DOI: 10.31038/MIP.2022332
Abstract
An investigation was conducted to gain essential information on the microbial ecology of endophytic bacteria in 17 corn plant varieties. Throughout the growing season, population dynamics of endophytic bacteria were discovered in the roots, leaves, and stems of corn plants. The bacterial density fluctuated from 104 to 105 colony-forming units per gram and increased as the plant grew, reaching the maximum at the heading stage. The mean bacterial populations were generally more in the roots and decreased in leaves and stems. This might be related to the soil serving as the source of endophytic bacteria and the colonization of roots by bacteria in the rhizosphere. These results collectively indicated that endophytic bacteria were not uniformly distributed in plant tissues. Moreover, the population was correlated with the growth period and plant parts. Our research would heighten interest in the research on endophytic bacteria, which may have potential value as a biofertilizer or biopesticide, thus providing a viable approach to sustainable agriculture.
Keywords
Corn, Endophytic bacteria, Population dynamics
Introduction
Zea mays L. is a staple food for half of the world’s population [1]. However, the plant can be seriously infected with many diseases, such as Bipolaris maydis [2], northern corn leaf blight (Spot), Fusarium stalk rot [3], head smut [4] ,and corn rust [5]. Chemical pesticides are widely used to protect corn against diseases. However, they also have many adverse effects, including pesticide residue, environmental pollution, disease resistance enhancement. Thus, interest in using biological control as an alternative approach via antagonistic microorganisms is increasing.
Endophytic bacteria are a group of bacteria that can be isolated from surface-sterilized tissues of asymptomatic plants [6]. Since the 1940s, more than 200 genera of endophytic bacteria from different plant tissues have been successfully categorized and reported from healthy plants [7,8]. And with over 300,000 species of land plant on earth is likely to host to one or more endophyte species [7,8]. Generally, each endophytic bacterium has a wide host range where most commonly isolated bacterial genera include Bacillus, Enterobacteria, Pseudomonas, Kosakonia, Methylobacterium, Microbacterium, Nocardioides, Pantoea, and Burkholderia [9-11]. These endophytic bacteria are important members of plant microbiome living asymptomatically in plant tissues and have attracted considerable attention as potential agents in their beneficial activities in terms of nutrient availability, plant growth hormones, and control of soil-borne and systemic pathogens [12,13]. The endophytic bacteria widely distributed in plant different tissues including roots, stems, leaf, flower, fruits, seeds, and pollens [12]. And the community structure depends on various factors, such as soil conditions, biological and abiotic stresses, as well as the genotype and age of plant [8,14-17].
Despite the beneficial effects of endophytes on plant growth, little is known about the population dynamics corresponding to the growing stage. Therefore, this study aimed to determine the distribution of endophytic bacteria at different growth periods in the roots, stems, and leaves of 17 corn varieties.
Materials and Methods
Corn Varieties
A total of 17 varieties of corn were used in this study: Dafeng123, Diandu 8, Zhongdan 815, Yunjin 2, Zhengda 615, Wugu 2, Wugu 3861, Yunrui 2, Yunrui 7, Yunrui 8, Yunrui 47, Yunrui 88, Yunrui 99, Yunrui 220, Yunyou 105, YR6, YR7. They were obtained from Seed Management Stations in Yunnan Province. A single field plot on the Yunnan Agricultural University farm was used to plant the different corn varieties. Meanwhile, 20 plants of each variety were grown in two rows, with 20 cm spacing between neighboring plants and a 60 cm long and 30 cm wide space between the rows.
Sample Preparation and Surface-disinfestation
Plants were sampled at three growth stages: seedling, elongation, and heading. On specific dates, one plant from each variety was randomly selected, manually uprooted, and washed thoroughly in running tap water to remove the adherent soil particles. Then, the third leaf from the top to bottom was removed, and a section of stem 20 cm above the ground was cut off. One gram of roots (5-10 cm below the soil line) was obtained. The plant materials of different varieties were transported in separate plastic sample bags to a laboratory where they were immediately surface-disinfested immediately [18].
