DOI: 10.31038/CST.2024934
Abstract
The risk of endometrial hyperplasia (EH) increases during adjuvant therapy of breast cancer (BC) with tamoxifen. Currently, the problem of endometrial hyperplasia and endometrial cancer due to long-term use of tamoxifen is relevant, since the incidence of endometrial pathology has a direct correlation with the duration of use of tamoxifen. In order to improve early detection of endometrial cancer and avoid unnecessary invasive procedures, surveillance by a gynecologist should be tailored to the risk of endometrial cancer in women who have had breast cancer.
We developed a prognostic model to determine the likelihood of developing a composite endpoint (polyp, endometrial hyperplasia, abnormal uterine bleeding) depending on anamnestic and genetic risk factors. A comprehensive association analysis using mathematical modeling allowed us to build a predictive model of the risk of developing such adverse events as endometrial hyperplasia, endometrial polyp and abnormal uterine bleeding. This prognostic model has demonstrated high diagnostic efficiency, which allows its implementation in the clinical practice of gynecologists.
Keywords
Tamoxifen, Endometrial hyperplasia, Endometrial polyp, Abnormal uterine bleeding, Predictive modeling
Introduction
Breast cancer (breast cancer) is hormone-dependent in 60-70% of cases. Due to the fact that estrogens enhance cell proliferation of hormone-dependent breast tumors, endocrine therapy with tamoxifen (TAM) or aromatase inhibitors (IA) is an important stage in the treatment of such patients [1,2]. According to the literature, taking tamoxifen for 5 years reduces the risk of breast cancer recurrence by 39% [3]. The use of tamoxifen may be limited due to the adverse drug reactions (ADR). It is known that the adjuvant breast cancer therapy with tamoxifen increases the risk of endometrial hyperplasia (EH) because tamoxifen acts as an antagonist of estrogen receptors on breast tissue and as an agonist on the endometrium [4]. According to Neven P. and Vernaeve H. 50% of women who received long-term TAM treatment experienced any adverse effects on the endometrium [5]. Currently, the problem of hyperplastic processes and endometrial cancer is especially relevant during long-term tamoxifen administration, since the frequency of endometrial pathology has a direct correlation with the duration of TAM administration [6]. The supervision of a gynecologist should be adapted to the risk of endometrial cancer in women who have undergone breast cancer in order to improve the early detection of endometrial cancer and avoid unnecessary invasive procedures.
The Purpose of the Study
To determine the prognosis of tamoxifen ADR including endometrial hyperplastic processes, endometrial polyps and abnormal uterine bleeding, based on mathematical modeling in conjunction with the carrier of polymorphic variants of genes of cytochrome P450 enzyme and drug transporter proteins.
Hypothesis
Women with breast cancer taking endocrinotherapy have predictors of the development of local gynecological symptoms that require additional attention from an obstetrician-gynecologist. These predictors are not only clinical factors, but also genetic determinants responsible for the metabolism and transport of tamoxifen.
Materials and Methods
A prospective clinical and epidemiological and simultaneous pharmacogenetic study involved 120 patients with luminal breast cancer of stage I-III who were on adjuvant TAM therapy. Anamnestic, clinical, laboratory and instrumental data obtained from a survey of patients and extracts from medical records (results of the last hospitalization) were analyzed. The collection of biological material for genetic research (double buccal scraping) was carried out simultaneously in the Clinic named after Professor Y.N. Kasatkin in 2018-2019 years in Moscow. Informed voluntary consent was signed by all participants of the study before taking the genetic material. Polymorphic variants of the CYP2D6, CYP2C, and CYP3A genes were studied: CYP2D6*4, CYP3A5*3, CYP2C9*2, CYP2C9*3, CYP2C19*2, CYP2C19*3, as well as the polymorphic marker of the ABCB1 gene (C3435T) encoding the P-glycoprotein. Polymorphic gene variants were determined by the polymerase chain reaction method in real time at the Russian Medical Academy of Continuing Professional Education (RMACPE) of the Ministry of Health of the Russian Federation. The study was approved by the Ethics Committee of the RMACPE of the Ministry of Health of the Russian Federation (Protocol No. 1 dated 17.01.2017) and was conducted in accordance with the legislation of the Russian Federation and international regulatory documents. The program SPSS Statistics 26.0 (USA) was used for statistical processing of the results. The normality of the distribution was checked by the Kolmogorov–Smirnov method with the Lilliefors correction. The intergroup differences were assessed using the Student’s t-test and the Mann-Whitney U–test. Comparative analysis was used using either Pearson’s χ2 or Fisher’s exact test. To form mathematical predictive models, the method of constructing a logistic function using binary logistic regression with step-by-step selection of factors and, if necessary, additional construction of ROC curves followed by ROC analysis was used.
