Abstract
Introduction: Vulvovaginal atrophy, as the most troublesome symptom complex within the more comprehensive clinical picture of Genitourinary Syndrome of Menopause, severely impacts the postmenopausal women’s quality of life, their self-respect as still attractive women, and possibly the women’s couple relationship. The same may happen at a younger age, with vaginal dryness as an occasional side effect of estro-progestin oral contraceptives, hormone-releasing vaginal rings, and other disorders leading to a deranged vaginal environment. Based on a sound rationale, a new medical device based on natural-origin polynucleotides and sodium hyaluronate formulated as vaginal ovuli may help over the long term with those frequent hassles of middle-aged and younger women.
Methods: Design: a survey investigation based on a questionnaire compiled by three investigators familiar with the new vaginal ovuli medical device in their everyday office practice. The investigators completed the survey based on outcomes in 45 ambulatory adult women (≥18 years old). All women had freely consulted the investigators to seek relief from vulvovaginal atrophy symptoms and vaginal dryness as a contraceptive side effect. The minimal inclusion and exclusion criteria helped simulate a real-world office situation. The survey followed a treatment cycle with a Class-III CE-marked medical device (Ovuli PNHA, Mastelli S.r.l., Sanremo, Italy—vaginal ovules with 0.25% polynucleotides, 0.25% non-cross-linked sodium hyaluronate, and 3% polycarbophil as functional ingredients). After the end of the self-administration cycle (one ovule intravaginally per day for two to four weeks), the survey’s goal was to assess the impressions of investigators and their patients about the safety and efficacy (symptom relief, restoration of a healthy vaginal environment) of the medical device in all conditions of vulvovaginal atrophy/vaginal dryness and dystrophic lesions/vaginal environment disorders. The device establishes a protective, moisturizing, and pH-controlled lubricating film onto the cervix and vaginal mucosa and promotes its physiological regenerative and reparative mechanisms. Assessment tools at baseline and after the end of the treatment cycle: Vaginal Health Index score (validated investigator-assessed five-item scale, with scores ranging from 5 to 25 and <15 as dryness and atrophy score threshold); subjective VVA symptom severity (vaginal dryness, vulvovaginal irritation/itching, vulvovaginal soreness, dyspareunia) assessed by the patients with the support of a series of five-point VAS scales (0=absent, 1=mild, 2=moderate, 3=severe, 4 = very severe).
Results: The surveyed investigators stated that the mean total VHI score significantly improved over the follow-up period, from 2.5 ± 0.94 to 3.6 ± 0.85 (p <0.001 vs. baseline). The mucosal thickness and integrity, showing severe or very severe atrophy in 13 surveyed women at baseline, presented no more than some mild atrophy in 91.1% of women, with 35.6% of surveyed women reverting to complete restitutio ad integrum. Vaginal dryness also markedly decreased from baseline, with 79% of cohort women improving to mild atrophy or symptom clearance and 21% reverting to restitutio ad integrum. Regarding the subjective symptom relief, the surveyed women reported a highly significant reduction of the mean total VAS score — cumulatively accounting for vaginal itching, vaginal burning, vaginal pain, pain/discomfort during intercourse, and vaginal dryness — from 3.5 ± 0.70 to 2.1 ± 0.73 (p <0.001 vs. baseline). The occasional mild local pain and irritation were of no clinical significance and rapidly transitory.
Conclusions: The novel formulation of polynucleotides and sodium hyaluronate as vaginal ovules efficiently counteract the symptom complex due to vulvovaginal atrophy in postmenopausal women, including younger women with contraindications for estrogen therapy and vaginal dryness as a contraceptive side effect. The study confirms that PNs, in synergy with hyaluronic acid, retain their innovative potential as non-pharmacological activators of physiological regenerative and reparative processes in vulvovaginal tissues. The study also confirms the novel device’s safety and the women’s compliance with treatment.