Leaf and Root Surface-disinfestation
For the isolation of leaf and root endophytes, 1 g of each sample was used and the plant parts were cut into small segments. The segments were surface sterilized by immersing them in 75% ethanol for 150 s, followed by immersion in sodium hypochlorite (1%, vol/vol) for 5 min. The samples were then rinsed with 10 mL sterilized distilled water to remove all chemical residues and were ground in a sterile mortar with 9 mL sterile distilled water (SDW).
Stem Surface-disinfestation
A stem section closest to the ground was surface-disinfected with 75% ethanol for 5 min and washed three times with SDW. After the epidermis was aseptically removed, 1 g of stem tissues was transferred to a sterile mortar and ground with 9 mL SDW.
To ensure that the plant surface had been thoroughly sterilized, each surface-disinfected stem, root, and leaf sample was first allowed to touch the surface of LB plates and coated with SDW before incubation at 30°C.
Bacterial Cultivation and Preservation
The ground tissues of each plant part were mixed with 9 mL SDW and ground further to obtain a tissue suspension. Bacteria from roots, stems, and leaves, respectively, were cultivated in 9-cm Petri dishes containing Lysogeny broth (LB) media (5 g/mL yeast extract, 10 g/mL tryptone, 10 g/mL sodium chloride, agar 15 g, 1000 mL ddH2O, pH 7.0-7.2). An aliquot of 200 µL tissue suspension was plated on the LB plates and incubated at 30°C for 36-48 h. A total of three plates were used for each plant part. The population of endophytic bacteria was estimated by counting the colonies appearing on the agar plates. The endophytic density was determined using the following formula [18]:
Endophytic density (CFU/g)=average number of colonies × dilution ratio × 5 (CFU: colony-forming units).
One representative from the numerous bacterial colonies with similar morphological characteristics on the culture plates was transferred to a fresh LB plate to establish a pure culture line for each bacterium strain isolated. Individual bacterial strains were transferred to LB liquid media and shaker-cultured at 180 rpm at 25°C until the media turned milky. The bacteria suspension was then transferred to 2 mL centrifuge tubes containing 40% glycerol and stored at -80°C.
Results
Bacteria recovered from surface-disinfested leaves, stems, and roots throughout the growing season were isolated on LB plates after incubating for two days. The density of bacteria colonies on LB agar plates did not change much with the extension of incubation time. The population density of bacterial endophytes in corn was influenced by the variety, origin of plant tissues, and growing stage.
Distribution of Endophytic Bacteria Taxa during Different Growth Periods
A different number of bacteria taxa were consistently isolated from the healthy plant organs of 17 different corn varieties (Table 1).
Table 1: Distribution of endophytic bacterial taxa in 17 corn varieties during different growth periods
|
Variety |
Seedling stage |
Elongation stage |
Heading stage |
|
Root Stem Leaf |
Root Stem Leaf |
Root Stem Leaf |
|
| Dafeng123 |
1 – 3 |
3 2 1 |
1 2 2 |
| Diandu 8 |
3 – 3 |
3 2 1 |
1 2 2 |
| Zhongdan 815 |
1 – 3 |
3 2 2 |
1 2 2 |
| Zhengda 615 |
1 – 3 |
2 1 1 |
1 2 1 |
| Wugu 2 |
2 – 3 |
2 2 2 |
1 1 3 |
| Wugu 3861 |
2 – 3 |
2 1 3 |
2 1 2 |
| Yunjin 2 |
3 – 4 |
2 1 3 |
1 1 1 |
| Yunyou 105 |
2 – 2 |
2 1 3 |
1 1 2 |
| Yunrui 2 |
1 – 3 |
2 1 3 |
2 1 2 |
| YR 6 |
0 – 2 |
3 1 1 |
1 1 2 |
| YR7 |
1 – 2 |
2 1 3 |
1 1 2 |
| Yunrui 7 |
1 – 3 |
2 1 2 |
2 1 3 |
| Yunrui 8 |
1 – 3 |
2 1 5 |
1 1 3 |
| Yunrui 47 |
1 – 2 |
2 1 2 |
1 1 2 |
| Yunrui 88 |
1 – 2 |
2 1 2 |
1 1 1 |
| Yunrui 99 |
2 – 3 |
2 2 2 |
1 1 1 |
| Yunrui 220 |
2 – 2 |
2 1 1 |
1 1 2 |
In the stems and roots, there were more taxa (2-3 taxa per corn variety) of endophytic bacteria in the elongation stage than in the seedling and heading stages. The number of bacteria taxa on the leaves was more in the seedling stage. In general, there were more taxa of endophytic bacteria in the leaves, followed by the roots.
