Abstract
In situ vaccination (ISV) is an established and growing cancer therapy strategy. Preclinical studies show that ISV antitumor efficacy is achievable by of plant virus nanoparticles (PVNPs), in which PVNPs are directly administered into the tumor. It had previously shown that some PVNPs are potent in inducing long-lasting antitumor immunity when used as an in situ vaccine. Here, we investigate a filamentous plant virus nanoparticle, potato virus Y (PVY), for in situ vaccination treatment of 4T1, the very aggressive and metastatic murine triple-negative breast cancer model. PVY used as ISV does not significantly slow down tumor progression. Data indicate that some PVNPs are more suitable for application as in situ vaccines than others; understanding the intricate differences and underlying mechanisms of immune-activation may set the stage for clinical development of these technologies.
Keywords
Immunotherapy, Cancer, In situ vaccination, Plant virus nanoparticle, Potato virus Y
Introduction
Over 120 years ago Dr. William Coley set the framework for cancer immunotherapy. It was directly administrated live and attenuated bacteria to tumor. This approach led to cancer regression and complete cures in subset of patients [1,2]. The mechanism is now partially understood to involve activating macrophages and lymphocytes, producing immune’s mediator such as cytokines and chemokines [3]. In this approach that known in situ vaccination (ISV), the tumor itself serves as the source of the antigen and what is introduced is an adjuvant [4]. One particular type of ISV involves the use of attenuated mycobacteria known as Bacille Calmette-Guérin (BCG), which has been the established standard of care for superficial bladder cancer for more than four decades [5]. Another type of ISV that has recently received FDA approval is talimogene laherparepvec (T-VEC) developed by Amgen. T-VEC is a therapeutic treatment that utilizes an attenuated herpes simplex virus. T-VEC is injected directly into identified tumors and its therapeutic effects are achieved through the recruitment and activation of immune cells through the secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) [6,7]. Recently, plant virus nanoparticles (PVNPs)-based nanotechnologies have provided evidence that can induce a strong immune response against tumors when administered as ISV [3,8,9]. The effectiveness of this approach has been demonstrated in mouse models of melanoma, breast cancer, ovarian cancer, and colon cancer [3,7,10,11]. The data suggests that antitumor effects leading to the development of immune memory and providing protection against tumor recurrence [11]. Investigations have revealed antitumor effects in these models was associated with activation of a broad spectrum of immune cells [3,12,13]. A subset of immune cell such as APCs, neutrophils have been become activated upon engulf PVNPs, resulting in an early inflammatory phase characterized by upregulation of pro-inflammatory cytokines, leukocyte recruitment [7,13]. Toward this end, have recently demonstrated that ISV with various shapes/sizes of PVNPs such as icosahedral cowpea mosaic virus (CPMV) [7], rod-like papaya mosaic virus (PapMV) [14] and tobacco mosaic virus (TMV) [7], bacillus alfalfa mosaic virus (AMV) [15] can induce immune-mediated antitumor responses. It has been demonstrated that filamentous such as potato virus X (PVX) also can exhibit antitumor effectiveness in the context of melanoma [16]. Here, we specifically asked whether other filamentous viruses qualify as in situ vaccine for cancer immunotherapy. In this regard, we employed a library of structurally similar plant virus; specifically, potato virus Y (PVY). PVY virions have a filamentous, flexuous form, with a length of 730 nm and a diameter of 12 nm and a single-stranded, positive sense RNA genome [17,18].
Material and Methods
Preparation and Characterization of PVY Nanoparticles
PVY was propagated within Nicotiana benthamiana plants and purified as previously reported [18]. Transmission electron microscopy (TEM) imaging was conducted to validate the integrity of PVY filaments. The Malvern Zetasizer (Malvern Instruments, Worcestershire, United Kingdom) was utilized to determine the electrostatic surface map and zeta potential measurements.
Determination of Inherent Immunogenicity
For the assessment of canonical pro-inflammatory cytokine IL-6 levels, the inherent immunogenicity was determined by previous method. Summary, purified PVY added in vitro cultures of peripheral human blood mononuclear cells (PBMCs) with a cell density of 106 cell/ml that were purified as previously reported.