Keywords
Dyspareunia, Hyaluronic acid, PNHA ovules, Polynucleotides, Vaginal dryness, Vulvovaginal atrophy
Introduction
The role of 17ß-estradiol in so many cellular pathways regulating vulvovaginal cell growth, barrier functions, and cell proliferation and trophism explains why menopausal hypoestrogenism may have a grievous impact on middle-aged women [1]. Unfortunately, women in advanced countries may expect, on average, to live more than three decades in postmenopausal condition, and about 27% to 84% of them will experience the most troublesome vulvovaginal atrophy (VVA) symptoms [2]. Severe vulvar irritation and burning, compromised vaginal lubrication with discomfort or frank dyspareunia during sexual activity, vaginal discharge, dysuria, and recurrent urinary tract infections may thus be unwelcome companions for many years. The consequences for daily living activities are dire for 75% of symptomatic women, according to the outcomes of the “Vaginal Health: Insights, Views & Attitudes” (“VIVA”) online survey of 3,520 postmenopausal women in six countries [3-6].
Combined with hyaluronic acid (HA), highly purified DNA polynucleotides (PNs), purified from male salmon trout gonads, already showed to improve genital atrophy, VVA symptoms, and the disrupted sexual life and couple relationship of menopausal women [7-11]. PNs showed a powerful restructuring effect on local connective tissues after infiltration in the dermis and tonaca propria of vulvovaginal tissues [9,10]. PNs act non-pharmacologically by progressively releasing small nitrogen precursors (nitrogen bases, nucleosides, nucleotides) in vulvovaginal tissues and showing persistent moisturizing effects.7 Now, a new handy medical device formulation of natural-origin PNs and strongly hydrating HA as vaginal ovuli (formulation henceforth labeled PNHA) promises to extend their combined benefits to other troubling vaginal environment disorders. Examples are the younger women’s vaginal dryness and dyspareunia occasionally observed as adverse effects of estro-progestin oral contraceptives, birth control vaginal rings releasing hormones, and especially progesterone-only contraceptive methods [12-14].
The paper reports on the outcomes of a retrospective survey centered on a questionnaire prospectively administered by the investigators, all gynecologists, to some of their patients who spontaneously sought help and office treatment for problems of menopausal vulvovaginal atrophy (VVA). Some younger women sought relief for their vaginal dryness as a side effect of estro-progestin oral contraceptives or birth control hormone-releasing vaginal rings.
All investigators already used the investigated PNHA device as described in the approved product information leaflet (IFU)-one PNHA ovule daily [15]. The survey study, performed after two to four weeks of everyday use of the intravaginal medical device, was the first within a long-term monitoring program of the device’s persistent efficacy and profile of known side effects and contraindications. Identifying unknown side effects or emergent risks was another purpose of the study and the ongoing long-term monitoring program. Resorting to real-world data, independently from the rigid inclusion and exclusion criteria of randomized clinical studies with their highly selected patient samples, is the clue that supports the clinical value of the investigation in daily practice.
Methods
Study Design
Conceived as a single-arm ambulatory cohort investigation of adults of both genders who had spontaneously sought specialist help for postmenopausal vaginal environment problems and vaginal dryness as a side effect of estro-progestin oral contraceptives and birth control hormone-releasing vaginal rings. Before the survey, following the investigators’ prescription, all individuals had self-administered, at home in a real-world setting, the monitored Class-III CE-marked medical device (Ovuli PNHA, Mastelli S.r.l., Sanremo, Italy: vaginal ovules with 0.25% polynucleotides, 0.25% non-cross-linked sodium hyaluronate, and 3% polycarbophil as functional ingredients). Dose and self-administration period: one ovule intravaginally per day for two to four weeks as stated in the approved product information leaflet (IFU) [15].
After the last ovule domiciliary self-administration, the survey, completed by the investigators, was purely observational with no active intervention. All subjects agreed to submit to the survey after being informed about its goals. Beyond monitoring the efficacy and safety outcomes, the reasons for vaginal treatment were also registered. Questionnaires allow information collection without time constraints for the investigator to answer questions thoroughly, faithfully, and more quickly than face-to-face interviews.
The office-based survey study respected the Helsinki Declaration and Good Clinical Practice principles. All study materials, which included informed consent forms and questionnaires, were preliminarily peer-reviewed for ethical problems.
