stimulatory and co- stimulatory signals by either of; antigen, mitogen and/or cytokine they may perform one or more of the following immune functions; (i) antibody production, (ii) antigen presentation, (iii) cytokine production, (iv) immune regulatory and (v) toleragenesis [17,18].
Regulatory B lymphocytes [B reg.]
Regulatory B lymphocyte express immunosuppressive functions via diverse mechanisms. Of which B reg. modulate immune responses through secretion of cytokines, IL10, IL35, and TGFB. Or by direct cell-cell contact dependent mechanisms. B reg. is important to human welfare both in health and disease. In disease, however, they are associated with abnormalities both in numbers and functions. B reg. accounts for 0.5% of human peripheral blood lymphocytes. And expressed a variety of protective and pathologic. They take part in the tissue transplantation tolerance and involved in creating immune prevealiged sites for the uterine environment and neonatal life [17-19].
Regulatory B Lymphocytes Subsets
Human regulatory B lymphocyte is heterogeneous in term of number, origin and localities as shown in the following (Table 3).
Table 3: The Subsets of Human B reg.*
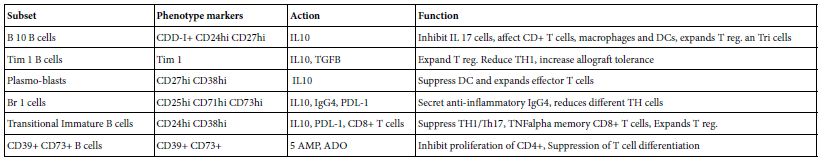
*Adapted from Menon et al. [17]
Mechanisms of Action of B reg
The operable mechanisms by which naïve B lymphocytes becomes B reg. are; (i) change in CD markers and surface immunoglobulins, (i) stimulation by IL10, (iii) stimulation by IL35 and TGFB, (iv) direct cell-cell contact dependent which might be attributed to CD 80 CD86 PD-L 1 CD40L and CDd 1 d, (v) granezyme dependent cell killing, (vi) anergic or tolerizing mechnisms and TLR-BCR engagement mechanisms [17,19].
B reg. in Human health and Disease
B reg. take part in; pathogenesis, protection and or promotion of human disease as in; cancer of lung, infections, malaria, allergic air way inflammation and transplantation Diabetes and pregnancy [17,19].
B reg. in Viral Infections
In human hepatitis B disease immature transition CD19+ CD24hi CD38hi and CD19+Il10+ Breg. involved in regulation of the antigen specific CD8+ Tcells. While, incaseofacquiredimmunedeficiency HIV disease, activated immature transtionalCD19+CD24hiCD38hi Breg. reduces the frequency of HIV infected individuals through suppression of CTL function which cause viral persistence. In Deng fever disease, CD19+CD24hi CD38hi reduces the severity of the disease. Neonatal CD5hiCD10-CD 1chiCD45RACd23loCd24hiC38loIgDloIgMlo Breg. produce IL10 that dampen the beneficial cytokine production by TH1 cells and contribute to severe disease [18].
Breg in COVID-19 Disease
In severe SARS-COV-2 infections the precursors of human transitional CD19hi CD38hi B reg. were reduced [19]. In a clinical setting in which two men vaccinee were enrolled in immune cell flow cytometery, it was evident that Breg was increased post first shot but reduced post to the second COVID-19 vaccine dosage [20].
Regulatory T lymphocytes
Cell Immunobiology
The CD4+ T lymphocytes may be differentiated into lymphocytes that have the ability to suppress other immune cells including T cell responses. These differentiated cells are known as regulatory T cells. Their development from naïve lymphocytes can either be thymic or extra-thymic. The thymic developed are natural T regs and stably expressFoxp3 transcription factor and is eligible for its immune suppressive function. The natural T regs are IL2 dependent and Foxp3 transcription factor producer. The surface markers of natural T regs are CD3+CD4+Cd25+ Foxp3+. While, the extra thymic developed T regs are of inducible nature. The surface markers of the inducible type 1 T regs are CD3+CD4+ [3,21].
Human Tregs Subsets
Three main subsets of T regs are known till date. One of which is natural and the other two were of inducible nature. The inducible subsets are either TGFB or IL10 induced subsets (Table 4).
Table 4: Human Regulatory Lymphocyte Subsets*

*LaRosa and Orange [3]
T regs Immune Functions
T regs expresses an array of immune functions including; negative regulation of T cell responses, sustaining immune tolerance, reserve immune homeostasis, taking part in establishment of lymphocyte anergic state [21].
T regs in Human Diseases
In human host microenvironment T regs. may express either protective or pathologic functions depending on the nature of the surrounding micro-environmental factors. Loss of function mutation in the Foxp3 gene lead to; inflammatory autoimmune disease, immune dysregulation, poly-endocrino-pathy, enteropathy and or graft versus host reaction GVHR. Such mutation may aggraviate human virus infections [21].