Cell Culture and Cell Viability Assay
Cell culture and a cell viability assay were performed using 4T1 cells (ATCC). These cells were cultured in RPMI, supplemented with 10% (v/v) fetal bovine serum (FBS, Atlanta Biologicals) and 1% (v/v) penicillin-streptomycin (penstrep, Life Technologies). The cells were maintained at a temperature of 37 °C and a CO2 concentration of 5%. Upon reaching confluency, the cells were detached with 0.05% (w/v) trypsin-EDTA (Life Technologies), seeded at a density of 2 × 103 cells/100 μL/well in 96-well plates, and incubated overnight at 37 °C and 5% CO2. On the following day, the cells were washed twice with PBS and exposed to PVY at concentrations of 0, 0.5, 1, 2.5, 5, 10, 25, 50, 75, 100 μg/ml for duration of 24 h, in triplicate. Cell viability was assessed using an MTT proliferation assay as suggested by the manufacturer (ATCC, catalog number: 30-1010K™).
Tumor Model
Female Balb/C mice of six weeks of age were acquired from the Pasteur Institute of Iran. Subcutaneous injection of the breast cancer cell line (4T1) was performed at the right side of the abdomen of a mouse with a seeding density of 106 cell/ml. The tumor volume was measured using a digital caliper and calculated using the equation volume=0.5 (length × width2). Mice were then randomly assigned to either the untreated group (PBS) or the group treated with PVY (n=4). In situ vaccination was conducted on day 10 post-tumor induction, where 100 μg of PVY in 50 μL of PBS was intratumorally injected with 72 h intervals. Tumor size was monitored daily, and mice were sacrificed when the tumor volume exceeded 1000 mm3, as per the approved protocol of the institutional animal care and use committee (IACUC) of Tabriz University of Medical Science.
Immunohistochemistry
Immunohistochemistry was performed using antibodies obtained from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA. The tumors were fixed in PBS and sent to the Sara-Co lab (https://www.sara-co.com) for sectioning and staining. Optic densities (OD) were quantified using Fiji image analysis software.
Statistical Analysis
Data analysis and chart generation were performed using GraphPad Prism 8 software for Windows (GraphPad Software, San Diego, CA, USA). Statistical significance was determined using two-way or one-way analysis of variance (ANOVA). A significance level of P≤ 0.05 was considered for comparisons between groups.
Results
Characterization of PVY
In this study, our objective was to investigate whether PVY could be utilized as an in situ vaccine against 4T1 tumors. PVY is filamentous virus with a length of 730 nm and a diameter of 12 nm. The ratios of RNA to protein (A260/280=1.7) indicated that the preparation consisted of uncontaminated and intact PVY (Figure 1A). Figure 1B displays the zeta potential of the PVY particles, which is recorded as-4.4 millivolts (Figure 1B).
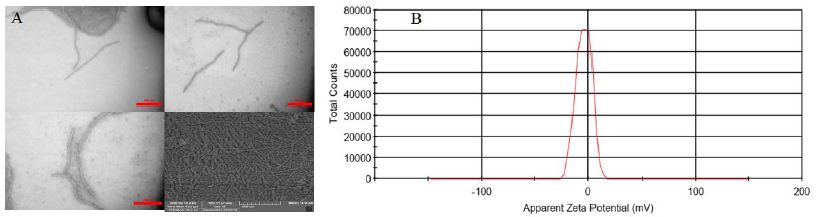
Figure 1: PVY characteristics A) Transmission electron microscopy confirms the structural stability of PVY particles. B) Dynamic light scattering was used to measure the zeta potential of PVY.
PVY is Immunogenic and Non-cytotoxic for Cancer Cells
4T1 cell line for evaluating the anticancer efficacy of the PVY nanoparticles (100 μg/ml) was used for cytotoxicity assay, the results did not exhibit direct cell toxicity on treated cells (Figure 2A). PVY possesses immunogenic properties and does not exhibit cytotoxic effects on cancer cells. When Human PBMCs were exposed to PVY for 24 hours, there was a significant increase in the expression of interleukin-6 (IL-6), a well-known pro-inflammatory cytokine, compared to the control cells that were left untreated (Figure 2B). This observation suggests that PVY inherently stimulates the immune cells.
