DOI: 10.31038/IDT.2024514
Abstract
Worldwide the major causes of viral hepatitis are 5 viruses: the RNA hepatitis A virus (HAV), the NA hepatitis B virus (HBV), the RNA hepatitis C virus (HCV), the RNA hepatitis delta viroid (HDV) and the RNA hepatitis E virus (HEV). Their epidemiology, life cycle, diagnosis, clinical course and associated diseases have been studied in great detail. Furthermore, effective treatment strategies and preventive measures have been developed and entered clinical practice.
lmportantly, with recent political commitments, policy updates and universal availability of highly effective preventive and therapeutic strategies against viral hepatitis B and C, respectively, low- and middle-income countries are scaling up their viral hepatitis prevention and therapy programs. ln this context, Egypt was leading the way for a public health approach to eliminate viral hepatitis C in October 2023.
While better tools and data than ever are now available to prevent, diagnose and treat viral hepatitis, including chronic hepatitis B and chronic hepatitis C and the recent political commitment of low- and middle-income countries with a high burden of viral hepatitis, such as China, lndia and Pakistan, the latest data from WHO show that hepatitis B and C are still a major public health challenge and far from the WHO goal of their elimination by 2030.
Keywords
Chronic viral hepatitis B and C, diagnosis, treatment, prevention, morbidity, mortality
Introduction
Worldwide, the causes of viral hepatitis are 5 hepatotropic viruses: the RNA hepatitis A virus (HAV), the DNA hepatitis B virus (HBV) [Figures 1 and 2], the RNA hepatitis C virus (HCV) [Figures 1 and 3], the RNA hepatitis delta viroid (HDV) [Figure 1] and the RNA hepatitis E virus (HEV). They infect the liver and can present with a broad spectrum of clinical signs and symptoms, ranging from an asymptomatic carrier state to acute/ fulminant hepatitis or chronic hepatitis with the potential to progress to liver cirrhosis and its sequelae, including hepatocellular carcinoma (HCC) [1]. Thus, viral hepatitis can be associated with significant morbidity and mortality and represents a global health care problem. ln the following, the history and epidemiology of viral hepatitis B [2-7] and hepatitis C [8-10], the world-wide burden of these diseases and the goals for their global elimination will be addressed.
Figure 1: Hepatitis B virus (HBV), hepatitis delta viroid (HDV), hepatitis C virus (HCV)
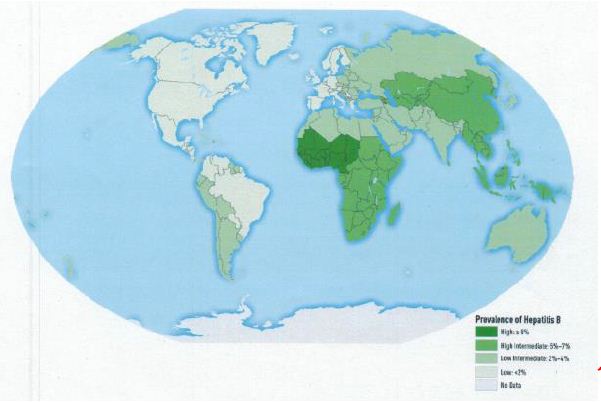
Figure 2: Worldwide prevalence of HBV infection in 2005 [13]
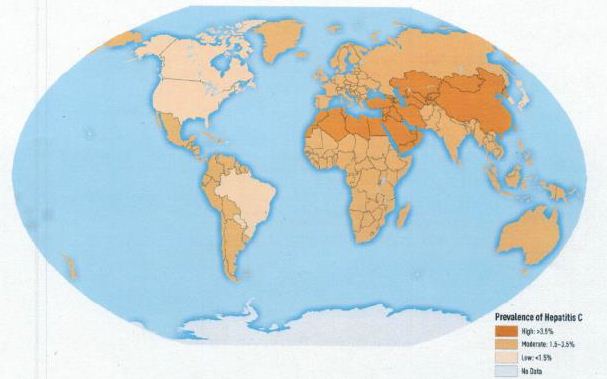
Figure 3: Worldwide prevalence of HCV infection in 2005 [14]
Combined, hepatitis B and C cause daily 3,500 deaths with increasing mortality and 6,000 new infections [1]. Worldwide, an estimated 254 million people are infected with hepatitis B and 50 million with hepatitis C. ln numerous countries, many people remain undiagnosed and even when diagnosed, the number of people receiving treatment is incredibly low. Although therapeutic agents are available at affordable prices, many countries do not take full advantage of this situation. Similarly, many infants do not receive the hepatitis B birth dose vaccination, despite the low cost of this intervention. Unfortunately, funding for viral hepatitis remains limited given the fact that viral hepatitis is about eight times more prevalent than HIV infection but receives less than one tenth of funding [1].