Tregs in COVID-19
In early stage of SARS-COV-2 infection, activated Tregs. Populations potently suppress the recruitment of immune cells such as Th1 a CD8+CTLs leading to reducing of the immune responses. In mild infection form, T regs becomes increased in numbers and could attenuate the inflammatory responses and quench the cytokine storm, this event could promotes recovery of the patients. When the infection form proceeds towards severity, Treg depletion can enhance the activation of pro-inflammatory immune cells and production of pro-inflammatory cytokine that leads to hyper-cytokinemia and lung injury [21]. The exact role played by T reg. in COVID-19 seemed to be controversial among different workers all over the world. In a study were 57 mild, severe and recovered patients, peripheral blood mononuclear cell were subjected to study Tregs CD25+ foxp3 has shown increase in proportion of these cell [22]. Other working group use to study 109 mild, severe and recovered patients T regs they were found Cd4+CD25+ CD127+ got increased in both proportion and numbers of Tregs [23]. No change in CD4+CD25+Foxp3+ Tregs have been found in a group of 17 moderate,27 severe and 8 control [24].
Show Case Analysis
A show case analysis was made for two published works; one concerning SARS-COV-2 infected patients in Japan and the other done on two COVID-19 vaccinee men. The investigation in both of the infected Table A and vaccinee Table B, tempted to analyze peripheral blood lymphocytes using flow cytometery and immune informatic approach of lymphocytes including T regs. Researchers reached results of different subsets of Tregs (Table 5) [20,25].
Table 5: Show case analysis of T reg subsets in COVID-19 infected and COVID-19 vaccinee
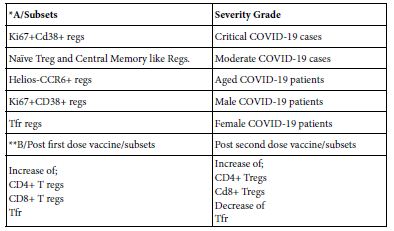
*Sondergraad et al. [25];**Gupta et al. [20]
Conclusion
In comparative Biologic sense regulatory lymphocytes as ordered in accordance with their chronological order of discovery are; T reg., Breg. and NK reg. They are at most immunosuppressive lymphocytes. Each of which may include typical or conventional and atypical or unconventional phenotypes. The main conventional regulatory lymphocytes are; Three T regs, six B regs and two Nk regs. The current newly reported regulatory lymphocytes are; Eight T regs, one Bregs and one NK regs. Hence the total number of these regulatory subsets are; Eleven T regs, seven B regs and three NK regs. These regulatory lymphocytes are of suggested basic sharing in common immune features as: (i) Natural or induced, (ii) initiated by activation and action mechanisms, (iii) mobilized by chemkines and activated by cytokines, (iv) own sets of surface inhibitory and/or stimulatory functions, (v) on function they may associated with age sex or severity of the disease, (vi) the consequences of their immune function in disease either promote protection or promote disease and vii- the mechanistic path can be of use as chemotherapeutic target. The regulatory lymphocytes shared essential roles in pathogenesis and immunology of COVID-19. Though therein presence of gaps in understanding of their exact interplay. The role of T regs in COVID-19 to date it is still in debate. Leem et al. [16] have done a landmark study of natural killer cell including NKregs in COVID-19 patients. Maucourant et al. [14] have been performing landmark investigation on NK immunotypes as related to severity, Bergantini et al. [13] did immunological signature of T cell and NK cells on hospitalized COVID-19 patients. They conclude that higher frequencies of NK and NKreg. Corresponded to lower frequencies of CD3+ and CD4+TCM in severe cases and B cells were not mapped. High frequencies of senescent and exhausted NK, and memory CD4+, CD8+ T cells associated with severe infection forms. B cells compartment were not mapped [Srivastava et al. [26]. Regulatory NK and T regs as well as B cells were investigated through mass flow cytometery with an emphasis on cell networking were tempted [25] performed profiling study to lymphocytes for NK, B and T cells including regulatory lymphocyte subsets have been reported in two vaccine men [Gupta et al. [20]. Hence full lymphocyte profiling using flow cytometery, single cell RNA sequencing and interactome studies including regulatory subsets as well as the deduced network in between lymphocyte and other immune cells to visualize the way they interacts between them and with other cells in mild, moderate, severe and deceased COVID-19 patients still need to uncovered. Thus it is being suggested.
References
- Wilson M, Rosen FS, Scholssman SF, Reinherz EL (1985) Ontogeny of human T and B lymphocytes during stressed and normal gestation; phenotypic analysis of umbilical cord lymphocytes from term and preterm infants. Clin Immunol Immunopathol 37: 1-12. [crossref]
- Luciveo G, Addario VD, Tannoia N, Dellosso A, Gambatesa V, et (1991) Ontogeny of human lymphocytes: Two color fluorescence analysis of circulatory lymphocyte subsets in fetuses in the second triemester. Fet Diag Ther 6: 101-106. [crossref]
- LaRosa DF, Orange TS (2008) I J Allergy Clin Immunol 121: S464-S369.
