DOI: 10.31038/SRR.2019212
Abstract
Objective: To determine whether the vocal folds length, along with the acoustic voice parameters measurements, can predict the moment of upcoming voice mutation and assess the process of a child’s maturation.
Study design: A cohort study started with examination of children at a premutation age, and a follow up 2.5 and 5 years later.
Setting: Referral center (Claros Otorhinolaryngology Clinic)
Subjects and methods: Children at a premutation age were examined, with a follow up at a mutation and postmutation age. During each visit a CT examination was performed to determine vocal folds length, followed by an examination of the acoustic voice parameters and a videolaryngoscopy and videostroboscopy. Obtained values were analyzed statistically to find the correlations between them and the reported age of mutation.
Results: 50 children (25 males aged 11.5, and 25 females aged 9.5, with a follow up 2.5 and 5 years later) were examined. A study started with 73 children, but 23 of them failed to attend the first or second follow up. Statistical significance was reported for a correlation between the age of mutation and loudness in boys aged 14 (r = 0.48, b = 0.31), vocal folds length in boys aged 14 (b = – 2.18), and loudness in boys aged 11.5 (b = -0.15); and for girls for a correlation between the age of mutation and decrease of fundamental frequency between ages 9.5 and 12 (r = 0.5, b = 0.01).
Conclusion: The parameters mentioned above have a correlation with the moment of mutation and might in future become an additional way of evaluating a child’s development.
Keywords
Voice mutation, Voice break, Vocal fold length, Voice acoustic parameters, Child’s development
Introduction
Proper development in a child can determine his/her educational, professional and emotional future. We watch carefully during child’s growth if this process is not disturbed. However, it is not easy to evaluate; especially during puberty, which is unique to every child and is determined by such an unpredictable and complex factor as the game of hormones [1]. Many authors agree that, while assessing the moment of puberty in girls is easy because of the presence of menarche and breast growth, it is more difficult in boys because of a lack of these concrete breaking moments and its extended character [2, 3]. It is well known that, in contrast, the situation is opposite with respect to mutation; i.e. it happens in a much more subtle way for girls, whilst for boys mutation happens suddenly, more dramatically, and more noticeably [2, 4, 5]. The interesting phenomenon of voice break during puberty has tempted many authors to evaluate the development of a child by assessing the age of voice break as a clean sign of puberty.1, 6, 7 Although exploring the subject of mutation by evaluating the acoustic parameters of the voice has received a reasonable amount of attention in the literature [6, 8–11], the other ways of predicting the time of mutation, especially by examining the length of vocal folds based on CT scans, are to the best of our knowledge underexplored. We conducted our research to address this gap.
Our Clinic is a widely known consultancy for professional opera singers of the Gran Teatro del Liceo in Barcelona, specializing in the issue of voice since 1970, and also providing medical support for a large number of Spanish children. The medical data presented in this article is the result of our work over the past five years. The objective of our research was to establish correlations between the change of vocal folds length and acoustic parameters and signs of voice break described by children and their parents, and therefore to determine which combination of parameters would be the best to evaluate a child’s development.
Changes in the vocal box over the growth of a child are the consequence of complex coordination between the respiratory, digestive and nervous systems [12], as well as anatomical, histological and neurological modifications [13]. In comparison to the adult larynx, the pediatric larynx has disadvantages in voice production on an anatomical level [4], insofar as the ratio of the membranous vocal fold length to the total vocal fold length is lower, the cartilaginous framework is less rigid, and the incidence and degree of posterior glottic chink is increased [14]. Histologically, the pediatric larynx also varies a lot compared to the adult one, which manifests mainly in increased cellularity and decreased cellular differentiation and organization [15], as well as in the lamina propria which begins as a monolayer [16, 17], and changes into a bilayer around the age of 10 and into a trilayer after puberty [18]. Some authors argue that the triple structure of lamina propria occurs already at the age of 7 [4, 19], however, it is widely accepted that the distribution and composition of the collagen and elastic fibers do not mimick those of adults until puberty [15, 16, 18, 20]. Other differences in the pediatric larynx include elevated overall subglottic pressure and recruitment of a greater percentage of pulmonary capacity [4, 21], which changes during mutation.
The reason for these changes lies mainly in the histological structure of the vocal fold – female and male vocal folds alike express androgen receptors in the cytoplasm of the laryngeal gland, progesterone receptors in the nuclei of the same cells, and estrogen receptors in the epithelial cells of the larynx [4, 22, 23], leading to muscle thickening, final development of the trilayered lamina propria, changes in elastin and collagen deposition between the layers, variable lubrication and vocal fold elongation [24]. The expression of the receptors is similar for boys and girls, however differences in the level of hormones between genders causes differences in vocal fold development. In contrast, the other histological features vary: there is more elastin in the cover than in the ligament of male vocal folds, while the elastin in the female lamina propria is more compact [20, 25]. These differences lead to enormous distinctions in the male and female mutations. Apart from the difference in its dynamics, for girls, voice break happens earlier [26–28], starting from the age of 10 and finishing about the age of 14 years, whilst for boys it happens around the age of 12–16 years [29], with some period of voice instability [30, 31]. For both genders it results in the enlargement of arytenoids, expansion of the laryngeal muscles and ligaments [32, 33], completing of the glottal closure, lengthening of the framework of the larynx, and lengthening and rounding of the vocal folds, 4 which is highly related to changes of the acoustic parameters. It is important to note that nowadays mutation occurs much earlier than in the past [2, 34]. This was widely described by Daw, who recorded the age of voice break in members of J. S. Bach’s choirs in Leipzig in 17271749 as being 18 years old [35].