Results and Discussion
Tamoxifen’s ADR structure contains both systemic and local ADR, and systemic ones dominate over local ones with the highest representation of tides as vasa-active symptoms from the autonomic nervous system (67.3%). Local gynecological symptoms are represented by endometrial hyperplasia (GE), abnormal uterine bleeding (AMC) and endometrial polyp (PE) are much less common (20.2%; 12.5% and 12.5%, respectively), but in total they amount to 45.2%, which, taking into account a low subjective assessment (frequent asymptomatic course, absence of complaints), requires close attention from obstetricians and gynecologists. The high percentage of local gynecological symptoms we obtained turned out to be close to the data of Neven P and Vernaeve H., indicating 50% of any adverse effects on the endometrium in women taking TAM [5]. Taking into account the high total frequency of occurrence of local gynecological symptoms (%EH+%AUB+%PE=45.2%) and the presence of reliable associative links between local and systemic ADR, as well as genetic and non-genetic parameters obtained earlier [7-9], we developed a prognostic model to determine the development of a combined endpoint (polyp, endometrial hyperplasia, abnormal uterine bleeding). The resulting model included 9 predictors, taking into account the determination coefficient of the Neidlekerk, included 40% of the factors determining the development of the combined endpoint, and was reliable (p<0.001). The observed dependence is described by the equation:
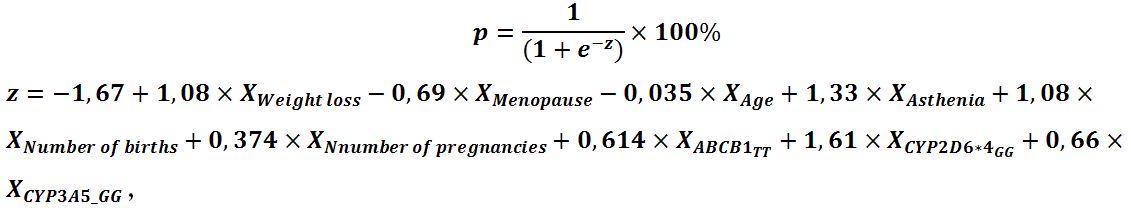
where p is the probability of developing a combined endpoint (polyp, endometrial hyperplasia, AUB) (in fractions of one); XWeight loss – Weight loss during TAM therapy, kg; XMenopause – menopause (0 – no, 1 – yes) ; XAge– age, years ; XAsthenia – asthenia (0 – no, 1- yes); Xnumber of births – number of births; Xnumber of pregnancies – number of pregnancies; XABCB1 3435_TT – TT genotype of polymorphic variant ABCB1 3435 (0 – no, 1 – yes); XCYP2D6_4_GG– GG genotype of polymorphic variant CYP2D6*4 (0 – no, 1 – yes); XCYP3A5_GG– GG genotype of polymorphic variant CYP3A5 (0 – no, 1 – yes).
Based on the values of the regression coefficients, factors such as weight loss, the presence of asthenia, an increase in the number of births, the presence of the TT genotype of the polymorphic variant ABCB1 3435, the presence of the genotype GG of the polymorphic variant CYP2D6*4, GG of the polymorphic variant CYP3A5 have a direct relationship with the probability of developing a combined endpoint. While the presence of menopause, an increase in the number of pregnancies in the anamnesis and an increase in age reduce the likelihood of developing a combined endpoint. Table 1 shows the parameters of the relationship of each of the predictors of the model, including both clinical and anamnestic data and genetic factors responsible for metabolism and transport of TAM, with the chances of developing a combined endpoint of local gynecological symptoms.
Table 1: Evaluation of the relationship between the predictors of the model and the chances of developing a combined endpoint of local gynecological symptoms.
|
Predictor |
COR (95% Cl) | p | AOR (95% Cl) |
p |
| Weight loss |
2.4 (0.93-6.13) |
0.07 | 2.94 (0.98-8.8) |
0.054 |
| Menopause |
0.55 (0.23-1.3) |
0.160 | 0.5 (0.14-1.8) |
0.302 |
| Age |
0.96 (0.9-1.01) |
0.113 | 0.97 (0.89-1.05) |
0.393 |
| Asthenia |
3.2 (1.3-7.3) |
0.009* | 3.78 (1.3-11) |
0.014* |
| Number of births |
1.8 (1.02-3.1) |
0.041* | 2.94 (1.13-7.66) |
0.027* |
| Number of pregnancies |
0.97 (0.76-1.2) |
0.804 | 0.689 (0.42-1.12) |
0.132 |
| ТТ ABCB1 3435 |
2.77 (1.16-6.6) |
0.021* | 1.85 (0.64-5.3) |
0.255 |
| GG CYP2D6*4 |
2.6 (1.07-6.4) |
0.035* | 5 (1.6-15.6) |
0.006* |
| GG CYP3A5 |
1.15 (0.49-2.7) |
0.746 | 1.9 (0.66-5.59) |
0.227 |
*The association with the predictor is statistically significant.
