DOI: 10.31038/CST.2024922
Abstract
Primary splenic angiosarcoma is an uncommon and fatal cancer that affects 2 out of every 10 million people worldwide, according to epidemiological incidence rates. For primary spleen angiosarcoma, there are not enough systematic clinical data available, there have been only 90 cases reported since 1950. In this study, we analyzed 25 publications published between January 1950 and November 2022, providing thorough information on 52 cases of main spleen angiosarcoma. We also described a case of primary spleen angiosarcoma with metastases to the liver and bone. This study will go into great length on the etiology, clinical manifestations, laboratory and pathological features, and available treatments for primary spleen angiosarcoma, with the purpose to provide a thorough review and guidance for clinical guidance of this rare disease.
Keywords
Spleen, Angiosarcoma, Systematic review, Clinical features, Pathology, Prognosis
Introduction
Primary splenic angiosarcoma is a rare and fatal neoplasm arising from vascular endothelial cells within the spleen; it was firstly described in 1879 by T. Langerhans. The incidence is 2 cases per 10 million people worldwide [1] and has a poor prognosis due to its high metastatic potential, it was reported that angiosarcoma has the poorest prognosis among all soft tissue sarcomas [2].
Case Report
A female patient, aged 43, was admitted to our hospital with complaints of “Upper left abdomen distension and fatigue persisting for a period of four months”. The patient had repeated discomfort and mild pain in the left upper quadrant for 4 months. There was an absence of any noticeable medical or familial history. Her blood pressure and breathing were normal at admission. There was a mild anemia visible in the conjunctiva. The cardiac rhythm was consistent, and the respiratory vibrations were visible. The abdomen was soft, and there were no swollen superficial lymph nodes, with spleen margin 2cm lower than the left rib. Clinical laboratory results revealed moderate anemia, thrombocytopenia, increased D-dimmer. Red blood cells 2.75×1012/L (normal range, 3.8-5.1×109/ L), hemoglobin 87 g/L (normal range, 115-150g/L), platelets 79×109/L (normal range, 125-350×109/L), D-dimmer 1058 ug/L (normal range, 0-550 ug/L). Liver and kidney functions were normal. The levels of serum tumor indicators, such as carbohydrate antigen 199 (CA199), carcinoembryonic antigen (CEA), α-fetoprotein (AFP), and carbohydrate-125 (CA-125), were all within the normal ranges. We also excluded hepatitis B, hepatitis C, syphilis, AIDS.
Electronic gastrography revealed multiple stomach and duodenal ulcers. Ultrasound Doppler showed a 7 cm × 6 cm mass in the spleen, the internal echo was less uniform, and the blood flow was rich. The abdominal CT showed a mass measuring 13×12cm in the spleen as the right red arrow indicated (Figure 1a), the patient had very large spleen, which oppressed the stomach, gastric cavity was significantly reduced as the left red arrow indicated, we also noticed obvious liver metastasis before surgery (Figure 1b). The patient received complete splenectomy and partial hepatectomy. One month after the surgery, abdominal CT showed tumor metastasis to gastric wall, as we can see from the CT image that some part of gastric walls was thick as the red arrow showed (Figure 1c).
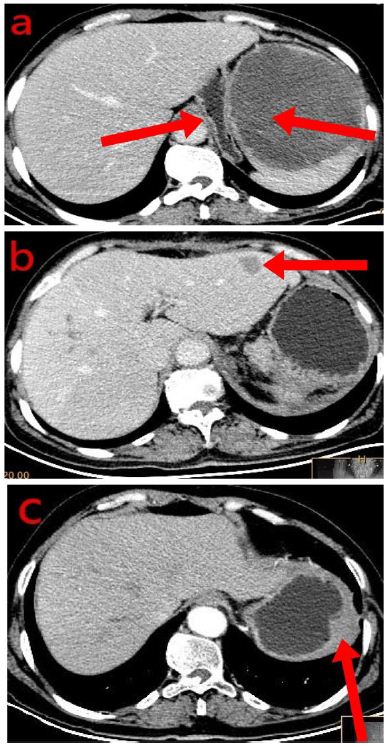
Figure 1: The abdominal CT showed a mass measuring 13 × 12 cm in the spleen as the right red arrow indicated (a), the patient had very large spleen, which oppressed the stomach, gastric cavity was significantly reduced as the left red arrow indicated, we also noticed obvious liver metastasis before surgery (b). The patient received complete splenectomy and partial hepatectomy. One month after the surgery, abdominal CT showed tumor metastasis to gastric wall, as we can see from the CT image that some part of gastric walls was thick as the red arrow showed (c).
