DOI: 10.31038/CST.2023834
The pancreas is made predominantly of acinar (exocrine aspect) and duct cells, while the islet cells (endocrine aspect) make up to 2% of the pancreas. Importantly, these cell types display remarkable plasticity and can alter cellular identity in response to injury, regeneration, and repair [1]. With the limitations due to ethical issues, much of our understanding of genes and molecular pathways that modulate the development, differentiation, and homeostasis of the pancreas originates from animal studies. Utilizing a newly developed conditional knockout (KO) mouse model, Kayal et al have recently reported that heparanase-2 (Hpa2) plays a critical role in acinar cell differentiation and protects the pancreas from malignant transformation and inflammation [2]. Heparanase is a unique enzyme due to its endoglycosidase activity, capable of cleaving heparan sulfate (HS) side chains of heparan sulfate proteoglycans (HSPG). HSPG are highly abundant in the extracellular matrix (ECM) and assist in assembling the major protein constituents of the ECM and basement membrane (i.e., laminin, fibronectin, collagen IV) into a three-dimensional, non-soluble matrix that provides structural support and biochemical cues to many cell types. Cleavage of HS by heparanase thus results in remodeling of the ECM, which in the pancreas results in impaired islet β cell survival [3]. These structural and biochemical alterations exert a profound impact on cell behavior including, among others, cell viability, differentiation, proliferation, migration and invasion. The latter is most often associated with increased metastatic capacity of tumor cells and augmented entry of immune cells into sites of inflammation. This, and many other mechanisms utilized by heparanase to promote tumorigenesis, have turned this enzyme into a promising drug target and heparanase inhibitors are currently being evaluated in clinical trials as anti-cancer drugs [4,5]. HPSE2, the gene encoding heparanase-2 (Hpa2), was cloned soon after the cloning of heparanase, based on sequence homology. Interestingly, Hpa2 lacks intrinsic HS-degrading activity, the hallmark of heparanase, yet retains the capacity to bind HS with high affinity, thereby competing for HS and inhibiting heparanase enzymatic activity capacity. Unlike the intense research effort devoted to exploring the significance of heparanase in cancer progression, very little attention was given to Hpa2. The emerging role of Hpa2 in autosomal recessive congenital disease called urofacial syndrome (UFS) [6,7], clearly indicates that Hpa2 plays a critical role in human disorders. To further explore the role of Hpa2 in tumorigenesis, Kayal et al generated a conditional Hpa2-knockout (KO) mouse. Interestingly, it was observed that the pancreas of Hpa2-KO female mice is smaller, presenting half the weight of wild-type pancreas when calculated relative to body weight. Importantly, heparanase enzymatic activity was dramatically increased in pancreatic tissue derived from Hpa2-KO mice vs. control, wt mice. Histological examination revealed significant morphological abnormalities in Hpa2-KO vs. wt pancreas. A large proportion of the Hpa2-KO pancreas appeared to consist of fat cells, replacing the pancreas acinar cells (Figure 1), possibly the result of acinar-to-adipocyte transdifferentiation (AAT) [8]. In addition, a substantial number of duct-like structures were observed only within the Hpa2-KO pancreas. These were stained positive for cytokeratin 19 and Sox 9 and exhibited high proliferative capacity. In addition, these structures deposited large amounts of collagen and were stained strongly with alcian blue that labels HS. Altogether, indicating that the Hpa2-KO pancreas undergoes acinar-to-ductal metaplasia (ADM) [9] and turns into fatty tissue. Fatty pancreas was first observed in the 1930s by imaging studies performed for other indications; it was thought to be an incidental finding and its clinical implications were not thoroughly investigated. In recent years, however, there has been accumulating evidence supporting the association of fatty pancreas with the development of pancreatic cancer as well as other pathologies of the human pancreas [10,11]. Kayal et al demonstrated that this pro-tumorigenic environment not only supports the growth of implanted cancer cells but, unlike wt mice, also leads to the development of pancreatic neoplasia once mice are exposed to conditions that elicit mutations (carcinogen) and prolonged inflammation (cerulein) [2]. These results strongly support the notion that Hpa2 functions as a tumor suppressor; in its absence, tissues become more prone to the development of pre-malignant and malignant lesions.Unlike female Hpa2-KO mice, the male Hpa2-KO pancreas did not exhibit accumulation of fat, AAT, and ADM. However, foci ofinflammation were readily detected within the Hpa2-KO pancreas of young (3-month-old) and older (8-month-old) male mice. Kayal et al, then, exposed wt and Hpa2-KO male mice to cerulein, best recognized for its capacity to induce acute pancreatitis. Importantly, it was found that Hpa2-KO male mice responded vigorously to cerulein, resulting in the accumulation of fat cells and ADM to an extent comparable with female Hpa2-KO pancreas [2]. Thus, within one day, the morphology of male Hpa2-KO pancreas approached the morphology observed in the female pancreas, implying that abnormal cellular and molecular mechanisms were already turned on in response to Hpa2 knockdown, awaiting induction. Collectively, it was concluded that Hpa2 functions to preserve the identity of acinar cells; deficiency of Hpa2 results in pre-neoplastic pancreas which, in response to further insults, develops into pancreatic neoplasia. It is hoped that the protective effects of Hpa2 against cancer and inflammation will be translated to the development of Hpa2-based therapeutic strategies.