- Zhang R, Dang X, Zhang Z, Yuan Y, Ren Y, et (2019) Comparison of transcriptional profiles in human lymphocyte cells irradiated with 12C ion beams at )-2.0Gy. Cancer Manag Res 11: 2363-2369. [crossref]
- National Human Genome Institue (2022) Lymphocytes.
- Lunemann A, Lunemann JD, Munz C (2009) Regulatory NK cells. Function In inflammation and auto-immunity. Med 15: 352-358. [crossref]
- Gross CC, Schulto-Mecklenbeck A, Wiendi H, Marcenaro E, Rosbo NKD, et al. (2016) Regulatory functions of natural killer cells in multiple seclerosis. Front Immunol 7:606.
- Neo SY, Wang B, Record J, Ma R, Chen X, et al. (2020) CD73 immune check point defines regulatory NK cells within tumor J Clin Invest 130: 1185- 1198. [crossref]
- Jiao G, Wang B (2016) NK subtypes in regulation of auto-immune liver Gasterointerol Res Pract 2016: 6903496. [crossref]
- Gallardo-Zapate J, Maldonado-Bernal (2022) Natural killer cellexhaustion in sars- cov-2 infections. Innat Immunty 28: 189-198. [crossref]
- Deng X, Terunuma H, Nieda M (2022) Exploring the utility of NK cells in covid-19. BioMedicine 10: 1002. [crossref]
- Di Vito C, Calcaterra F, Coianiz N, Terzoli S, Voza A, et (2022) Natural killer cells in sars-cov-2 infection: pathogenesis and therapeutic implicatons. Frot Immunol 13: 888248. [crossref]
- Bergantini L, d’Alessandro M, Cameli P, Cavallaro D, Gangi S, et (2021) NK and T cell immunological signature in hospitalized patients with covid-19. Cells 10: 3182. [crossref]
- Maucourant C, Filipovic I, Ponzetta A, Aleman S, Cornillet M, et (2020) Natural killer cell immunity related to covid-19. Sci Immunol 5: eabd6832. [crossref]
- Borner K, Teichmann SA, Quardokus EM, Gee JC, Browne K, et al. (2021) Anatomical structures,cell types and biomarkers of human reference atlas. Nat Cell Biol 23: 1117-1128. [crossref]
- Leem G, Cheon S, Lee H, Choi SJ, Jeong S, et al. (2021) Abnormality in NK cell populations is prolonged in severe covid-19 J Allerg Clin Immunol 148: 996- 1006. [crossref]
- Menon V, Hussell T, Shuwa HA (2020) Regulatory B cells in respiratory health and diseases. Imunol Rev 299: 61-73. [crossref]
- Upasani V, Rodenhule-Zybert I, Cantaert T (2021) Review: Antibody independent functions of B cells during viral Plos Pathogens 17: e1009708. [crossref]
- Abebe EC, Dejenie TA, Ayele TM, Baye NG, Teshome AA, et (2021) The role of regulatory B cells in health and disease: A systematic review. J Inflam Res 14: 74-84. [crossref]
- Gupta S, Su H, Agarwal S (2020) Immune response to sars-cov-2 vaccine in two Int Arch Allergy Immunol 183: 350-359.
- Wang Y, Zheng J, Islam MS, Yang Y, Hu Y, et al. (2021) The role of CD4+Foxp3+ regulatory T cell in the immune-pathogenesis of covid-19:implication for treatment. Int J Biol Sci 17: 1507-1520. [crossref]
- Galvan-Pena S, Leem J, Chowdhary K, Michelson DA, Vijaykumar B, et al. (2020) Profound T reg pertuberations with covid-19 bioRxiv 416180.
- Chen X, Haung J, Chen J, Huang Y, Jiang X, et (2020) Characterisitics of immune cells and cytokines in patients with coronavirus didsease-19 in Guangzhou China. Human Immunol 81: 702-708. [crossref]
- Meckiff BJ, Ramire-Suasteyal C, Fajardo V, Chee SJ, Kusnadi A, et al. (2020) Imbalance of regulatory and cytokines of sars-cov-2 CD4+ T cells in covid-19. Cells 183: 1340-1353. [crossref]
- Sondergraade JN, Tulyeu J, Edahira R, Shira Y, Yamaguchi Y, et (2022) Regulatory T cells are central lubs for age, sex, and severity associated networks during covid-19. MedRxivan 22268711.
- Srivasta R, Dhanushkodi N, Prakash S, et al. (2022) High frequency phenotypically and functionally senescent and exhausted CD58+CD57+PDL+ NK cell sars- cov-2 specific memory CD4+ and CD8+ T cells associated with severe disease in unvaccinated covid-19 patients.