We performed an exploratory laparoscopy for her, the neoplasm was located within the central region of the moderately enlarged spleen, and there was no obvious invasion and metastasis to the liver. We performed splenectomy, the cut-off was sent for pathology examination. The spleen was 20cm × 14cm × 10cm, red with foci bleeding, there was an 8 cm × 7 cm × 7 cm neoplasm within it. Under the microscope, tumor cells were arranged into spindles and coincide with each other into irregular revascularization, the nuclear was large, deep stained, nuclear mitotic phase were common, the tumor was polymorphic and composed of spindle cells and multinucleated giant cells arranged in a storiform pattern, which mimics undifferential polymorphic sarcoma. H&E stain, ×10 (Figure 2A left) and H&E stain, ×40 (Figure 2A right). Tumor cells were arranged into spindles and coincide with each other into irregular revascularization. H&E stain, ×40 (Figure 2B left) and H&E stain, ×200 (Figure 2B right). The nuclear was large, deep stained, nuclear mitotic phase were common. H&E stain, ×40 (Figure 2C left) and H&E stain, ×100 (Figure 2C right). The immunohistochemical analysis revealed that the tumor cells exhibited positive expression of endothelial markers of CD34++, FVIII++ and viimentin++ (Figure 3).
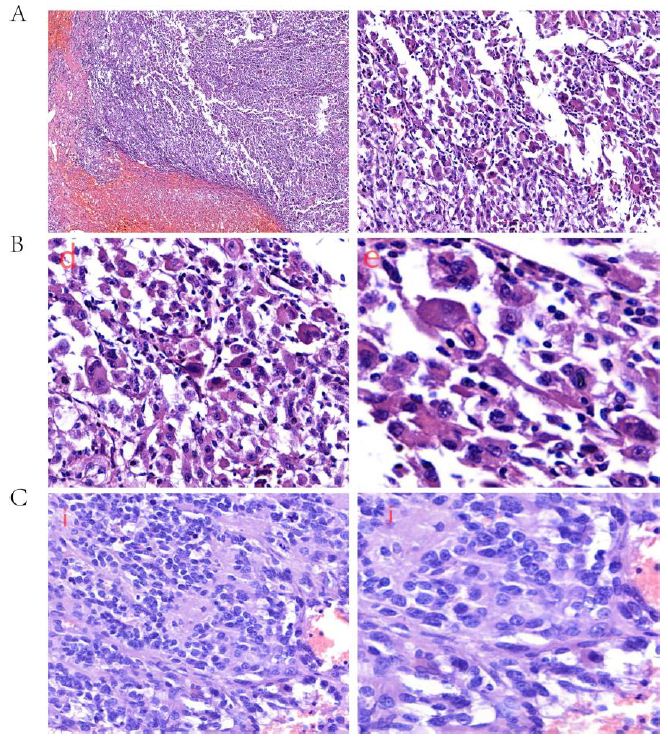
Figure 2: Histopathological examination revealed that the tumor was polymorphic and composed of spindle cells and multinucleated giant cells arranged in a storiform pattern, which mimics undifferential polymorphic sarcoma. H&E stain, ×10 (A left) and H&E stain, ×40 (A right). Tumor cells were arranged into spindles and coincide with each other into irregular revascularization. H&E stain, ×40 (B left) and H&E stain, ×200 (B right). We noticed red with foci bleeding. The nuclear was large, deep stained, nuclear mitotic phase were common. H&E stain, ×40 (C left) and H&E stain, ×100 (C right).