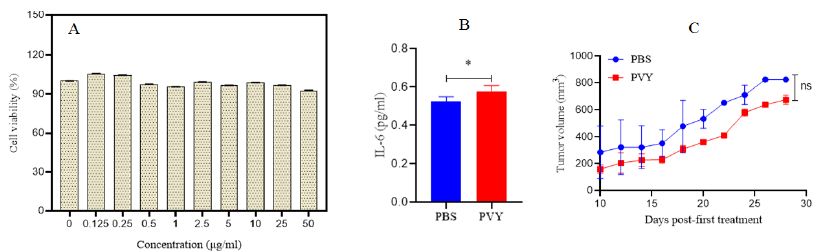
Figure 2: A) Cell viability of 4T1 cancer cell line exposed to PVY. B) Human PBMCs exposed to PVY produce elevated levels of IL-6 pro-inflammatory cytokine in vitro. C) Therapeutic efficacy of PVY in a breast tumor model. Balb/C mice inoculated with 1 × 106 4T1 cells followed by 100 µg of PVY, once tumors were approximately 100> mm3, 10 days post-inoculation. Tumor growth followed by measuring volume (n=4).
PVY ISV Treatment has not Potent Efficacy in Treating the 4T1 Model
PVY treatment was done 5 times for each mouse in three days intervals starting 10 days after 4T1 cell injection and tumors well established. Control group mice were injected with PBS on the same schedule. The tumor growth (volume) was no significantly delayed in PVY treated groups compared to untreated control (Figures 2C). Inflammatory cytokines were assayed by Immunohistochemistry. Intra-tumor concentrations of pro-inflammatory cytokines, IFN-β, IL-1β, IL-12 andIL-6 don’t significantly increased in PVY-treated animals compared to untreated control animal tumors (Figures 3).
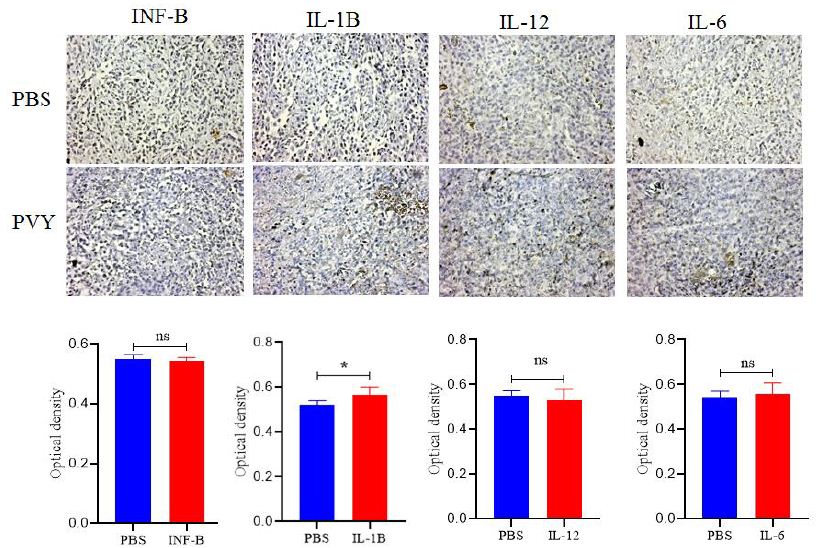
Figure 3: Immunohistochemistry don’t reveal dramatic changes in cytokines associated with treatment. Tumor sections stained for INF-β, IL-1β, IL-12 and IL-6. Quantitative analysis was performed using Fiji software to determine relative optical densities of the stained sections (p<0.05), magnification 20.