Materials and Methods
The study protocol was acknowledged, reviewed and approved by the internal ethics committee of our medical center, Claros Otorhinolaryngology Clinic Institutional Review Board. All of the parents and children were informed about the examination technique and provided written informed consent. We examined children of a premutation age, with a follow up 2.5 and 5 years later (at the mutation and postmutation age). Exclusion criteria were: vocal fold pathologies, history of neck trauma, previous intubations or laryngeal, head and neck or torso surgeries that have caused changes in vocal folds structure. For the power of a test equal to 0.9 (90%), the smallest sample size was calculated for each checked independent variable, and for statistically significant variables it varied from 10 to 41.
As advised by the pediatric voice assessment guidelines and European Laryngological Society (ELS), subjective and instrumental acoustic evaluations of the voice and aerodynamic performance, as well as visual evaluation of the larynx, were performed [36–38]. During each of the three appointments that the child attended, the voice parameters were measured by a speech- language pathologist. We chose these specific parameters based on advice from the literature: fundamental frequency as the basic, classical objective parameter of the voice [13], vocal range as quite a broad parameter, and because of that a strong sign of a voice disorder if pathological, shimmer and jitter as described as non-invasive, relatively easily applicable and objective [13, 39–41], and, furthermore, highly related to voice problems and dysphonia, [36, 42–44] loudness because it is believed to be a necessary parameter to objectify the result of checked jitter and shimmer [39, 45, 46], and maximum phonation time because it is believed to be the simplest, most easily measured aerodynamic parameter of phonation [47]. To perform the examinations we used sustained vowels taking examples from the approved authors [13, 39, 48, 49]. The vocal recording was performed in accordance with the Union of European Phoniatricians recommendations, with the child in a standing position, in a silent room, with noise level no higher than 40 dB, and with a microphone placed in front of the mouth at a 30 cm distance [50]. We used a microphone from Bruel & Kjaer Rhino-larynx Stroboscope—Type 4914 (Bruel & Kjaer Sound & Vibration, Denmark). All children were examined and recorded in the same conditions. Based on approved literature we defined the norms of all checked parameters [47, 51–59], and we compared them to obtained values. Finally, an ENT consultant examined the vocal folds during every visit to exclude any pathologies, performing a videolaryngoscopy with a rigid endoscope followed by a videostroboscopy (Hopkins II telescope 70 degrees, Karl Storz, Germany).
On every single visit, every 2.5 years, after parents and children provided written informed consent again, CT scanning was performed the way confirmed to be accurate before in our different study [60], using Philips Brilliance ICT 256 (Medical Systems, Netherlands), in the supine position, from the level of the frontal to the level of the aortic arch. Acquisition parameters consisted of a tube current—250 mA, 120 kV, 128×0.625 detector collimation, 0.75-second rotation time, pitch 0.993, scan field of view of250, standard resolution, raw slice thickness – 1 mm. For laryngeal evaluation we added a set of axial reconstruction 2×2 angled through C4 C6 disc spaces. The reconstruction interval was 0.5 mm and the slice thickness was 1 mm. Using standard CT software, a radiologist measured the precise length of the vocal folds in the axial view of the glottis, the longitudinal size of the glottis was estimated in a midsagittal plane (from anterior to a posterior boundary), and in the axial plane, and the length of vocal folds was measured between the anterior commissure and the most posterior part of vocal folds.
Finally, after the third examination (five years after the initial one), the children and their parents answered a survey. The first questions included gender, current age and presumed age of mutation. The following parts of the survey included questions about signs of mutation and voice problems during voice break. The next set of questions related to the age of menarche and breast growth for girls and the age of the first signs of puberty for boys. Lastly, they were asked to complete with the speech-language pathologist the GRBAS scale, which gives scores from 0 to 3 for hoarseness, roughness, breathiness, asthenia, and vocal strain [61].
Data was then implemented into Statistica 13.1 (StatSoft Poland, Cracow) software. Statistical significance was reported at the alpha level of 0.05. P value below 0.05 was considered significant. While analyzing the data we performed the Pearson correlation coefficient test, as well as an analysis of multiple regression and simple linear regression. Correlation coefficients were interpreted to determine whether the effect size was low (correlation coefficient-O.lO), medium (correlation coefficient~0.30) or high (correlation coefficient~0.50). Hypothesis tests were designed as two-tailed. A hypothesis null was formulated as HO: there is no correlation between the change of vocal folds length or acoustic parameters and the moment of voice break (r = 0, b = 0), against the alternative hypothesis H1: there is a correlation (r≠0, b≠0). We created graphs and classification trees to present our findings. The power of the test was determined, and the confidence intervals (Cl) were established for the obtained values.
Results
50 children of a premutation age were our final study group (25 males and 25 females) with a follow up 2.5 and 5 years later (in the mutation and postmutation age). Exactly half of them were males examined at age 11.5, age 14 and age 16.5, and the other half were females examined at age 9.5, 12 and 14.5.