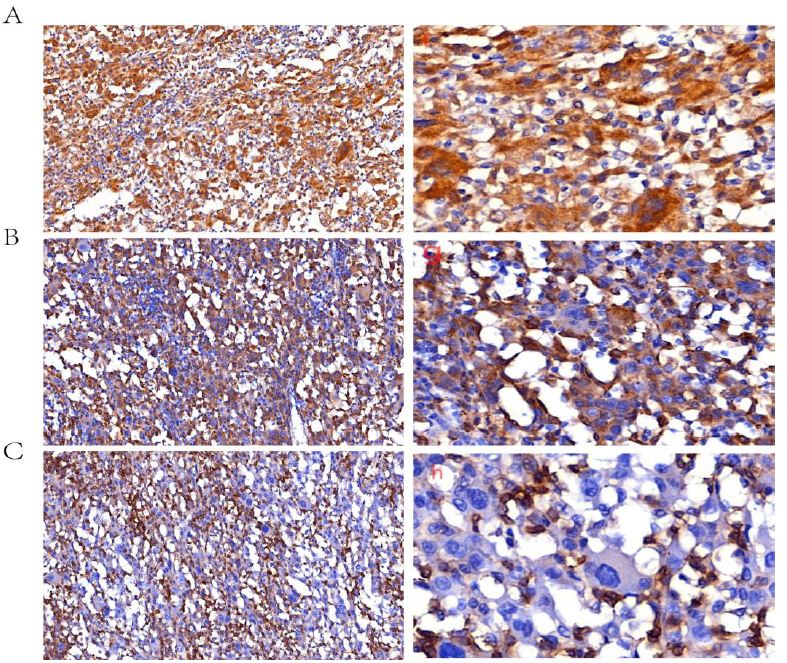
Figure 3: Immunohistochemical stains were performed with a panel of monoclonal antibodies. It showed positive for ALK, ×40 (A left) and ×200 (A right); CD68, ×40 (B left) and ×200 (B right), and negative for CD45, ×40 (C left) and ×200 (C right).
It was negative for desmin, LCA, cytokeratin, lysozyme and S100. Liver nodules were confirmed by immunohistochemical analysis to be homologous with spleen.
The patient received postoperative 4 cycles of IFO and paclitaxel chemotherapy. Although the patient was still alive 5 month after surgery, she experienced body weight loss and cachexia.
Systematic Review
Primary splenic angiosarcoma accounts for 10% of all primary splenic malignancies and 2.6% of all angiosarcoma cases [3]. Benign spleen vascular neoplasm [4] include hematoma, lymphangioma, hemangioma, extra-medullary hematopoiesis (EMH), and sclerosing angiomatoid nodular transformation (SANT). Malignant spleen vascular neoplasms include angionsarcoma, myeloma, lymphoma, and metastases tumors. Littoral Cell angiomas have malignant potential, although they were once considered benign [4]. Spleen angiosarcoma derives from the spleen sinus endothelial cells, extramedullary hematopoietic is typical.
Etiology
Arsenic, vinyl chloride, ionizing radiation, and chemotherapy for lymphoma are among the potential causes [5,6]. Nonetheless, certain research indicates that splenic angiosarcoma arises from pre-existing benign tumors, including hemangioma or hemangio-endothelioma. In the 1970s scientists had explored correlation between vinyl chloride exposure and angiosarcoma, and an estimated 25%-30% of angiosarcoma was related to direct or indirect contact with vinyl chlorideare. Tumor suppressor TP53 and K-RAS gene mutation is found to be responsible for more than 60% of angiosarcoma. The most common mutation sites are axon 1 of K-RAS, and exons 5, 6, 7 and 8 of the TP53 gene. However, gene mutations in angiogenesis signaling are also found to occur in nearly 40% of angiosarcoma, which would reinforce the therapeutic hypothesis to target angiogenesis signaling in angiosarcoma.
Clinical Features
Different from studies that there are more male patients than female, the cases included 24 males and 28 females [7], aged 2 years old [8,9] to 89 years old, the average age at presentation is 54.5 years, with a median age of 49 years. There was a statistically significant difference in the mean age at presentation between females (57 years) and boys (46 years). The most two youngest patients were 2 years old and 7 years old, there were no significant radiation or chemical exposure, gene mutations may play a more important role for these two patients.
The clinical manifestations of PSA vary significantly [10], we made a summary of clinical features of primary splenic angiosarcoma which included 52 cases (Table 1). The most common symptoms at presentation include left upper quadrant pain /abdominal pain (n=33, 63%), chronic weight loss/anorexia/anemia/fatigue (n=22, 42%), complete or incomplete spleen rupture (n=12, 23%). Other less common symptoms include gastrointestinal tract bleeding (n=4, 8%) hemoptysis (n=1, 2%), right flank pain (n=1, 2%). Thrombocytopenia occurs in case 32 and 37; both patients had bone metastasis and moderate to severe anemia.
Table 1: Clinical features of primary splenic angiosarcoma (52 cases).
We included and analyzed 24 males and 28 females, aged 2 years old to 89 years old, with an average age of 54.5 years at presentation (median, 49 years). The mean age at presentation for females was statistically significantly older (57 years) than men (46 years). The most two youngest patients were 2 years old and 7 years old, there were no significant radiation or chemical exposure, gene mutations may play a more important role for these two young patients.