Observational Efficacy Assessments
Primary Efficacy Endpoint
Relieving objective signs and restoring the healthy vaginal state is paramount in managing vulvovaginal atrophy and disorders of the vaginal environment. The improvement of vaginal signs and symptoms from baseline, evaluated by the investigators with the help of the validated Vaginal Health Index, was the observational primary efficacy endpoint (assessment: total VHI score change from baseline after the last PNHA-based vaginal ovule). First described in 1995, the VHI is a five-point investigator-assessed scoring scale extensively used in clinical trials, which considers the lack of mucosal moisture, vaginal elasticity and volume of vaginal secretions, intravaginal pH, and the evidence of vaginal petechiae and bleeding (Table 1 [16,17]. The ranges of VHI subscale scores are 1 = none, 2 = poor, 3 = fair, 4 = good, and 5 = excellent, with the total VHI score between 5 and 25. Total VHI scores below 15 signal vaginal atrophy [15]. The unimodal symmetric distributions of outcomes on five-point Likert-like assessment tools like the VHI subscales minimize the statistical liability of skewed J- and U-shaped distributions; outcomes assessed on five-point scales also have lower means, floor, and ceiling effects. At the same time, the regression analysis shows that these scales explain a significant fraction of the variation in floor and ceiling effects while minimizing the contribution of unknown factors [16].
Table 1: Vaginal Health Index (Bachmann et al.); * Lower scores: progressively more severe atrophy [16]
|
Score |
Overall elasticity * |
Secretions type and consistency |
pH |
Mucosal epithelium |
Moisture |
| 1 | None | None | 6.1 | Petechiae noted before contact | None; mucosa inflamed |
| 2 | Poor | Scant, thin yellow | 5.6−6.0 | Bleeds with light contact | None; mucosa not inflamed |
| 3 | Fair | Superficial, thin white | 5.1−5.5 | Bleeds with scraping | Minimal |
| 4 | Good | Moderate, thin white | 4.7−5.0 | Not friable, thin mucosa | Moderate |
| 5 | Excellent | Normal (white flocculent) | ≤4.6 | Not friable, normal mucosa | Normal |
Secondary Efficacy Endpoint
Subjective assessment by patients of symptom severity (vaginal dryness, vulvovaginal irritation/itching, vulvovaginal soreness, dyspareunia) with the support of a series of impromptu five-score Visual Analog Scales (henceforth VAS, 0=absent, 1=mild, 2=moderate, 3=severe, 4 = very severe) [18].
Safety
Based on spontaneous reporting over the whole study period. The questionnaire included closed and open questions to identify known side effects, describe their clinical presentation, severity, and duration, and detect any previously unknown adverse event.
Statistics
The sample size estimation took advantage of the G*Power statistical program version 3.14 [19]. The estimate, after a literature review of the efficacy of comparable products, was based on a fictional cumulative VAS symptom score and a conservative assumption of the mean cumulative VAS symptom score at the end of the treatment course (60% improvement). Under these assumptions, the statistical power (two-tailed) to detect a significant difference in a treatment cohort of at least 42 women would have minimized the risk of false-negative type II errors (ß-risk = 0.92).
Descriptive statistics: tabulated as means ± standard errors of the mean (SEM). Inferential statistics: mean total VHI score and subscores as primary efficacy parameter and self-assessed symptom severity VAS scores as secondary efficacy parameter: non-parametric Wilcoxon test [18]. All statistical analyses, two-tailed with a 5% significance level, were performed with the StatPlus analysis program Version v7 [20].
Results
The three participating clinical investigators, all experienced gynecologists already prescribing the PNHA vaginal ovules, enrolled 45 ambulatory menopausal and non-menopausal women with VVA symptoms. All women completed the study fully compliant with the protocol and domiciliary vaginal ovule self-administration with only a few transitory and mild local adverse effects and without dropouts. Table 1 illustrates the women’s demographics, menopausal or non-menopausal conditions, and previous use of estrogens. The subgroup of menopausal women had been in menopause for a range of 2 to 30 years and had experienced VVA symptoms for periods ranging from 1 month to 20 years (Figure 1). Often together with oral or topical estrogens, 16 cohort women (35.6%) had used other topical and parenteral formulations and techniques to counter their VVA symptoms—other injectable polynucleotide and hyaluronic acid gels, hyaluronate topical formulations, vaginal moisturizers, lubricants, and electroporation and radiofrequency biostimulation procedures.