While analyzing the correlations between all the obtained variables and the age of mutation with the Pearson correlation coefficient test, we reported statistical significance in the correlation between the age of mutation and loudness in boys aged 14 (positive correlation coefficient r = 0.48, 0.48, 95%, CI:0, 10–0.73, P = .015, power of the test = .7). Multiple regression analysis showed statistical significance in the correlation between the age of mutation and vocal fold length in boys aged 14 (negative coefficient b = -2.18, P = .044), as well as loudness in boys aged 11.5 (negative coefficient b = -0.15, P = .047), loudness in boys aged 14 (positive coefficient b = 0.31 , P = .022), and loudness in boys aged 16.5 (negative coefficient b = -0.31, P = .040\ however, loudness in boys aged 16.5 cannot be treated as a predictor of mutation, which presumably had occurred earlier). The effect size for these coefficients was R2 = 0.88 (0.88, 95%, CI: 0.72–0.93) and the Cohen’s coefficient was f2 = 7.33. Deeper analysis of these calculations is shown in the classification trees (Figures 1 and 2).
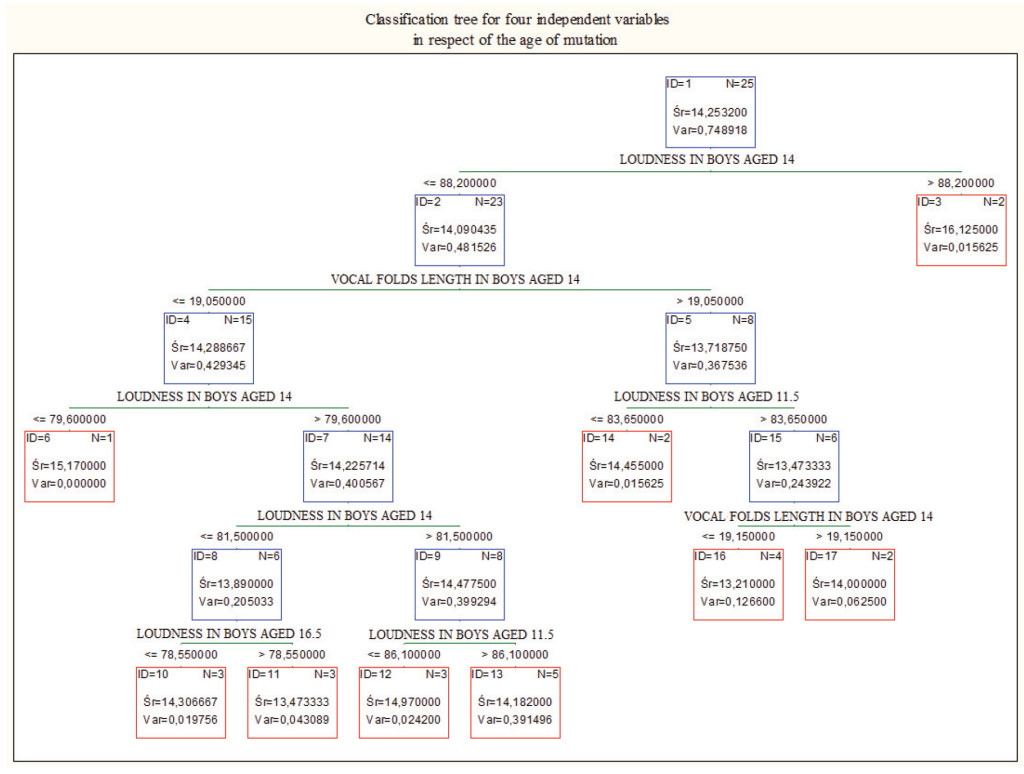
Figure 1. Classification tree for loudness in boys aged 11, 5, loudness in boys aged 14, loudness in boys aged 16.5, and vocal folds length in boys aged 14.
Loudness in boys aged 14. and subsequently vocal folds length in boys aged 14, differentiate cases the best. Combined, they are a good prediction of the age of mutation.
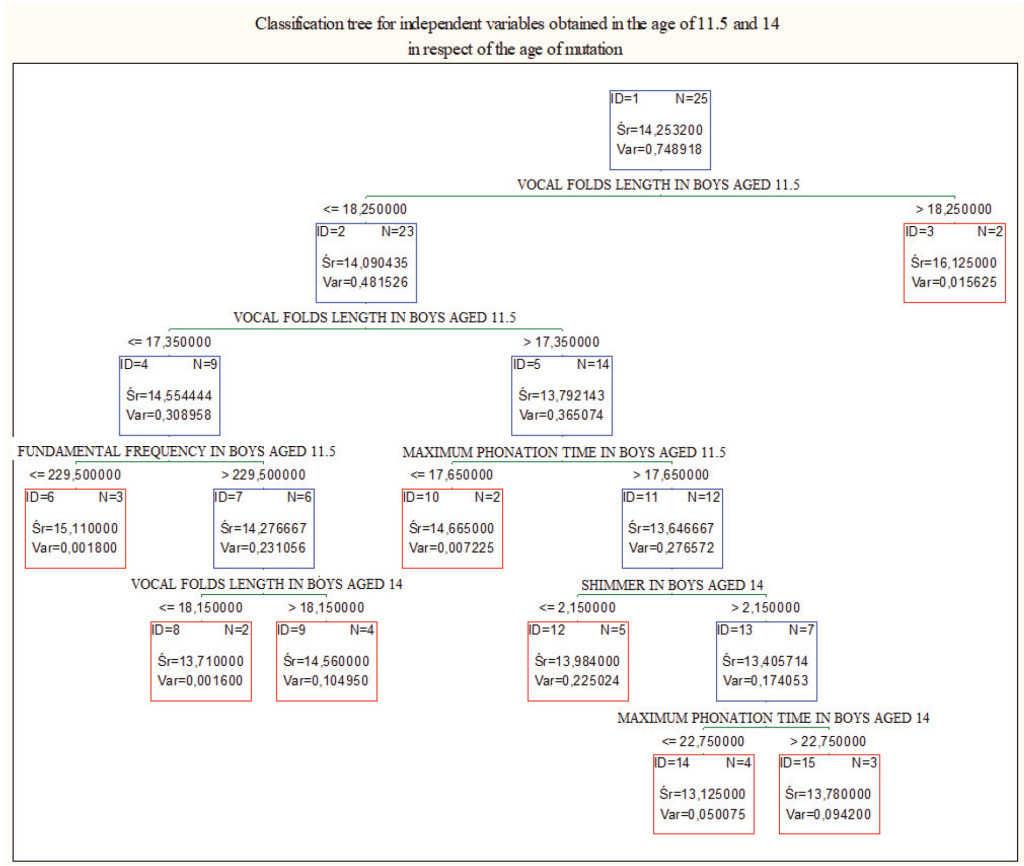