The clinical manifestations of PSA vary significantly. The most common symptoms at presentation include left upper quadrant pain /abdominal pain (n=33, 63%), chronic weight loss/anorexia/ anemia/fatigue (n=22, 42%), complete or incomplete spleen rupture (n=12, 23%). Other less common symptoms include gastrointestinal tract bleeding (n=4, 8%) hemoptysis (n=1, 2%), right flank pain (n=1, 2%). Thrombocytopenia occurs in case 32 and 37; both patients had bone metastasis and moderate to severe anemia.
Physical examination revealed splenomegaly in 29 patients and hepatomegaly in 7 patients. Spleen rupture occurred in 12 patients (n=12, 23%). The most common physical finding was splenomegaly (71%). 17 of 21 patients were reported to have anemia. There were 9 patients without obvious physical findings on admission. The most common metastatic sites in descending order were to the liver (31/52), lung (24/52), lymph nodes (19/52), bone (15/52), adrenal glands (6/52), gastrointestinal tract (6/52), brain (4/52). Diaphragm and stomach were less involved compared with the listed above, 4 patients were found to be widely metastatic at diagnosis.
|
Case no. |
Age/gender | Admission symptoms | Physical findings | Sites of metastasis | treatment |
follow-up OS |
| 1 | 36/M | LUQ Pain | HSM | Liver/Lung/LNs | Surg Chem | 5 mo |
| 2 | 73/M | Abd pain/fatigue/GI bleeding | HSM,F | GI tract | Surg | 2 mo |
| 3 | 76/F | LUQ Pain | SM | Liver/Lung/LNs/Bone | Surg Rad | 7 mo |
| 4 | 51/M | Abd pain/fatigue/fever | NONE | Liver/Lung/LNs | Surg | 9 mo |
| 5 | 73/F | LUQ Pain | SM | Liver[1]/Lung | Surg Rad Chem | 3 mo |
| 6 | 45/F | Abd pain/Spleen rup | SM | Lung/LNs/Bone/Adrns | Surg Rad Chem | 4 mo |
| 7 | 60/M | Abd pain/fatigue | SM | Liver/Lung/Adrns/Diaphragm | Surg Rad | 4 mo |
| 8 | 33/F | Abd pain/Spleen rup | SM | Liver/Lung/LNs | Surg Rad | 4 mo |
| 9 | 29/M | Abd pain/fever | SM | Liver | Surg | 4 mo |
| 10 | 50/M | Abd pain/Spleen rup | SM | Liver/Lung/Adrns/Stomach | Surg | 24 mo |
| 11 | 75/F | Fever/weight loss/fatigue | NONE | Liver/Lung/LNs/Brain | Surg Rad | 10 yrs |
| 12 | 68/M | LUQ Pain | NONE | Widely metastasis | Surg | 8 mo |
| 13 | 27/M | LUQ Pain | SM | Lung/LNs/LNs/Bone/Adrns | Surg Rad | 3 mo |
| 14 | 89/F | LUQ Pain/fatigue | SM | Liver/Bone | NONE | 29 mo |
| 15 | 46/M | Abd Pain/fever/fatigue/hemoptysis | HSM | Widely metastasis | Surg | 1 mo |
| 16 | 85/F | Abd pain/Spleen rup | SM | Liver/Lung/LNs/Stomach /Brain | Surg | 1 mo |
| 17 | 32/M | Spleen rup/Fever/weight loss/fatigue | SM | Liver/Lung/LNs | Surg | 27 mo |
| 18 | 55/M | Found during lymphoma work-up | SM | Liver | Surg | 8yrs |
| 19 | 56/M | Abd pain | SM | Liver/Lung/Bone/Brain /Stomach | Surg Rad | 25 mo |
| 20 | 68/M | Abd pain/weekness/weight loss | SM | Liver/Lung/Bone/LNs | Surg Rad | 21 mo |
| 21 | 59/M | LLQ Pain/fatigue/spleen rup | SM | Widely metastasis | Surg | 29 mo |
| 22 | 64/F | Anorexia | LUQ Pain | Not alaviable | Not alaviable | Not alaviable |
| 23 | 65/M | Abd pain/weekness | NONE | Liver/Lung/Bone/LNs/Soft tissues | Surg Rad | 22 mo |
| 24 | 62/F | Asymptomatic | NONE | Widely metastasis | Surg | 1 mo |
| 25 | 64/F | LLQ Pain/fatigue /spleen rup | NONE | Liver/Lung/Bone/Brain[2] | Surg | 9 mo |
| 26 | 68/F | Abd pain/weekness | NONE | Liver/Lung/LNs/Adrns/Brain | Surg | 1 mo |
| 27 | 69/F | LUQ Pain/weekness/spleen rup | SM | Liver/Lung/Bone | Surg | 12 mo |