Discussion
We have elected filamentous plant virus, namely PVY as ISV for the first time. Intratumoral injection of the viral nanoparticles (VNPs) achieved with the combination of virus genome and multivalent capsid proteins (wild virus) with diameters of approximately 12 nm and long 700nm (Figure 1A). In order to assess their inherent immunogenicity, we introduced of the PVYs into in vitro cultures of PBMCs extracted from human. After duration of 24 hours, we measured the level of pro-inflammatory cytokine, IL-6 secreted using ELISA (Figure 2B). We have observed that in the context of dermal 4T1 in mice, the utilization of PVY as an in situ vaccine don’t leads to a suboptimal antitumor immune response against breast tumors. In contrast, it could not be showed with the effectiveness of CPMV, AMV, and PapMV. However, it did observe a relative similarity with PVX. When comparing the formulation of PVY with PVX, no apparent differences in antitumor efficacy were observed [16]. It demonstrated that there was no statistical difference in tumor growth rate or survival time between PVX-DOX complex versus PVX or DOX alone, but PVX+DOX did significantly slow tumor growth rate versus PVX and DOX alone [16]. Furthermore, PVX in mixture with OVA did not prove to be an effective adjuvant. Unexpectedly, no differences in IgG titers between mice immunized with individual OVA and a composition of OVA+PVX were revealed [19]. Furthermore, TMV used as an in situ vaccine elicits a weak antitumor immune response against melanoma [7]. Analysis of cytokines indicated no statistical difference. Prominent cytokine included IL-6, this cytokine also play crucial roles in inducing pro-inflammatory responses to infection and inflammation. In stark contrast, PVY elicits an immune response at in vitro, characterized by the production of pro-inflammatory cytokine, primarily IL-6. It has been observed that IL-6 signaling is associated with attributes that promote tumor growth, such as the ability to control the differentiation of macrophages and neutrophils into their tumor-promoting phenotypes, specifically M2 and N2 [20]. Our results don’t show very significant stimulation of INF-β, IL-1β, IL-12 and IL-6cytokines by PVY in situ vaccination. These cytokines are particularly intriguing due to their significance in eliciting immune responses against tumors through the mediation of T cells [21]. Regardless of their origin, interferons possess the ability to potentially exert antitumor effects either directly or indirectly [22]. The cytokine IL-12 holds great importance in the realm of cancer immunotherapy as it has the capability to activate immune cells with antitumor properties, including those that combat melanoma, by stimulating IFN-γ signaling and production [23]. It can exhibit that PVY can be suitable for epitope presentation. It is demonstrated that the preS1 epitope displayed on PVY (chimeric PVY CP particles) can immunize of mice with a strong anti-preS1 immune response, even in the absence of adjuvants [24].
Conclusion
Plant virus-based materials have emerged as innovative in situ vaccines, potentially initiating an immune response against tumors. These nanoparticles interact with innate immune sensors, reprogramming the immunosuppressive tumor microenvironment to an immune-activated state. This reactivation of the cancer-immunity cycle leads to systemic elimination of cancer cells through the adaptive immune system. It has been documented that CPMV, PapMV, PVX, TMV, and AMV-based ISV can induce anti-tumor responses, reduce tumor growth, and improve survival rates when administered directly to the tumor site. In this study, we present evidences that PVY-based ISV, in compare with them, no efficacy or immune-stimulation in a mouse model. Nevertheless, it can exhibit that PVY can be suitable for epitope presentation. Further research is required to fully elucidate the mechanism of plant viral in situ vaccines.
References
- Coley WB (1914) The treatment of malignant inoperable tumors with the mixed toxins of erysipelas and bacillus prodigiosus. Weissenbruch.
- McCarthy EF (2006) The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. The Iowa Orthopaedic Journal 26: 154. [crossref]
- Lizotte P, Wen A, Sheen M, Fields J, Rojanasopondist P, et al. (2016) In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nature Nanotechnology 11: 295-303. [crossref]
- Shahgolzari M, Fiering S (2022) Emerging potential of plant virus nanoparticles (PVNPs) in anticancer immunotherapies. Journal of Cancer Immunology 4: 22. [crossref]
- Kamat AM, Flaig TW, Grossman HB, Konety B, Lamm D, et al. (2015) Consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nature Reviews Urology 12: 225-35. [crossref].