The COVID-19 pandemy severely affected strategies aimed at the elimination of viral hepatitis B and C
The COVID-19 pandemy urged many countries worldwide to adjust their health care priorities. ln particular, the COVID-19 pandemy affected 10 out of 38 WHO focus countries for the viral hepatitis response (China, lndia, lndonesia, Nigeria, Pakistan, Ethiopia, Bangladesh, Vietnam, Philippines and the Russian Federation). Among these 10 countries which account for about 80% of the global disease burden of viral hepatitis B and C, nearly two thirds were very much restricted in their viral hepatitis programs [1]. Together with a universal access to diagnosis, treatment and prevention by the special effort of the African Region, it is the goal to regain the momentum for achieving the Sustainable Development Goals.
Key findings of the WHO Global Hepatitis Report 2024. Overall, 304 million people were living with hepatitis B and C in 2022: an estimated 254 million (84%) with hepatitis B and an estimated 50 million (16%) with hepatitis C. Half the burden of chronic hepatitis is among people between 30 and 54 years old. Approx. 58% of all patients had a history of medical injections or other medical procedures, of newborns and children at risk for mother-to-child transmission of hepatitis B, of indigenous populations and mobile and migrant populations from countries with higher prevalence rates as well of key populations, such as people who inject drugs, people in prison or other closed settings, and men who have sex with men.
According to recent data from 187 countries [1] the estimated number of deaths from viral hepatitis increased from 1.1 million in 2019 to 1.3 million in 2022. 83% were caused by hepatitis B and 17% by hepatitis C. The estimated number of individuals newly infected by viral hepatitis declined from 2.5 million in 2019 to 2.2 million in 2022. Of these, 1.2 million (55%) were infected by hepatitis B and 1.0 million (45%) by hepatitis C. This reduction is due to hepatitis B and C prevention through immunization against hepatitis B and safe injection practices and the initial impact of novel curative antivirals against hepatitis C. Both HBV vaccination and cure of hepatitis C by widely available directly active antiviral agents (DAAs) are central for a sustainable viral response. Taken together, deaths from viral hepatitis B and C, unfortunately, increased from 2019 to 2022 while infections decreased.
Diagnosis, treatment and prevention of hepatitis B and C is still too low to achieve their elimination by 2030. By the end of 2022, 13% of people have been diagnosed with hepatitis B and only about an estimated 3% (7 million) have received long-term antiviral therapy, e.g., adefovir, entecavir, lamivudine, telbivudine, tenofovir disoproxil fumarate and tenofovir alafenamide [1-7].
Between 2015 and 2022, globally 36% of individuals with hepatitis C infection were diagnosed and 20% received curative treatment, e.g., genotype-specific or pangenotypic drugs or drug combinations (DAAs), After decades of interferon-based therapeutic strategies, the availability of DAAs has revolutionized the treatment of patients with chronic hepatitis C of any genotype with HCV elimination rates approaching 95-100% after treatment for 8-12 weeks [8-10]. The DAAs include protease inhibitors (e.g., telaprevir, boceprevir, asunaprevir, simeprevir, faldaprevir), non-nucleoside polymerase inhibitors (e.g., deleobuvir, filibuvir, setrobuvir, tegobuvir), NS5A inhibitors (e.g., daclatasvir, ledispavir) and NS5B polymerase inhibitors (e.g., sofosbuvir, mericitabine).