| 28 | 49/M | LUQ Pain/asthenia | SM | Liver/Lung/LNs | Surg | 8 mo |
| 29 | 45/F | LUQ Pain/Anemia | SM | Liver/Lung/LNs | Surg | 5 mo |
| 30 | 46/F | LUQPain/anorexia | HSM | Liver/Lung/Bone/LNs | Surg | 3 mo |
| 31 | 55/M | LUQ Pain/hemorrhagic ascitis | HSM Fever | Liver/Lung/Bone | Surg | 35 days |
| 32 | 26/F | LUQ Pain/severe anemia/thrombocytopenia | HSM Fever | Liver/Lung/Bone | Surg | 12 mo |
| 33 | 65/F | Asyptome | NONE | Lung/Bone | Surg | 13 yrs |
| 34 | 77/F | Abd Pain Spleen rup | SM | Liver/Lung/LNs | Surg | 2 weeks |
| 35 | 13/F | LUQ Pain Anemia | SM | No metastasis | Surg | 18 mo |
| 36 | 25/F | Anemia | SM | Bone/LNs | Surg | 12 mo |
| 37 | 61/M | Anemia Leukocytosis Thrombocytopenia | SM | Liver/Bone /LNs | Surg | 5 yrs |
| 38 | 82/F | Left Pleural effusion | SM | Diaghpram | Surg | 8 mon |
LUQ, left upper quadrant; LLQ, left lower quadrant; Abd, abdomen; GI, gastrointestinal; rup, rupture; HSM, hepatosplenomegaly; SM, splenomegaly; LNs, lymph nodes; Adrns, adrenals; Surg, surgery Chem, chemeotherapy Rad, radiotherapy
Upon physical examination, 29 individuals had splenomegaly, while 7 patients presented hepatomegaly. Spleen rupture occurred in 12 patients (n=12, 23%). Spleen rupture can be further classified into complete and incomplete subcapsular rupture. Complete spleen rupture often leads to fetal hemorrhagic shock, whereas incomplete subcapsular rupture may not be obviously detected on admission and may not be that fetal. This could be the reason for the lack of a correlation between spleen rupture and clinical result. Splenomegaly accounted for 71% of all physical findings. Anemia was found in 17 out of 21 individuals.
There were 9 patients without obvious physical findings on admission. The most common metastatic sites in descending order were to the liver (31/52), lung (24/52), lymph nodes (19/52), bone (15/52), adrenal glands (6/52), gastrointestinal tract (6/52), brain (4/52). Diaphragm and stomach were less involved compared with the listed above, 4 patients were found to be widely metastatic at diagnosis. This might be explained by tumor cells transferred through the blood to most common distant organs like liver, lung, adrenal glands, and bone marrow [11,12] etc. A more concise summary of the detailed clinical features of 52 spleen primary angiosarcoma patients was made (Table 2).
Table 2: The most common symptoms at presentation for patients of primary splenic angiosarcoma.
This table concisely summarizes the detailed clinical features of 52 spleen primary angiosarcoma patients. The most common symptoms at presentation include LUQ Pain/ abdominal pain (n=33,63%), chronic weight loss/anorexia/anemia/fatigue (n=22,42%), complete or uncomplete spleen rupture (n=12,23%). Other less common symptoms at presentation include GT tract bleeding, hemoptysis, thrombocytopenia.
|
Symptoms at presentation |
Number (n) |
Percentage (%) |
| LUQ Pain/abdominal pain |
33 |
63 |
| GT tract bleeding |
4 |
8 |
| Complete or uncomplete Spleen rupture |
12 |
23 |
| Chronic weight loss/anorexia/anemia/fatigue |
22 |
42 |
| Hemoptysis |
1 |
2 |
| Thrombocytopenia |
2 |
4 |
| Asymptomatic |
1 |
2 |
| Total |
52 |
100 |
Thrombocytopenia occurs in case 32, 37, both with bone metastasis and moderate/severe anemia. LUQ, left upper quadrant; GI, gastrointestinal.