Population Dynamics of Endophytic Bacteria
Effect of the Growing Season on the Population Density
Endophytic bacteria were found during the entire growth period, and its population dynamics in different tissues changed accordingly. Based on the number of colonies on LB agar plates, the seedling bacterial population from field-grown corn was 103-104 CFU/g; however, it increased with the age of the plants, reaching up to 105 CFU/g in the heading stage (Figure 1).
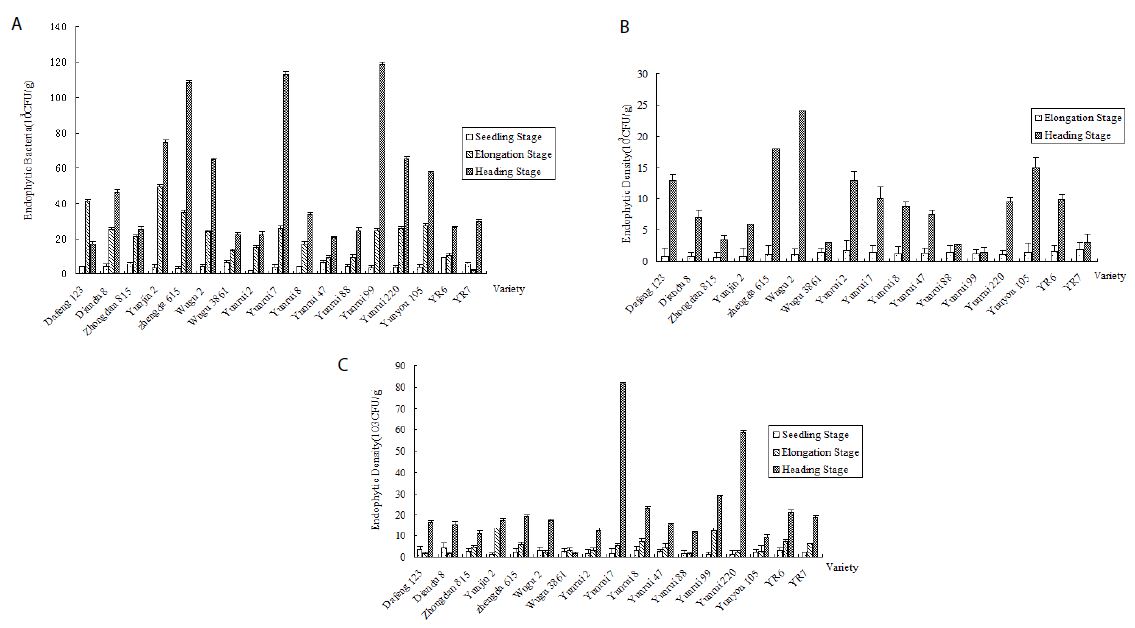
Figure 1: Population quantity of endophytic bacteria in three growth periods of corn. a: Endophytic bacteria in corn root. b: Endophytic bacteria in corn stem. c: Endophytic bacteria in corn leaf.
Effect of Plant Tissues on the Population Density
In all corn varieties, the endophytic bacterial density of corn was the highest in the roots, followed by the leaves, and lowest in the stems (Figure 2).