Vaccination against HBV infection, a cost-saving strategy in countries with high and intermediate endemicity, was applied to an estimated 45% of newborns within 24 hours after birth. Coverage varies between 18% in the African region and 80% in the Western Pacific Region [1].
To date, the global response to viral hepatitis B and C is off-track towards the global elimination of viral hepatitis and far below the global targets for eliminating viral hepatitis by 2030 [1-11]. Major public health activities are expected to reduce the incidence of chronic viral hepatitis by 95%, mortality by 65% and the cost by 15%. The benefits of achieving these global targets will save 2.85 million lives, avert 9.5 million new infections and 21 million cases of cancer. Looking to 2050, this will save nearly 23 million lives and prevent nearly 53 million new viral hepatitis infections and 15 million cases of cancer [1].
Summary and Perspectives
Overall, the worldwide prevalence of hepatitis B and C decreased from 2019 to 2022 while the deaths from these infections increased. ln 2022 about 1.3 million people died from chronic viral hepatitis, similar to the number of deaths from tuberculosis. lmportantly, the COVID-19 pandemy severely affected hepatitis services. The 2024 WHO report [1] presents information on access to health products from 38 WHO focus countries for viral hepatitis response. These countries account for about 80% of the global disease burden of hepatitis B and C. These 38 countries include 10 that account for nearly two thirds of the global burden: China, lndia, lndonesia, Nigeria, Pakistan, Ethiopia, Bangladesh, Viet Nam, Philippines and the Russian Federation. Universal access to prevention, diagnosis and treatment in these countries by 2026 together with a special effort in the African region should enable the global response to gain momentum for the elimination of HBV and HCV infections and their associated morbidities and mortalities by 2030.
The recent WHO report on the global health sector strategies for the period 2022-2030 [11] focuses on their implementation to achieve progress and to fill gaps in the worldwide elimination of HBV and HCV lnfection [12].
Conflict of interests
The author declares no conflict of interest.
Financial disclosure
The author has no financing to disclose.
Acknowledgement
The excellent contribution of Mr. Alain Conard to the content and formatting of the manuscript is gratefully acknowledged.
References
- Global hepatitis report 2024: action for access in low- and middle-income World Health Organization, Geneva 2024.
- Dusheiko G, Agarwal K, Maini MK (2023) New approaches to chronic hepatitis N Engl J Med 388: 55-69. [crossref].
- Naggie S, Lok AS (2020) New therapeutics for hepatitis B: the road to cure. Ann Rev Med 72: 93-105[crossref].
- Sarin SK, Kumar M, Lau GK, et (2016) Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int 10: 1-98. [crossref]
- European Association for the Study of the Liver (2017) Clinical Practice guidelines on the management of hepatitis B virus J Hepatol 67: 370- 398. [crossref]
- Terrault NA, Lok ASF, McMahon BJ, et (2018) Update on prevention. diagnosis, and treatment of chronic hepatitis B. Hepatology 67: 1560-1599. [crossref]
- Yardeni D, Chang K-M, Ghany MG (2023) Current best practice in hepatitis management and understanding long-term prospects for Gastroenterology 164: 42-60. [crossref]
- HCV guidance: recommendation for testing, managing and treatment. Joint panel from the American Association of the Study of Liver Diseases and the Infection Disease Society of America. http://www.hcvguidelines.org/ (accessed on January 01, 2020)
- Spearman CW, Dusheiko GM, Hellard M, et (2019) Hepatitis C. Lancet 394: 1451- 1466.
- Koroumalis E, Voumvouraki A (2022) Hepatitis C virus: Approach to who really needs treatment. World J Hepatol 14: 1-44. [crossref]
- Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period of 2022-2030. Geneva: World Health Organization 2022.
- Thomas DL (2019) Global elimination of chronic hepatitis, N Engl J Med 380: 2041-2050
- Ott J, Stevens GA, Groeger J, et al. (2012) Global epidemiology of hepatitis B infection: New estimates of age-specific HBsAg prevalence and Vaccine 30: 2212-2229. [crossref]
- Mohd Hanafiah K, Groeger J, Flaxman AD, et (2013) Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence Hepatology 57: 1333-1342. [crossref]