Diagnosis
The benign lesions found in the spleen include hemangiomas, hematomas, and sclerosing angiomatoid nodular transformation. Primary and metastatic lesions comprise a variety of malignant conditions, including lymphoma, angiosarcoma, and pleomorphic sarcoma. Metastases and lymphomas are included in this study because of their variety and importance, despite their tendency to exhibit hypo enhanced lesions in in comparison to the surrounding parenchyma. Littoral cell angiomas, formerly considered benign, are now being individually investigated due to recent research indicating their potential malignancy [4].
Splenic lesions are frequently observed and frequently occur by chance. Hemangioma, hematoma, lymphangioma, extra- medullary hematopoiesis (EMH), and sclerosing angiomatoid nodular transformation (SANT) are all examples of benign splenic vascular neoplasms [13]. Among the uncommon splenic entities are focal EMH, focal myeloma, angiomyolipoma, and SANT. The most prevalent malignant non-hematolymphoid malignancy of the spleen is primary spleen angiosarcoma. Other malignant conditions that affect the spleen include lymphoma, myeloma, and metastases. We’ll discuss on the clinical manifestation, important imaging results, and correlations of benign, neoplastic, and malignant conditions that might affect the spleen [14].
T. Langerhans initially described spleen angiosarcoma in 1879. The variability of clinical symptoms and diagnostic values related to splenic angiosarcoma is considerable. However, a significant proportion of the patients (75%) have stomach pain, while approximately 25% to 33% exhibit rupture of the affected organ [15,16].
Angiosarcomas are high-grade vascular tumors associated with poor prognosis due to their aggressive nature [17,18]. Because they tend to be aggressive, angiosarcomas are high-grade vascular tumors with a bad prognosis. A timely splenectomy and cytotoxic chemotherapy after an early diagnosis may be useful treatment options, according to anecdotal findings.
Splenomegaly was observed in 85% of patients during macroscopic examination. Distinct lesions were observed in 88% of patients upon sectioning, exhibiting a range of characteristics including well-defined solid nodules as well as poorly characterized areas of necrosis and bleeding related to cystic spaces. The tumors exhibited heterogeneity at a microscopic level, although all instances exhibited a focal vasoformative component that was bordered by atypical endothelial cells. The study revealed the presence of solid sarcomatous, papillary, and epithelioid development patterns. In two cases, the component of the solid sarcomata had similarities to fibrosarcoma, whereas in one case, it displayed similarities to malignant fibrous histiocytoma. Frequent observations included hemorrhage, necrosis, hemosiderin, extramedullary hematopoiesis, and intracytoplasmic hyaline globules.
The findings from a series of immunohistochemical experiments indicated that a significant proportion of tumors had immunoreactivity for a minimum of two markers associated with vascular differentiation (CD34, FVIIIRAg, VEGFR3, and CD31), as well as at least one marker indicative of histiocytic differentiation (CD68 and/or lysozyme) [19]. In all cases, metastases occurred at some point throughout the disease’s course [20-22]. After the last follow-up, only two patients remained alive, one with disease after 8 years and the other without disease at 10 years. Of the 26 patients, 26 died of their condition despite rigorous therapy. [23]. In summary, primary spleen angiosarcoma is a highly aggressive tumor that, in nearly all cases, results in mortality [24]. According to immunohistochemistry study, most of the spleen angiosarcomas co-express histiocytic and endothelial markers, indicating that some tumors may arise from spleen lining cells [25].
The diagnosis was based on histopathological results. Microscopically, the tumors were heterogenous; hemosiderin, hemorrhage, necrosis, and extramedullary hematopoiesis were frequently identified [26]. Differentiating benign vascular tumors from malignancies with modest atypia was typically challenging. The ability of tumors to generate blood vessels was frequently impaired in cases of severe atypia [27]. Only instances with moderate or severe cytology abnormal exhibited the presence of peculiar tumor giant cells. The immunohistochemical analysis demonstrated that tumor cells exhibited immunoreactivity for markers associated with vascular differentiation, including CD34, FVIIIRAg, and CD31. Additionally, vimentin was found to be commonly positive, while S-100 protein and cytokeratin were generally observed to be negative. Abnormal laboratory findings included anemia, leukocytosis, and thrombocytopenia. Moderate to severe anemia and thrombocytopenia would indicate bone metastasis on most occasions. Many tumor markers, including AFP, CEA, CA-125, and CA19-9, were either slightly increased or within normal ranges. Radiologists should take note of an enlarged spleen that shows low attenuation on computed tomography, as well as a single or numerous heterogeneous nodular masses in the liver.