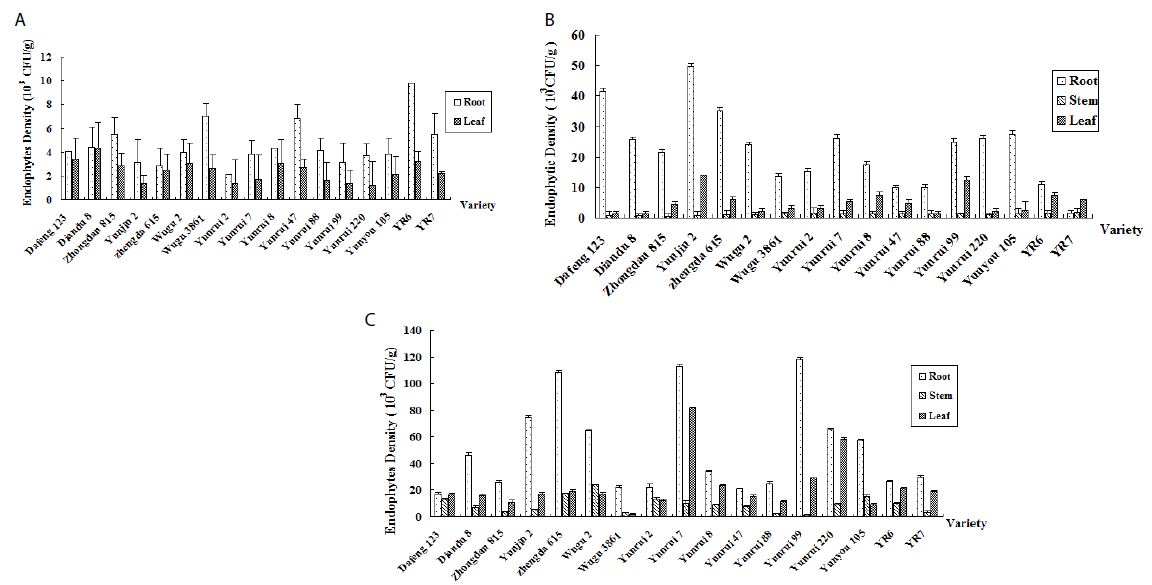
Figure 2: Population quantity of endophytic bacteria in different tissues. a: Endophytic bacteria in the seedling stage. b: Endophytic bacteria in the elongation stage. c: Endophytic bacteria in the heading stage.
Endophytic populations in corn leaves and stems remained at 103 CFU/g for most of the growing season and increased to 104 CFU/g in the heading or post-harvest stages. Root tissues harbored more endophytic bacteria than other plant tissues. For the remainder of the growing season, the root’s bacteria population in the heading stage ranged from 104-105 CFU/g.
Discussion
Extensive knowledge regarding the density and diversity of endophytic bacteria colonizing plant tissues is essential in understanding the indigenous endophytic bacterial community and the assessment of endophytes as potential sources for plant growth promotion and biological control of plant diseases. However, an accurate estimation of the total number of endophytic bacteria is often difficult. This is due to the heterogeneous distribution of bacteria within the plant tissues and the tendency of some bacteria to clump together in their secreted mucilage [19] or adhere to various particles, including the plant cell wall components [20]. Researchers have also found that the density of indigenous endophytic bacteria was approximately 105 CFU/g in the root, but 104 CFU/g and 103 CFU/g, respectively, in the stem and leaf [18-20]. Similarly, the endophytic population in corn stem and root was 104-106 CFU/g for most of the growing season [18,21].