Table 1: Women’s demographics, menopausal condition, and oral and topical estrogen use. SEM: Standard Error of the Mean.
| Age | Mean ± SEM (years) | 55.4 ± 10.29 |
| Median (years) | 56 | |
| Range (years) | 25-74 | |
| Menopause | Yes (%) | 29 (64.4) |
| Years in menopause ± SEM | 9.2± 6.64 | |
| Years with VVA symptoms ± SEM | 6.6 ± 5.46 | |
| No (%) | 16 (35.6) | |
| Oral estrogens | Yes (%) | 7 (15.6) |
| No (%) | 38 (84.4) | |
| Topical estrogens | Yes (%) | 5 (11.1) |
| No (%) | 46 (88.9) | |
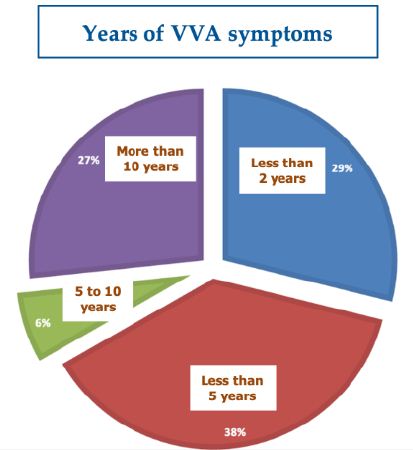
Figure 1: Years with VVA atrophy symptoms in the 45-strong women’s cohort
At baseline, the VVA symptoms appeared to severely affect the women’s sexual life, with 31 menopausal and non-menopausal women (68.9%) reporting reduced sexual activity with decreased sexual interest and desire in 27 (60.0%).
The VVA signs and symptoms which most commonly induced the investigators to prescribe the PNHA intravaginal ovules were, in decreasing frequency, vaginal dryness (80% of cohort women), vaginal burning (40% of women), vaginal itching (37.8% of women), vaginal pain (31.1% of women), often during intercourse (24.4% of women), and vaginal bleeding during intercourse (15.6% of women). Symptoms co-existed very commonly. Previous genital surgery and dysuria were other significant reasons for prescribing the PNHA ovules (6.7% and 2.2% of women, respectively).
Efficacy
At baseline, the mean VHI score was 12.68, beyond the VVA threshold, while the mean total VHI score (mean of all VHI subscale scores) was 2.5 ± 0.94, once again describing a severe VVA picture. Figure 2 shows the highly significant improvement in the mean total VHI score at the study end compared with baseline, although always with some persisting atrophy signs and symptoms. Figure 3 analytically illustrates the improvements vs. baseline for each VHI subscore after treatment with the PNHA ovules.
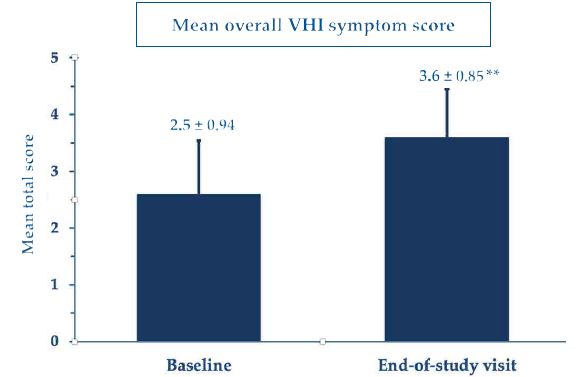
Figure 2: Mean total Vaginal Health Index scores (± standard errors of the mean); **p<0.001 vs. baseline
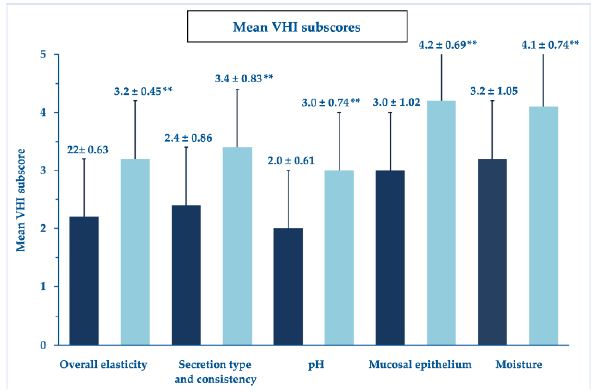
Figure 3: Mean VHI subscores (± standard errors of the mean); **p<0.001 vs. baseline
Looking more in detail into the mucosal thickness and integrity (Figure 4), the ultimate basis for bleeding and other symptoms, thirteen women showed severe or very severe atrophy of the vaginal epithelium at baseline (scores: 1 or 2); after the topical PNHA treatment, 91.1% of women showed no more than a condition of mild atrophy (scores: 4 or 5), with 35.6% of them reverting to complete restitutio ad integrum (score: 5).