Figure 2. Classification tree for all the values obtained in boys aged 11.5 and 14. In these age groups vocal folds length in boys aged 11.5 was the best parameter differentiating cases
In the same calculations for girls, we reported statistical significance in the correlation between the age of mutation and the age of first menstruation (positive coefficient r = 0.84, 0, 84, 95%, CI:0, 66–0, 92, P<.001. power of the test = 1) and increase of fundamental frequency between the age of 9.5 and 12 years old (negative coefficient r = -0.5, 0, 5, 95%, CI:0.13–0.74, P = , 011, power of the test = .75). Since the correlation coefficient between the age of the mutation and the age of the first menstruation was high, we could perform an analysis of simple linear regression, results of which are shown in Figure 3. Multiple regression analysis also showed a statistically significant correlation between the age of mutation and the age of the first menstruation (positive coefficient b = 0.7, P<.001). and increase of fundamental frequency (negative coefficient b = -0.01, P = , 039), which is in agreement with previous calculations. The effect size for these coefficients was R2 = 0.76 (0.88, 95%, CI:0.54–0.86) and the Cohen’s coefficient was f2 = 3.1. Further analysis is shown in the classification trees (Figures 4 and 5).
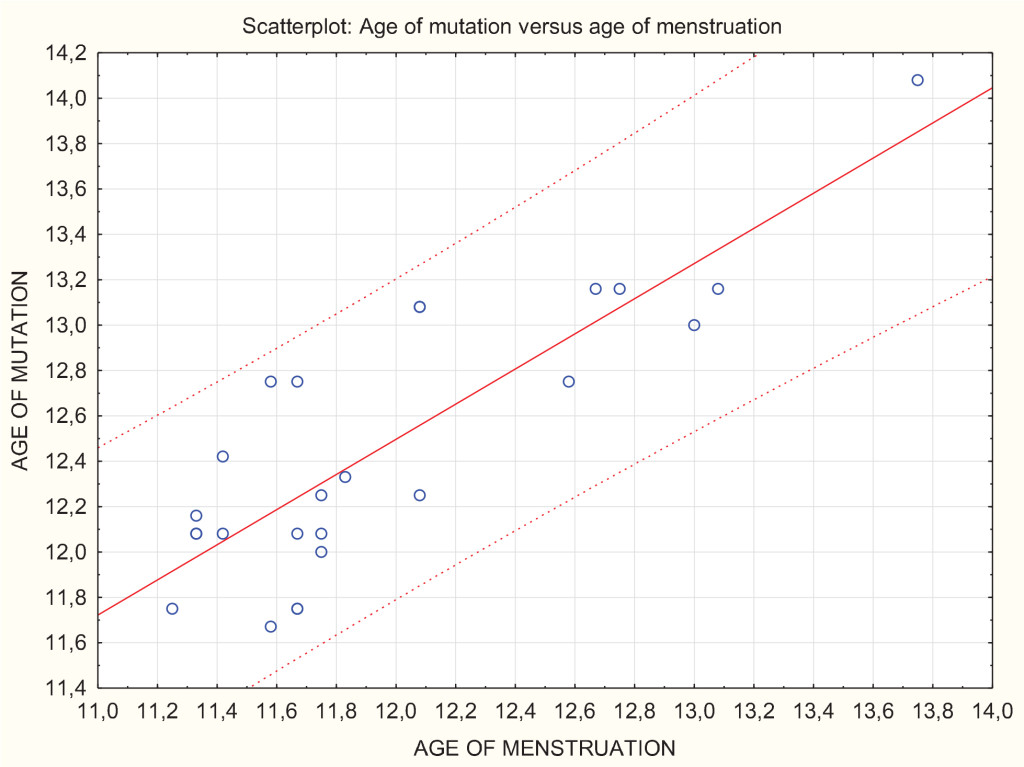
Figure 3. Scatterplot showing analysis of simple regression and a prediction zone of 95% for the mutation age. We can see that, e.g., for the menstrual age of 12 (axis x), with 95% of probability the mutation will occur between the age of 11.8 and 13.2 (axis y).