Imaging Manifestations
The imaging results showed an enlarged spleen with lobules, as well as heterogeneous nodules that had significant and uneven enhancement following contrast injection and spontaneously hyper dense regions [28]. Bone lesions with visible veins and arteries were present, in addition to metastases to the liver and lungs [29,30]. Lymphoma and metastases often exhibit hypo-enhancing lesions compared to the surrounding tissue [4]. The CT and MR imaging results indicated that the lesions had a hemorrhagic character and contained higher levels of iron [31].
Treatment Options
Early clinical symptoms for spleen primary malignancy are not typical [13]. Most patients were diagnosed at an advanced stage and had poor prognosis. Considering the treatment options, timely identification and immediate removal of the spleen before it ruptures and peritoneal dissemination is crucial for long-term survival, so early detection is a hot topic for angiosarcoma [5]. There have been controversies on the use of chemotherapy and radiotherapy in primary spleen angiosarcoma. Adjuvant cytotoxic chemotherapy can be effective treatment options for multiple metastases that cannot be excised. Kamocki [15] reported a 57-year-old woman with spleen angiosarcoma and liver metastasis received 8 rounds of weekly-based paclitaxel before undergoing metastatic liver resection; this was followed by a pathological full response. But overdose chemotherapy may impair immune function and acerate progression of tumors, in our 52 cases, there were four patients survived more than 9 years, none of them received chemotherapy or radiotherapy. This might e ascribed to complete resection of tumor. Radiotherapy is helpful for doubtful regional resection; radiation on bone metastasis is helpful for the relieving pain.
We reported a case of a 46-year-old female patient who had liver metastases and primary splenic angiosarcoma (PSA); she died soon 35 days following surgery. Clinicians can obtain a thorough review from this case report, the systematic review, and the in-depth analysis of 52 instances. When the patient exhibits symptoms such as upper abdomen pain and abnormalities in the blood cell count (such as anemia, leukocytosis, thrombocytopenia, and/or high erythrocyte sedimentation rate), it is advisable to consider the possibility of PSA. Furthermore, imaging evaluation is crucial for the timely detection of PSA.
Availability of Data and Materials
The data generated in the present study may be requested from the corresponding author through 109274952@qq.com
Authors’ Contributions
Conception and design were performed by Rui Wang. Data analysis and interpretation were performed by Yuan Fang and Jingqiu Zhang. Manuscript writing was performed by Rui Wang and revised by Jingqiu Zhang. Final approval of manuscript was performed by all authors who read and approved the final manuscript.
Acknowledgement
None
Grant Support
Rui Wang is founded by China Scholarship Council (202206920039). This research was supported by funds from Natural Science Foundation of Suqian Science and Technology Bureau (K201903, Z2018076, Z2018213 and Z2022065). Jiangsu Association for Science and Technology (JSTJ-2022-004).
Ethics Approval and Consent to Participate
The patient reported in this study was told for the purpose and process of this study and had written informed consent according to the guidelines of the hospital’s human associated research.
Patient Consent for Publication
Not applicable.
Competing Interests
The authors indicated no potential conflicts of interest.
References
- Batouli A (2016) Primary Splenic Angiosarcoma: Clinical and Imaging Manifestations of This Rare Aggressive Neoplasm. Curr Probl Diagn Radiol 45(4): 284-7. [crossref]
- Coppola S (2017) Demanding Diagnosis of Splenic Angiosarcoma as Cause of Delayed Treatment of Spontaneous Splenic Rupture: A Case Report and Literature Case Rep Surg 3(4): 625-632. [crossref]
- Alexandrino H (2013) Rupture of splenic angiosarcoma: a rare cause of spontaneous BMJ Case Rep 6(4): 225-232. [crossref]
- Bowerson M (2015) Hot spleen: hypervascular lesions of the Abdom Imaging 40(7): 796-813. [crossref]
- Ferreira BP (2012) Systemic therapy in primary angiosarcoma of the spleen. Rare Tumors 4(4): e55. [crossref]
- Alexandrino H (2013) Rupture of splenic angiosarcoma: a rare cause of spontaneous haemoperitoneum. BMJ Case Rep 5(7): 796-813. [crossref]
- Coppola S (2017) Demanding Diagnosis of Splenic Angiosarcoma as Cause of Delayed Treatment of Spontaneous Splenic Rupture: A Case Report and Literature Case Rep Surg 2(3): 625-632. [crossref]
- den Hoed ID (2005) Metastasized angiosarcoma of the spleen in a 2-year-old Pediatr Hematol Oncol 22(5): 387-90. [crossref]
- Mejri A (2015) Pediatric Primary splenic angiosarcoma: A very rare disease. Tunis Med 93(4): 266-8.