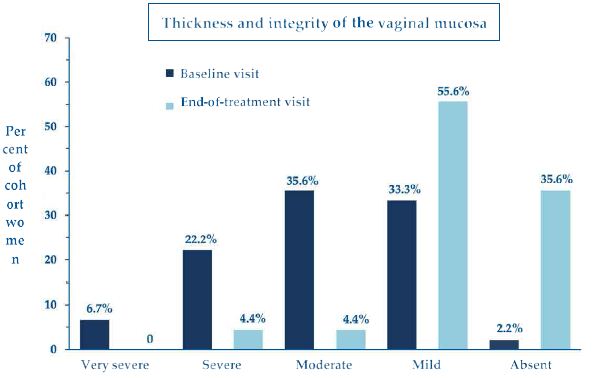
Figure 4: Percent of cohort women with compromised vaginal mucosal thickness and integrity of variable clinical severity (scores 1 to 5) at baseline and end-of-treatment assessment visits.
Regarding the clinical severity of vaginal dryness, the most bothersome and frequently reported symptom of atrophy, it also markedly decreased from baseline in all cohort women (Figure 5), with 79% of them improving to a condition of mild atrophy or symptom clearance (scores: 4 or 5), and 21% of them reverting to complete restitutio ad integrum (score: 5).
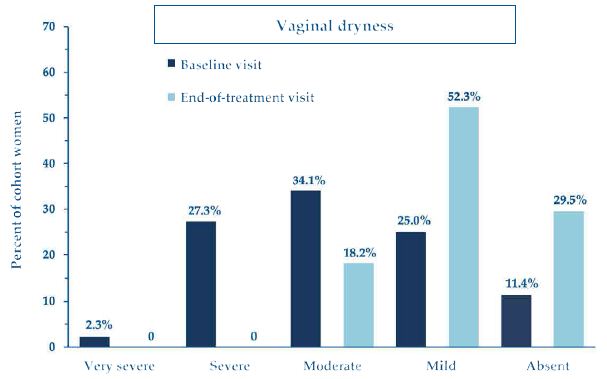
Figure 5: Percent of cohort women who reported vaginal dryness of variable clinical severity (scores 1 to 5) at baseline and end-of-treatment assessment visits.
Safety
The investigators and treated women invariably deemed the procedure manageable and handy without unexpected technical difficulties, troubles, and discomfort. The repeated insertion of the PNHA ovules was well tolerated, with a few transitory and mild local adverse effects—local burning in 15 cohort women (mild 31.8%, moderate 2.3%), vulvovaginal itching in 16 women (mild 31.8%, moderate 4.5%), and local discomfort in 19 women (mild 36.4%, moderate 6.8%). According to investigators, two women reported some mild vaginal blood losses, which disappeared in 6 to 10 days and were unrelated to the PNHA topical treatment. There were no unexpected technical difficulties. The few transitory and mild local adverse effects (local burning, vulvovaginal itching, and local discomfort) were of no clinical significance. They resolved spontaneously in a few hours without further therapy. There were no unexpected side events or complications (Figures 6 and 7).