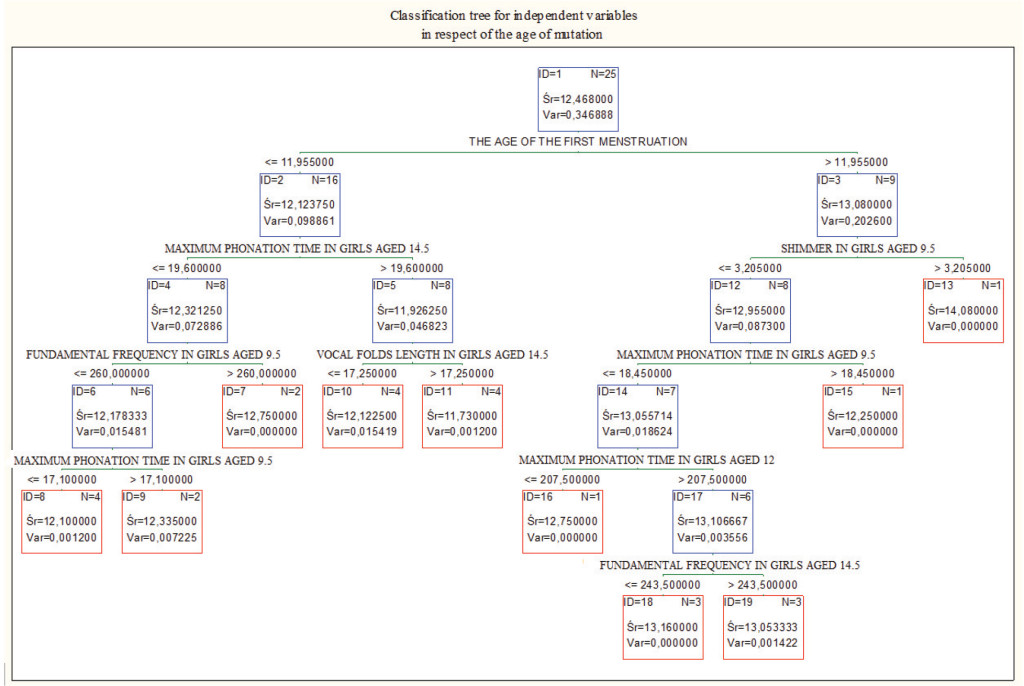
Figure 4. Classification tree for the values obtained in girls aged 9.5, 12 and 14.5. Age of the first menstruation was the best parameter differentiating cases in respect of the mutation age.
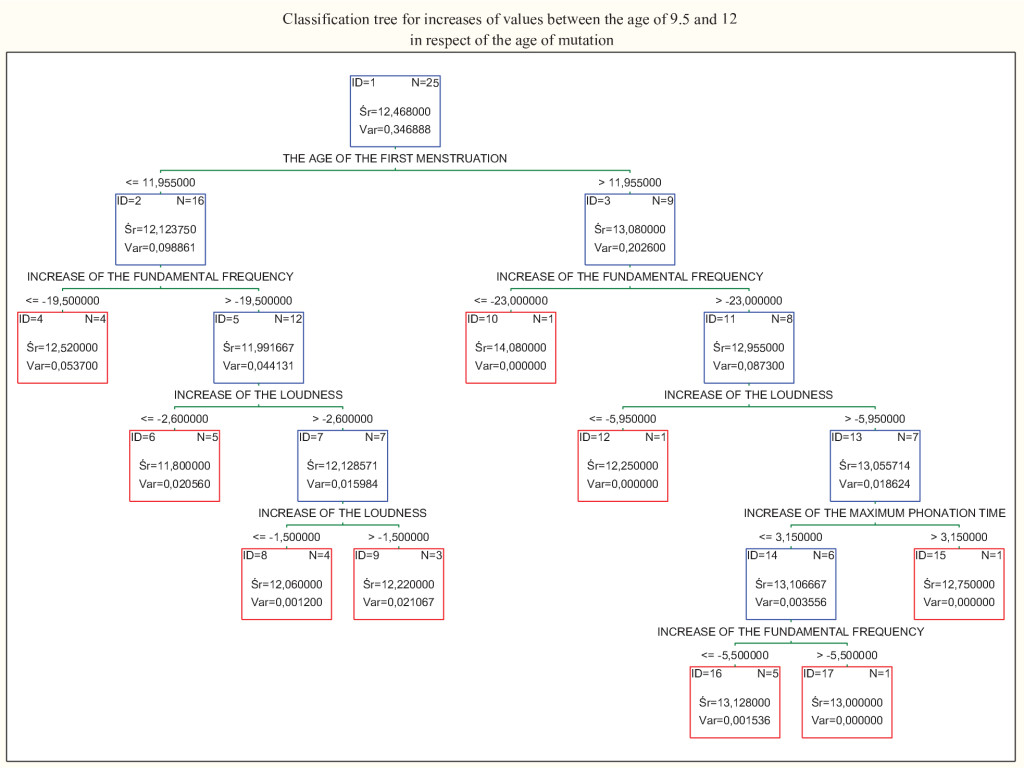
Figure 5. Classification tree for the increases of the values obtained in girls between ages 9.5 and 12. Age of the first menstruation was the best parameter differentiating cases.
Discussion
The main aim of our study was to find the parameter which correlates the best with the age of mutation, and therefore could possibly serve to predict the age of mutation and evaluate development of the child. We took under further considerations only the values with confirmed statistical significance.
With respect to boys, our calculations showed a statistically significant correlation between the age of mutation and loudness in boys aged 14. vocal fold length in boys aged 14, and loudness in hoys aged 11.5. For loudness in hoys aged 14 the correlation coefficient was positive, which tells us that the louder a child sings at the age of 14, the later he has the mutation. For loudness in boys aged 11.5 and vocal fold length in boys aged 14 the correlation coefficient was negative, which tells us that the louder boy sings at the age of 11.5, the lower the age of mutation, and – most importantly – the longer vocal folds are at the age of 14, the sooner the mutation will start. This is especially interesting in the case of boys, because despite of the obvious vocal folds lengthening with age, male mutation is well accepted to be a sudden and steep change, [2, 4, 5] with periods of higher voice interrupted by periods of lower voice, [30,31] and is dependent on numerous systematic changes described above, therefore it is not simply related to the change of vocal folds length.