To confirm the results obtained, the most optimal value of the predictive function P was additionally determined using ROC analysis and a ROC curve was constructed (Figure 1).
Figure 1 is a ROC curve characterizing the dependence of the forecast of the combined endpoint on the value of the logistic function P. The area under the ROC curve was 0.827±0.043 (95% CI: 0.743-0.911). The value of the logistic function P at the cut-off point was 0.273. Patients with P values equal to 0.273 or higher were predicted to have a high risk of developing EH, endometrial polyp and AUB, and with P <0.273, a low risk. The sensitivity of the model at the selected cut-off point value was 82.4% (28 correct predictions out of 34 cases of combined endpoint), specificity was 72.9% (51 correct predictions out of 70 cases of absence of combined endpoint development). The overall diagnostic efficiency is 76%. According to the results, independent predictors of the development of the combined endpoint are the presence of asthenia, the number of births, the presence of the genotype GG polymorphic variant CYP2D6*4, the genotype TT polymorphic variant ABCB1 3435 and GG polymorphic variant CYP3A5.
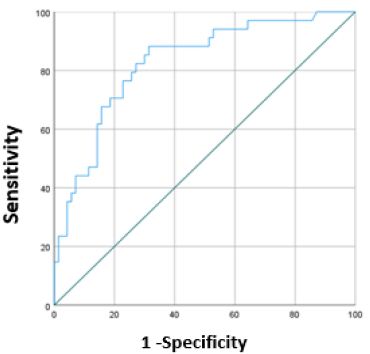
Figure 1: A ROC curve characterizing the dependence of the forecast of the combined endpoint on the value of the logistic function P.
Conclusion
The conducted complex associative analysis allowed us, using mathematical modeling, to construct a prognostic model of the risk of developing combined local gynecological symptoms, such as endometrial hyperplasia, endometrial polyp and AUB. These local gynecological symptoms are a natural manifestation of the pharmacodynamic effects of tamoxifen, as an agonist of estrogen receptors on the endometrium in women with breast cancer. The result obtained determines the need for increased alertness of obstetricians and gynecologists regarding endometrial hyperplastic processes in women with breast cancer undergoing tamoxifen endocrinotherapy and, accordingly, the development of measures for their prevention. In addition, this predictive model has demonstrated high diagnostic effectiveness, which allows it to be implemented in the clinical practice of an obstetrician-gynecologist, including through medical decision support programs.
References
- Huang B, Warner M, Gustafsson JA (2015) Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol Cell Endocrinol. [crossref]
- Rugo HS, Rumble RB, Macrae E, et al. (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol. [crossref]
- Early Breast Cancer Trialists’ Collaborative G (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet. [crossref]
- Chernukha GE, Dumanovskaia MR (2013) Sovremennyie predstavleniia o giperplazii endometriia. Akusherstvo i ginekologiia.
- Neven P, Vernaeve H (2000) Guidelines for monitoring patients taking tamoxifen treatment. Drug Saf. [crossref]
- Chekalova MA, Shabanov MA, Zakharova TI, Kolpakova MN (2010) Significance of ultrasound morphological comparisons in the complex ultrasound diagnosis of the cervix uteri. Tumors of Female Reproductive System.
- Golubenko EO, Savelyeva MI, Sozaeva Zh A, Korennaya V V, Poddubnaya IV, Valiev T T, Kondratenko S N, Ilyin M V (2023) Predictive modeling of adverse drug reactions to tamoxifen therapy for breast cancer on base of pharmacogenomic testing. Drug Metab Personalized Ther. [crossref]
- Savelyeva MI, Golubenko EO, Sozaeva ZA, et al. (2022) Analysis of the complications of endocrine therapy with tamoxifen in breast cancer: clinical and pharmacogenetic aspects. Prospective pharmacogenetic cohort study. Journal of Modern Oncology.
- Golubenko EO, Savel’yeva MI, Sozayeva ZHA, et al. (2022) Klinicheskoe znacheniie geneticheskogo polimorfizma fermentov metabolizma i transporterov tamoksifena pri rake molochnoi zhelezy: rezul’taty populiatsionnogo kogortnogo issledovaniia. Farmateka.