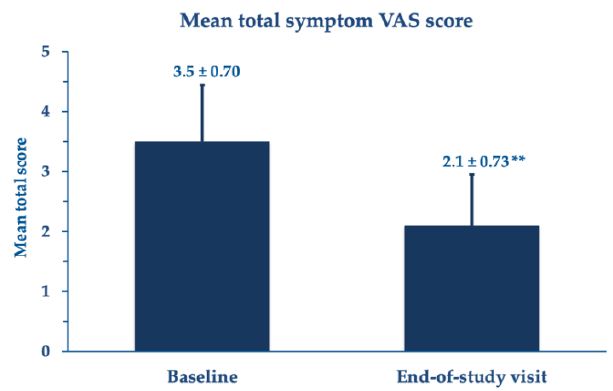
Figure 6: Mean total symptom VAS scores (± standard errors of the mean); **p<0.001 vs. baseline
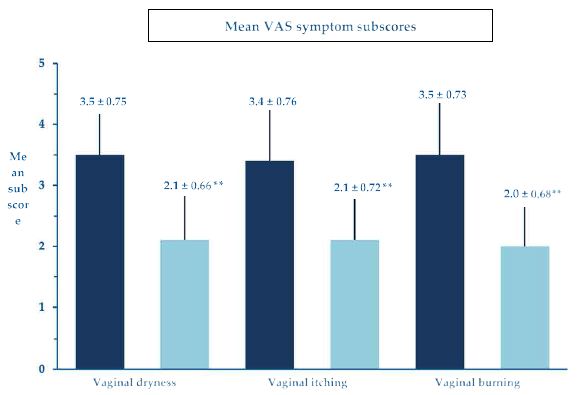
Figure 7: Mean VAS scores (± standard errors of the mean) for some representative symptoms; **p<0.001 vs. baseline.
Discussion
Real-world investigations aim to provide reliable insights into conditions analogous to everyday clinical practice [21]. Within a long-term monitoring program, the PNHA vaginal ovule formulation confirmed its efficacy and lack of unknown and troublesome side effects with once-daily dosing in menopausal VVA and other non-menopausal and post-surgical disorders with vaginal dryness and other symptoms, as stated in the Information for Use leaflet.
In this first step of the ongoing real-world, long-term monitoring program of the once-daily PNHA vaginal ovule medical device, the formulation proved to be effective with no intolerably bothersome side effects in vulvovaginal atrophy and other menopausal and non-menopausal or post-surgery disorders.
The phenotypic expression of postmenopausal vulvar involution — depletion of labia majora adiposity, blundering of interlabial sulci, loss of pigmentation and hair, reduced density of sweat and sebaceous production, preputial retraction with clitoral exposure and chronic irritation, and overall dysfunction of the vaginal ecosystem — and related symptoms are a burden on the woman’s self-confidence and self-image. Microscopically, the fragmentation and fusion of elastin fibers, collagen hyalinization, and extracellular matrix depletion are the markers of the VVA picture [2,22]. Moreover, vaginal dryness is a frequent side effect of estro-progestin oral contraceptives and hormone-releasing vaginal rings in up to 12.7% to 30.4% of women after three menstrual cycles, especially with preparations with the lowest synthetic estrogen content [23].
The hydrophilic polynucleotide polymers of the PNHA formulation reorganize in vulvovaginal tissues into a three-dimensional gel that binds water with a moisturizing and volume-increasing effect that synergizes with the potent HA hydrating effect [7-11]. Over the longer term, the polynucleotide component of the PNHA device passively replenishes the fibroblast pool of nitrogen bases, nucleosides, and nucleotide precursors and supports the dermal fibroblast viability, thus facilitating the production of new collagen fibers—the rationale for exploring the PNHA option to antagonize the menopausal and non-menopausal VVA and vaginal dryness [7-10].
The survey study had two main problems: compensating for the lack of a control group and the ß-risk of failing to detect a significant difference in semiquantitative scores, compared with baseline, at the end of the self-administration at home. However, the impressive efficacy outcomes reported by surveyed investigators and women are unlikely to be incidental findings and likely compensate for the first bias. Moreover, the cohort size of the enrolled women, more numerous than what was estimated to reduce to almost zero the ß-risk under the conservative assumption of a 60% efficacy, compensated for the second bias.
All surveyed clinical signs and symptoms showed highly significant improvements over the follow-up period, demonstrating that the newly introduced PNHA ovules are an effective option in all forms of vaginal atrophy, independently of cause. The clinically meaningful relief of VVA objective signs was not associated with more than some occasional undefined discomfort and mild burning of no clinical significance.