It is worth emphasizing, that the correlation between the age of mutation and loudness in hoys aged 14 was confirmed to be statistically significant by all the statistical tests that we performed, and also was the best value differentiating the cases in respect of the age of mutation in the classification tree (Figure 1), therefore it is a variable worth special attention.
Our study also revealed interesting findings in girls. The calculations showed a statistically significant negative correlation between the age of mutation and increase of fundamental frequency between the age of 9.5 and 12. Which means that the more fundamental frequency drops between the age of 9.5 and 12 years old, the later mutation occurs. We have also confirmed a statistically significant positive correlation between the age of mutation and age of the first menstruation. Which means that the sooner the first menstruation appears, the sooner the voice break starts. This is not a surprising result; however, it gave us the opportunity to deepen our statistical analysis. Figure 3 illustrates an analysis of simple regression and the prediction zone of 95% for the mutation age, which means it allows us to predict with 95% of probability the age of voice break knowing the age of menstruation. This way of illustrating the correlation has the potential to be extremely useful in everyday medical and choral practice. The effect size of our results measured by the correlation coefficients was high.
It is important to point out that we have to consider the possible lack of precision in radiological measurements, however it is worth noting that our CT examinations had especially high resolution parameters, and that the CT scans were analyzed multiple times, in different views and planes. A further limitation of our study might be uncertainty about the proper understanding of our instructions during the acoustic examinations (which uncertainty accompanies scientists in every study involving children [62]), however, the age of children involved in our study was not so low as to make this a major concern.
Although several studies have investigated the subject of mutation and ways of predicting it, there is still room to explore it further. Decoster et al. investigated changes in acoustic parameters in girls, however boys were not the subject of the study [2]. Hacki and Heitmiiller, as well as Boltezar et al., did address the subject of mutation, yet in relation to acoustic voice parameters, not vocal folds length [6, 31]. Similarly, numerous studies examined acoustic voice parameters in pediatric population, though other examinations, such as vocal folds length measurements, were beyond the scope of the research [8–11]. Rogers et al. evaluated vocal fold growth as a function of age in a large group of patients [7], however using measuring sticks in total anaesthesia, and emphasized that it might have lengthened the vocal folds [63]. There are several other studies investigating vocal folds length in relation to age, however measurements were performed post mortem, and therefore did not reflect the actual conditions of the living human being’s body [18, 64–67]. Hollien, similarly as in our study, has used radiological imaging; however he has used X-ray images, which are less precise than the CT scans used in our research [68]. Thus, we are tempted to claim that our research is original, and to the best of our knowledge explores aspects not addressed before, adding an important contribution to still not exhausted research about a child’s development and the subject of mutation.
Conclusion
In the academic pursuit of knowledge, evaluating the proper maturation of a child has a special place of a particular concern. This is unsurprising, given that childhood can determine the future of a young human being. However, as we are all different, it is also difficult to determine whether development is proper, and, at the same time, so easy to miss the red flags. Undoubtedly, the period of puberty is the most challenging, both for the human body, which goes through multiple changes, and for scientists, who try to establish reference points to make the evaluation of maturing easier. We believe this hunt is never finished. In the future, one way to asses this might be a routine examination of vocal folds length and acoustic voice parameters. Our study attempted to make our contribution in bringing this future closer.
Acknowledgement
The authors report no conflicts of interest. The authors report no financial and material support for the research and the work reported in the manuscript.
References
- Fenichel P (2012) Delayed puberty. Endocr Dev 22: 138–159. [crossref]
- Decoster W, Ghesquiere S, Van Steenberge S. Great talent (2008) excellent voices-no problem for pubertal girls? Logoped Phoniatr Vocol 33: 104–112.
- Mumm R, Hermanussen M, Scheffler C (2016) New reference centiles for boys’ height, weight and body mass index used voice break as the marker of biological age. Acta Paediatr 105: e459–463. [crossref]
- Kerschner JE, Merati AL (2008) Science of Voice Production from Infancy Through Adolescence. In: Hartnick CJ, Boseley ME. Pediatric Voice Disorders. 1st edn. San Diego, California: Plural Publishing Inc; Pg No: 23–29.
- McAllister A, Sjölander P (2013) Children’s voice and voice disorders. Semin Speech Lang 34: 71–79. [crossref]
- Hacki T, Heitmiiller S (1999) Development of the child’s voice: premutation, mutation. Int J Pediatr Otorhinolaryngol 49: 141–144.
- Rogers DJ, Setlur J, Raol N, Maurer R, Hartnick CJ (2014) Evaluation of true vocal fold growth as a function of age. Otolaryngol Head Neck Surg 151: 681–686. [crossref]
- Böhme G, Stuchlik G (1995) Voice profiles and standard voice profile of untrained children. J Voice 9: 304–307. [crossref]
- McAllister A, Sederholm E, Sundberg J, Gramming P (1994) Relations between voice range profiles and physiological and perceptual voice characteristics in ten-year-old children. J Voice 8: 230–239.