The present study systemically revealed the effects of tissue-types corresponding to different growing periods on structures and densities of endophytic bacteria. Obtained results showed that there was no significant difference in endophytic bacterial community structure among maize varieties. The overall trend was that the endophytic bacteria species were the most abundant in leaves and the least in stems. Previous studies have demonstrated that endophytic bacterial diversity variation is associated with physicochemical properties of soil and atmospheric conditions [18,22-24]. Soil microorganisms can migrate through the xylem track to colonize the plant tissues and the roots play an essential role for the retrieval of microbiome community from soil [25-27]. Therefore, this might be a response to the similar soil type, growing climate condition, as well as the agricultural practices. A dynamic infection process could begin in the rhizosphere (especially at the site of lateral root emergence), followed by endophytic colonization of the roots and subsequent ascending endophytic migration into the stem base, leaf sheath, and leaves [28]. However, it is still unknown if the vascular tissues only serve as a transport channel for endophytic bacteria or if bacteria multiply within the vascular system. In the latter case, many bacteria within the vessels might lead to plugging and, therefore, could induce plant pathogenicity. This may explain why endophytic bacteria are usually found in relatively low numbers within the vascular tissues. Therefore, the hypothesis is supported that bacterial endophytes originate in the rhizosphere and proceed into stem tissue and leaves via the vascular system. Moreover, our study found that the bacterial population from field-grown corn was increased as the plant grew, reaching the highest in the heading stage. The heading stage signals that the crops change from vegetative to reproductive growth and is in a critical period for determining crop yield. Endophytic bacteria have been demonstrated to improve plant growth by producing phytohormones, including IAA and gibberellic acid. In addition, endophytic bacteria have been shown to induce plant disease resistance [18,29]. These biological functions can provide a healthy growth environment for the plants and increase their ability to absorb water and minerals from the soil.
Our results provided more insights by demonstrating that the endophytic bacterial community was dynamic and influenced by biotic factors, with the plant itself being one of the significant factors. The total number and taxa of endophytic bacteria isolated from corn in this study suggest that internal corn tissues harbor diverse microbial flora. Screening endophytic bacteria adds to the list of bacteria as potential plant growth promotors and biological control agents. More useful endophytic bacteria are expected to be discovered as more crops are studied.
Acknowledgements
Not applicable
Author Contributions
Yueqiu He conceived and designed the study and experiments; Liwei Guo and Pengfei He performed the experiments; Pengfei He analyzed the data; Liwei Guo wrote the manuscript; and all authors contributed to the final draft of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
References
- Lobo LLB, Dos Santos RM, Rigobelo EC (2019) Promotion of maize growth using endophytic bacteria under greenhouse and field conditions. Aust J Crop Sci 13(12): 2067-2074. [crossref]
- Wei J-K, Liu K-M, Chen J-P, Luo P-C (1988) Pathological and physiological identification of race C of Bipolaris maydis in China. Phytopathol 78: 550-4. [crossref]
- Saravanakuma K, Li Y, Yu C, Wang Q, Wang M, Sun J, Gao J, Chen J (2017) Effect of Trichoderma harzianum on maize rhizosphere microbiome and biocontrol of Fusarium Stalk rot. Sci Rep 7: 1771. [crossref]
- Wang W, Chao Q, Zhang N, Ye J, et al. (2014) A maize wall-associated kinase confers quantitative resistance to head smut. Nat Genet 47(2): 151-159. [crossref]
- Groth J, Zeyen R, Davis D, Christ B (1983) Yield and quality losses caused by common rust (Puccinia sorghi Schw.) in sweet corn (Zea mays) hybrids. J Crop Prot 2: 105-111. [crossref]