- Johnson DB, Puzanov I, Kelley MC (2015) Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 7: 611-9. [crossref].
- Murray AA, Wang C, Fiering S, Steinmetz NF (2018) In situ vaccination with cowpea vs tobacco mosaic virus against melanoma. Molecular Pharmaceutics 15: 3700-16. [crossref]
- Shukla S, Wang C, Beiss V, Cai H, Washington T, et al. (2020) The unique potency of Cowpea mosaic virus (CPMV) in situ cancer vaccine. Biomaterials Science 8: 5489-503. [crossref].
- Shahgolzari M, Pazhouhandeh M, Milani M, Yari Khosroushahi A, Fiering S (2020) Plant viral nanoparticles for packaging and in vivo delivery of bioactive cargos. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 12: e1629. [crossref]
- Kerstetter-Fogle A, Shukla S, Wang C, Beiss V, Harris PL, et al. (2019) Plant virus-like particle in situ vaccine for intracranial glioma immunotherapy. Cancers 11: 515. [crossref]
- Gautam A, Beiss V, Wang C, Wang L, Steinmetz NF (2021) Plant viral nanoparticle conjugated with anti-PD-1 peptide for ovarian cancer immunotherapy. International Journal of Molecular Sciences 22: 9733. [crossref].
- Wang C, Fiering SN, Steinmetz NF (2019) Cowpea mosaic virus promotes anti‐tumor activity and immune memory in a mouse ovarian tumor model. Advanced Therapeutics 2: 1900003. [crossref]
- Shahgolzari M, Dianat-Moghadam H, Fiering S (2022) Multifunctional plant virus nanoparticles in the next generation of cancer immunotherapies. In: Seminars in Cancer Biology 86: 1076-85. [crossref]
- Lebel M-È, Chartrand K, Tarrab E, Savard P, Leclerc D, et al. (2016) Potentiating cancer immunotherapy using papaya mosaic virus-derived nanoparticles. Nano Letters 16: 1826-32.[crossref]
- Shahgolzari M, Pazhouhandeh M, Milani M, Fiering S, Khosroushahi AY (2020) Alfalfa mosaic virus nanoparticles-based in situ vaccination induces antitumor immune responses in breast cancer model. Nanomedicine 16: 97-107. [crossref]
- Lee KL, Murray AA, Le DH, Sheen MR, Shukla S, et al. (2017) Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano Letters 17: 4019-28. [crossref]
- Della Bartola M, Byrne S, Mullins E (2020) Characterization of potato virus Y isolates and assessment of nanopore sequencing to detect and genotype potato viruses. Viruses 12: 478. [crossref]
- Quenouille J, Vassilakos N, Moury B (2013) P otato virus Y: a major crop pathogen that has provided major insights into the evolution of viral pathogenicity. Molecular Plant Pathology 14: 439-52. [crossref]
- Evtushenko EA, Ryabchevskaya EM, Nikitin NA, Atabekov JG, Karpova OV (2020) Plant virus particles with various shapes as potential adjuvants. Scientific Reports 10: 10365.
- Chen L, Wang S, Wang Y, Zhang W, Ma K, et al. (2018) IL-6 influences the polarization of macrophages and the formation and growth of colorectal tumor. Oncotarget 9: 17443. [crossref]
- Lee S, Margolin K (2011) Cytokines in cancer immunotherapy. Cancers 3: 3856-93. [crossref]
- Parker BS, Rautela J, Hertzog PJ (2016) Antitumour actions of interferons: implications for cancer therapy. Nature Reviews Cancer 16: 131-44. [crossref]
- Seo S, Kim K, Park S, Suh Y, Kim S, et al. (2011) The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Therapy 18: 488-95. [crossref]
- Kalnciema I, Skrastina D, Ose V, Pumpens P, Zeltins A (2012) Potato virus Y-like particles as a new carrier for the presentation of foreign protein stretches. Molecular Biotechnology 52: 129-39. [crossref]