- Sorenson DN (1989) A fundamental frequency investigation of children ages 6–10 years old. J Commun Disord 22: 115–123. [crossref]
- Pedersen MF, Moller S, Krabbe S, Bennett P, Svenstrup B (1990) Fundamental voice frequency in female puberty measured with electroglottography during continuous speech as secondary sex characteristic. Int J Pediatr Otorhinolaryngol 20: 17–24.
- Baken RJ, Orlikoff RF (1997) Voice measurement: is more better. Logop Phoniatr Vocol 22: 147–151.
- Nicollas R, Garrel R, Ouaknine M, Giovanni A, Nazarian B, et al. (2008) Normal voice in children between 6 and 12 years of age: database and nonlinear analysis. J Voice 22: 671–675. [crossref]
- Pabon JPH, McAllister A, Sederholm E, Sundberg J (2000) Dynamics and voice quality information in the computer phonetograms of children’s voices. In: Wide P, (ed). Child voice Stockholm: KTH Voice Research Centre Pg No: 85–100.
- Hartnick CJ, Rehbar R, Prasad V (2005) Development and maturation of the pediatric human vocal fold lamina propria. Laryngoscope 115: 4–15. [crossref]
- Hirano M, Sato K (1993) Histological color atlas of the human larynx. San Diego, California: Singular Publishing Group, USA.
- Hartnick CJ, Rehbar R, Prasad V (2005) Development and maturation of the pediatric human vocal fold lamina propria. Laryngoscope 115: 4–15. [crossref]
- Hirano M, Kurita S, Nakashima T (1983) Growth, development, and aging of human vocal folds. In: Bless DM, Abbs JH, ed. Vocal fold physiology. San Diego, California: College-Hill.
- Boseley ME, Hartnick CJ (2006) Development of the human true vocal fold: depth of cell layers and quantifying cell types within the lamina propria. Ann Otol Rhinol Laryngol 115: 784–788. [crossref]
- Chan RW, Fu M, Young L, Tirunagari N (2007) Relative contributions of collagen and elastin to elasticity of the vocal fold under tension. Ann Biomed Eng 35: 1471–1483. [crossref]
- Stathopoulos ET, Sapienza CM (1997) Developmental changes in laryngeal and respiratory function with variations in sound pressure level. J Speech Lang Hear Res 40: 595–614. [crossref]
- Newman SR, Butler J, Hammond EH, Gray SD (2000) Preliminary report on hormone receptors in the human vocal fold. J Voice 14: 72–81. [crossref]
- Brunings JW, Schepens JJ, Peutz-Kootstra CJ, Kross KW (2013) The expression of estrogen and progesterone receptors in the human larynx. J Voice 27: 376–380. [crossref]
- Kuhn MA (2014) Histological changes in vocal fold growth and aging. Curr Opin Otolaryngol Head Neck Surg 22: 460–465. [crossref]
- Kahane JC (1988) Histologic structure and properties of the human vocal folds. Ear Nose Throat J 67: 322, 324–325, 329–30. [crossref]
- Marshall WA, Tanner JM. Puberty. In: Falkner F, Tanner JM (1986) Human Growth-A Comprehensive Treatise 2: Postnatal Growth Neurobiology. 2nd ed. Plenum, New York: Springer, Pg No: 171–210.
- Tanner JM (1981) Growth and maturation during adolescence. Nutr Rev 39: 43–55. [crossref]
- Bogin B (1999) Patterns of human growth. (2nd edn) Cambridge University Press, London.
- Balasubramaniam RK, NN (2017) Voice Mutation During Adolescence in Mangalore, India: Implications for the Assessment and Management of Mutational Voice Disorders. J Voice 31: 511.e29–511.e33.
- Fuchs M, Fröehlich M, Hentschel B, Stuermer IW, Kruse E, et al. (2007) Predicting mutational change in the speaking voice of boys. J Voice 21: 169–178. [crossref]
- Boltezar IH, Burger ZR, Zargi M (1997) Instability of voice in adolescence: pathologic condition or normal developmental variation? J Pediatr 130: 185–190. [crossref]
- Fitch WT, Giedd J (1999) Morphology and development of the human vocal tract: a study using magnetic resonance imaging. J Acoust Soc Am 106: 1511–1522. [crossref]
- Ferlito A (2000) In: Ferlito A., ed. Diseases of the Larynx. London: Oxford; New York: Arnold. Oxford Univeristy Press.
- Gackle L (2000) Understanding voice transformation in female adolescents. In: Welch G, ed. Bodymind and Voice. Minnesota, USA: The Voice Care Network; Pg No: 739–744.
- Daw SF (1970) Age of boys’ puberty in Leipzig, 1727–49, as indicated by voice breaking in J.S. Bach’s choir members. Hum Biol 42: 87–89. [crossref]
- Cohen W, Wynne DM, KubbaH, McCartney E (2012) Development of a minimum protocol for assessment in the paediatric voice clinic. Part 1: Evaluating vocal function. Logoped Phoniatr Vocol 37: 33–38.
- Kelchner LN, Brehm SB, de Alarcon A, Weinrich B (2012) Update on pediatric voice and airway disorders: assessment and care. Curr Opin Otolaryngol Head Neck Surg 20: 160–164. [crossref]
- Hartnick C, Boseley M (2008) Pediatric Voice Disorders: Diagnosis and Treatment. San Diego, CA: Plural Publishing.
- Brockmann-Bauser M, Beyer D, Bohlender JE (2014) Clinical relevance of speaking voice intensity effects on acoustic jitter and shimmer in children between 5;0 and 9; 11 years. Int J Pediatr. Otorhinolaryngol 78: 2121–2126.