- Aqil B, LK Green, S Lai (2014) Primary splenic angiosarcoma associated with anemia, leukocytosis and thrombocytopenia. Ann Clin Lab Sci 44(2): 217-21. [crossref]
- Wang C (2004) Bone marrow metastasis of Pathol Res Pract 200(7-8): 551-5.
- Anoun S (2014) Primary splenic angiosarcoma revealed by bone marrow Turk J Haematol 31(4): 408-10. [crossref]
- Kohutek F, L Badik, B Bystricky (2016) Primary Angiosarcoma of the Spleen: Rare Diagnosis with Atypical Clinical Case Rep Oncol Med 5(3): 490-526. [crossref]
- Thipphavong S (2014) Nonneoplastic, benign, and malignant splenic diseases: cross- sectional imaging findings and rare disease entities. AJR Am J Roentgenol 203(2): 315-22. [crossref]
- Kamocki Z (2013) Primary splenic angiosarcoma – the same diagnosis yielding two different clinical pictures. Case report. Contemp Oncol (Pozn) 17(2): 218-21. [crossref]
- Alexandrino H (2013) Rupture of splenic angiosarcoma: a rare cause of spontaneous BMJ Case Rep, (3): 315-322. [crossref]
- Thipphavong S (2014) Nonneoplastic, benign, and malignant splenic diseases: cross- sectional imaging findings and rare disease entities. AJR Am J Roentgenol 203(2): 315-322. [crossref]
- Kamocki Z (2013) Primary splenic angiosarcoma – the same diagnosis yielding two different clinical pictures. Case report. Contemp Oncol (Pozn) 17(2): 218-21. [crossref]
- Falk S, J Krishnan, JM Meis (1993) Primary angiosarcoma of the spleen. A clinicopathologic study of 40 Am J Surg Pathol 17(10): 959-70. [crossref]
- Wang C (2004) Bone marrow metastasis of Pathol Res Pract 200(7-8): 551-5. [crossref]
- Yang KF (2016) Primary splenic angiosarcoma with liver metastasis: A case report and literature World J Gastroenterol 22(12): 3506-10. [crossref]
- Plotnik AN (2008) Splenic angiosarcoma metastasis to the J Clin Neurosci 15(8): 927-9. [crossref]
- Neuhauser TS (2000) Splenic angiosarcoma: a clinicopathologic and immunophenotypic study of 28 cases. Mod Pathol 13(9): 978-87. [crossref]
- Plotnik AN (2008) Splenic angiosarcoma metastasis to the J Clin Neurosci 15(8): 927-9. [crossref]
- Neuhauser TS (2000) Splenic angiosarcoma: a clinicopathologic and immunophenotypic study of 28 cases. Mod Pathol 13(9): 978-87. [crossref]
- Serra JT (2016) [Myelophthisis and kasabach merrit syndrome as initial manifestation of splenic angiosarcoma]. Rev Fac Cien Med Univ Nac Cordoba 73(4): 297-301. [crossref]
- Bowerson M (2015) Hot spleen: hypervascular lesions of the Abdom Imaging 40(7): 2796-813. [crossref]
- Ha HK (1994) Primary angiosarcoma of the CT and MR imaging. Acta Radiol 35(5): 455-8. [crossref]
- Batouli A (2016) Primary Splenic Angiosarcoma: Clinical and Imaging Manifestations of This Rare Aggressive Neoplasm. Curr Probl Diagn Radiol 45(4): 284-7. [crossref]
- Serra JT (2016) [Myelophthisis and kasabach merrit syndrome as initial manifestation of splenic angiosarcoma]. Rev Fac Cien Med Univ Nac Cordoba 73(4): 297-301. [crossref]
- Batouli A (2016) Primary Splenic Angiosarcoma: Clinical and Imaging Manifestations of This Rare Aggressive Neoplasm. Curr Probl Diagn Radiol 45(4): 284-7. [crossref]