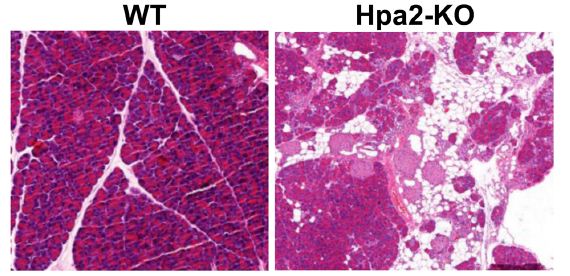
Figure 1: H&E staining of pancreatic tissue sections showing the morphology of wild-type (wt) vs. Hpa2-KO pancreas and demonstrating the replacement of acinar cells by fat cells
Funding
These studies were generously supported by research grants awarded by the Israel Science Foundation (ISF-1021/19); The Israel Cancer Association (ICA), the US-Israel Binational Science Foundation (BSF2021059); and the Technion Integrated Cancer Center (TICC) Rubinstein scholarship (to YK).
References
- Grimont A, Leach SD, Chandwani R (2022) Uncertain Beginnings: Acinar and Ductal Cell Plasticity in the Development of Pancreatic Cancer. Cell Mol Gastroenterol Hepatol 13: 369-82. [crossref]
- Kayal Y, Barash U, Naroditsky I, Ilan N, Vlodavsky I (2023) Heparanase 2 (Hpa2) – A new player essential for pancreatic acinar cell differentiation. Cell Death Dis 14: 465. [crossref]
- Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ (2012) Heparan sulfate and heparanase play key roles in mouse beta cell survival and autoimmune diabetes. J Clin Invest 122: 132-41. [crossref]
- Dredge K, Brennan TV, Hammond E, Lickliter JD, Lin L, Bampton D, et al. (2018) A Phase I study of the novel immunomodulatory agent PG545 (pixatimod) in subjects with advanced solid tumours. Br J Cancer 118: 1035-41. [crossref]
- Galli M, Chatterjee M, Grasso M, Specchia G, Magen H, Einsele H, et al. (2018) Phase I study of the heparanase inhibitor roneparstat: an innovative approach for ultiple myeloma therapy. Haematologica 103: e469-e72. [crossref]
- Daly SB, Urquhart JE, Hilton E, McKenzie EA, Kammerer RA, Lewis M, et al. (2010) Mutations in HPSE2 cause urofacial syndrome. Am J Hum Genet 86: 963-9. [crossref]
- Pang J, Zhang S, Yang P, Hawkins-Lee B, Zhong J, Zhang Y, et al. (2010) Loss-of-function mutations in HPSE2 cause the autosomal recessive urofacial syndrome. Am J Hum Genet 86: 957-62. [crossref]
- Bonal C, Thorel F, Ait-Lounis A, Reith W, Trumpp A, Herrera PL (2009) Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology 136: 309-19 e9. [crossref]
- Parte S, Nimmakayala RK, Batra SK, Ponnusamy MP (2022) Acinar to ductal cell trans-differentiation: A prelude to dysplasia and pancreatic ductal adenocarcinoma. Biochim Biophys Acta Rev Cancer 1877: 188669. [crossref]
- Khoury T, Sbeit W (2022) Fatty Pancreas and Pancreatic Cancer: An Overlooked Association? J Clin Med [crossref]
- Truong E, Pandol S, Jeon C (2022) Uniting epidemiology and experimental models: pancreatic steatosis and pancreatic cancer. EBioMedicine 79: 103996. [crossref]