- Oates J, Kirkby R (1980) An acoustical investigation of voice quality disorders in children with vocal nodules. Australian J HumanCommDis 8: 28–39.
- Meal lister A, Sundberg J, Hibi SR (1998) Acoustic measurements and perceptual evaluation of hoarseness in children’s voices. Logoped Phoniatr Vocol 23: 27–38.
- Lopes LW, Barbosa Lima IL, Alves Almeida LN, Cavalcante DP, de Almeida AA (2012) Severity of voice disorders in children: correlations between perceptual and acoustic data. J Voice 26: 819.e7–819.el2.
- Shah RK, Engel SH, Choi SS (2008) Relationship between voice quality and vocal nodule size. Otolaryngol Head Neck Surg 139: 723–726. [crossref]
- Valadez V, Ysunza A, Ocharan-Hernandez E, Garrido-Bustamante N, Sanchez-Valerio A, et al, (2012) Voice parameters and videonasolaryngoscopy in children with vocal nodules: a longitudinal study, before and after voice therapy. Int J Pediatr Otorhinolaryngol 76: 1361–1365.
- Brockmann-Bauser M (2012) Improving Jitter and Shimmer Measurements in Normal Voices. Schulz-Kirchner Verlag GmbH Idstein 205.
- Brockmann-Bauser M, Drinnan MJ (2011) Routine acoustic voice analysis: time to think again? Curr Opin Otolaryngol Head Neck Surg 19: 165–170. [crossref]
- Pribuisiene R, Uloza V, Kardisiene V (2011) Voice characteristics of children aged between 6 and 13 years: Impact of age, gender, and vocal training. Logopedics Phoniatrics Vocology 36: 150–155.
- Glaze L, Bless D, Milenkovic P, Susser R (1988) Acoustic characteristics of children’s voice. J Voice 2: 312–319.
- Cohen SR, Perelman N, Nimni ME, Mahnovski V, Cheung DT (1993) Whole organ evaluation of collagen in the developing human larynx structures and adjoining anatomic structures (hyoid and trachea). Ann Otol Rhinol Laryngol 102: 655–659.
- Shutte HK, Seidner W (1983) Recommendations by the Union of European Phoniatricians (UEP): Standardizing voice area measurement/phonetography. Folia Phoniatr 35: 286–288.
- Bennett S (1983) A 3-year longitudinal study of school-aged children’s fundamental frequencies. J Speech Hear Res 26: 137–141. [crossref]
- Stathopoulos ET, Sapienza C (1993) Respiratory and laryngeal measures of children during vocal intensity variation. J Acoust Soc Am 94: 2531–2543. [crossref]
- Finnegan DE (1985) Maximum phonation time for children with normal voices. Folia Phoniatr (Basel) 37: 209–215. [crossref]
- Horii Y (1983) Automatic analysis of voice fundamental frequency and intensity using a VisiPitch. J Speech Hear Res 25: 467–471.
- Banik A, Arya S, Kant A (2015) Vocal Parameters in Children between 4 To 12 Years of Age: An Attempt to Establish a Prototype Database. International Journal of Scientific and Research Publications 11: 446–453.
- Ting HN, Chia SY, Manap HH, Ho AH, Tiu KY, Abdul Hamid B (2012) Fundamental Frequency and Perturbation Measures of Sustained Vowels in Malaysian Malay Children Between 7 and 12 Years Old. J Voice. 26: 425–30.
- Bonet M, Casan P (1994) Evaluation of dysphonia in a children’s choir. Folia Phoniatr Logop 46: 27–34. [crossref]
- Williamson G (2006) Human communication: A linguistic introduction. 2nd ed. Billingham, UK: Speech Language Services.
- Maturo S, Hill C, Bunting G, Ballif C, Maurer R, et al. (2012) Establishment of a normative pediatric acoustic database. Arch Otolaryngol Head Neck Surg 138: 956–961. [crossref]
- Claros P, Sobolewska AZ, Domenech-Claros A, Claros-Pujol A, Pujol C, et al. (2018) CT-based Morphometric Analysis of Professional Opera Singers’ Vocal Folds. J Voice 21: 30614–30618.
- Sundberg J (2000) Emotive transforms. Phonetica 57: 95–112. [crossref]
- Brockmann-Bauser M, Beyer D, Bohlender JE (2014) Clinical relevance of speaking voice intensity effects on acoustic jitter and shimmer in children between 5;0 and 9; 11 years. Int J Pediatr Otorhinolaryngol 78: 2121–2126.
- Su MC, Yeh TH, Tan CT, Lin CD, Linne OC, et al. (2002) Measurement of adult vocal fold length. J Laryngol Otol 116: 447–449. [[crossref]
- Eckel HE, Sprinzl GM, Sittel C, Koebke J, Damm M, et al. (2000) Anatomy of the glottis and subglottis in the pediatric larynx]. HNO 48: 501–507. [crossref]
- Eckel HE, Sittel C (1995) Morphometry of the larynx in horizontal sections. Am J Otolaryngol 16: 40–48. [[crossref]
- Friedrich G, Kainz J (1988) Morphometry of the larynx in horizontal sections: normal data for the quantitative evaluation of current imaging technics in German], Laryngol Rhinol Otol (Stuttg) 67: 269–274.
- Kahane JC (1978) A morphological study of the human prepubertal and pubertal larynx. Am J Anat 151: 11–19. [crossref]
- HOLLIEN H (1960) Some laryngeal correlates of vocal pitch. J Speech Hear Res 3: 52–58. [crossref]