- Hallmann J, Quadt-Hallmann A, Mahaffee W, Kloepper J (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43: 895-914. [crossref]
- Smith SA, Tank DC, Boulanger L-A, Bascom-Slack CA, et al. 2008. Bioactive endophytes warrant intensified exploration and conservation. PLOS ONE 3(8): e3052. [crossref]
- Ali M, Ali Q, Sohail MA, Saleem MH, Hussain S, Zhou L (2021) Diversity and taxonomic distribution of endophytic bacterial community in the rice plant and its prospective. Int J Mol Sci 22: 10165. [crossref]
- Long HH, Sonntag DG, Schmidt DD, Baldwin IT (2010) The structure of the culturable root bacterial endophyte community of Nicotiana attenuata is organized by soil composition and host plant ethylene production and perception. New Phytol 185(2): 554-567. [crossref]
- Afzal I, Shinwari ZK, Sikandar S, Shahzad S (2019) Plant beneficial endophytic bacteria: mechanisms, diversity, host rangeand genetic determinants. Microbiol Res 221:36-49. [crossref]
- Bünger W, Jiang X, Müller J, Hurek T, Reinhold-Hurel B (2020) Novel cultivated endophytic Verrucomicrobia reveal deep-rooting traits of bacteria to associate with plants. Sci Rep 10: 8692. [crossref]
- Dudeja SS, Suneja-Madan P, Paul M, Maheswari R, Kothe E (2021) Bacterial endophytes: Molecular interactions with their hosts. J Bas Microbiol, 1-37. [crossref]
- Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71: 4951-59. [crossref]
- Hardoim PR, Hardoim CCP, van Overbeek LS, van Elsas JD (2012) Dynamics of seed-borne rice endophytes on early plant growth stages. PLOS ONE 7(2): e30438. [crossref]
- Ding T, Melcher U (2016) Influences of plant species, season and location on leaf endophytic bacterial communities of non-cultivated plants. PLOS ONE 11(3): e0150895. [crossref]
- Papik J, Folkmanova M, Polivkova-Majorova M, Suman J, Uhlik O (2020) The invisible life inside plants: deciphering the riddles of endophytic bacterial diversity. Biotechnol Adv 44:107614. [crossref]
- Cherif-Silini H, Thissera B, Bouket AC, et al. (2019) Durum wheat stress tolerance induced by endophyte Pantoea agglomerans with genes contributing to plant functions and secondary metabolite arsenal. Int J Mol Sci 20 (16): 3989. [crossref]
- Cun H, Munir S, He P, Wu Y, et al. (2022) Diversity of root endophytic bacteria from maize seedling involved in biocontrol and plant growth promotion. Egyptian Journal of Biological Pest Control, 32:129. [crossref]
- Dong Z, Canny MJ, Mccully ME, Roboredo MR (1994) A nitrogen-fixing endophyte of sugarcane stems (A new role for the apoplast). Plant physiol 105: 1139-47. [crossref]
- Fisher P, Petrini O, Scott HL (1992) The distribution of some fungal and bacterial endophytes in maize (Zea mays L.). New Phytol 122: 299-305. [crossref]
- Mcinroy JA, Kloepper JW (1995) Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant soil 173: 337-42. [crossref]
- Landa M, Cottrell MT, Kirchman DL, Blain S, Obernosterer I (2013) Changes in bacterial diversity in response to dissolved organic matter supply in a continuous culture experiment. Aquat Microb Ecol 69(2):157-168. [crossref]
- Correa-Galeote D, Bedmar EJ, Arone GJ (2018) Maize endophytic bacterial diversity as affected by soil cultivation history. Front Microbiol, 9: 484. [crossref]
- Penuelas J, Rico L, Ogaya R, Jump AS, Terradas J (2012) Summer season and long-term drought increase the richness of bacteria and fungi in the foliar phyllosphere of Quercus ilex in a mixed Mediterranean forest. Plant Biol 14: 565-575. [crossref]
- Liu H, Carvalhais LC, Crawford M, et al. (2017) Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front Microbiol 8: 2552. [crossref]
- Okunishi S; Sako K, Mano H, Imamur A, Morisaki H (2005) Bacterial flora of endophytes in the maturing seed of cultivated rice (Oryza sativa). Microbes Environ 20(3): 168-177. [crossref]
- Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17, 478-486. [crossref]
- Chi F, Shen S-H, Cheng H-P, Jing Y-X, Yanni YG, Dazzo FB (2005) Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl Environ Microbiol 71: 7271-8. [crossref]
- Munir S, Li Y, He P, et al. (2020) Unraveling the metabolite signature of citrus showing defense response towards Candidatus Liberi-bacter asiaticus after application of endophyte Bacillus subtilis L1-21. Microbiol Res 234: 126425. [crossref]