In conclusion, improving VVA-related objective signs means that the novel PNHA vaginal ovule medical device is an effective, safe, and well-tolerated non-hormonal therapeutic option in postmenopausal women with VVA and all women unwilling or unable to consider estrogen therapy. In general, the new PNHA device appears effective, independently of age, in all situations of vaginal dryness and a deranged vaginal environment for whatever cause.
References
- Cotreau MM, Chennathukuzhi VM, Harris HA, et al. (2007) A study of 17beta-estradiol-regulated genes in the vagina of postmenopausal women with vaginal atrophy. Maturitas 58: 366-376. [crossref]
- Nappi RE, Martini E, Cucinella L, et al. (2019) Addressing vulvovaginal atrophy (VVA)/Genitourinary Syndrome of Menopause (GSM) for healthy aging in women. Front Endocrinol (Lausanne) 10: 561. [crossref]
- NAMS Position Statement. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause 27: 976-992.
- Nappi RE, Davis SR (2012) The use of hormone therapy for the maintenance of urogynecological and sexual health post WHI. Climacteric 15: 267-274. [crossref]
- Davis SR, Lambrinoudaki I, Lumsden M, et al. (2015) Menopause Nat Rev Dis Primers 1: 15004.
- Shifren JL, Zincavage R, Cho EL, Magnavita A, Portman DJ, et al. (2018) Women’s experience of vulvovaginal symptoms associated with menopause. Menopause 26: 341-349. [crossref]
- Colangelo MT, Govoni P, Belletti S, Squadrito F, Guizzardi S, et al. (2021) Polynucleotide biogel enhances tissue repair, matrix deposition, and organization. J Biol Regul Homeost Agents 35: 355-362. [crossref]
- Bartoletti E, Cavallini M, Maioli L, et al. (2020) Introduction to Polynucleotides Highly Purified Technology. Aesthetic Medicine 6: 43-47.
- Palmieri IP, Raichi M (2019) Biorevitalization of postmenopausal labia majora, the polynucleotide/hyaluronic acid option. Obstet Gynecol Rep 3.
- Palmieri IP, Raichi M (2022) Vulvar rejuvenation with polynucleotides HPT® and benefits on postmenopausal sexual life disruption. Obstet Gynecol Rep 6. [crossref]
- Angelucci M, Frascani F, Franceschelli A, Lusi A, Garo ML (2022) Climacteric 25: 490-496.
- Sabatini R, Cagiano R (2006) Comparison profiles of cycle control, side effects and sexual satisfaction of three hormonal contraceptives. Contraception 74: 220-223. [crossref]
- Mares P, Hoffet M, Rousseau O, Ripart-Neveu S (2001) Vaginal dryness. Rev Prat 51: 155-158.
- Shah MB, Hoffstetter S (2010) Contraception and sexuality. Minerva Ginecol 62: 331-347. [crossref]
- Newest Gyn ovuli PNHA product information leaflet (IFU).
- Bachmann G (1995) Urogenital ageing: an old problem newly recognized. Maturitas S1-S5. [crossref]
- Cagnacci A, Xholli A, Sclauzero M, Venier M, Palma F, et al. (2019) Vaginal atrophy across the menopausal age: results from the ANGEL study. Climacteric 22: 85-89. [crossref]
- Ettinger B, Hait H, Reape KZ, Shu H (2008) Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause 15: 885-889. [crossref]
- Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods 41: 1149-1160. [crossref]
- Statistical software downloaded from https://www.analystsoft.com/en/products/statplus/#statfeatures.
- Miksad RA, Abernethy AP (2018) Harnessing the power of real-world evidence (RWE): a checklist to ensure regulatory-grade data quality. Clinical Pharmacology & Therapeutics 103: 202-205. [crossref]
- Pérez-López FR, Phillips N, Vieira-Baptista P, Cohen-Sacher B, Fialho SCAV, et al. (2021) Management of postmenopausal vulvovaginal atrophy: recommendations of the International Society for the Study of Vulvovaginal Disease. Gynecol Endocrinol 37: 746-752. [crossref]
- Roumen FJM (2008) Review of the combined contraceptive vaginal ring, NuvaRing. Ther Clin Risk Manag 4: 441-451. [crossref]