Abstract
Aim: Traumatic brain injury (TBI) was associated with increased plasma serotonin 2A receptor (5-HT2AR) autoantibodies in adults who experienced neurodegenerative complications. We tested whether the baseline presence of plasma serotonin 2A receptor (5-HT2AR) autoantibodies was a significant predictor of the two-year rate of cognitive decline in middle-aged and older adult TBI.
Methods: Plasma from thirty-five middle-aged and older adult veterans (mean 65 years old) who had suffered traumatic brain injury was subjected to protein-A affinity chromatography. One-fortieth dilution of the resulting immunoglobulin (Ig) G fraction was tested for binding (in ELISA) to a linear synthetic peptide corresponding to the second extracellular loop region of the human 5-HT2A receptor. All available patients completed baseline and two-year follow-up neurocognitive tests of memory (St Louis University Mental Status), processing speed (Digit Symbol Substitution Test) and executive function (Trails-making Test, Part B). Change in cognitive performance was computed as (two-year – baseline) raw test score.
Results: Eighteen patients completed both baseline and two-year follow up neurocognitive tests. TBI patients harboring plasma 5-HT2AR autoantibodies at the baseline examination (n=13) did not differ significantly in their baseline clinical characteristics (age, education level) compared to TBI patients lacking baseline plasma autoantibodies (n=5). Plasma serotonin 2AR antibody-positive patients experienced a significantly greater post-baseline decline in performance on the St Louis University Mental Status test (P=0.0118) and in the Digit Symbol Substitution Test (P=0.011), but not in Trails-making Part B (P=0.129) compared to serotonin 2AR antibody-negative patients. In multivariable linear regression analyses that adjusted for age, baseline presence of plasma 5-HT2AR autoantibody was a significant predictor of the two-year rate of decline in memory, and processing speed. Binding of plasma autoantibody to the serotonin 2A receptor peptide in the enzyme linked immunosorbent assay was also significantly higher (at 1/160th titer of the protein-A eluate= 1 µg/mL IgG) in TBI patients harboring vs. those not harboring baseline plasma 5-HT2AR autoantibodies.
Conclusion: These data suggest that plasma 5-hydroxytryptamine 2A receptor autoantibodies which were increased in approximately two-thirds of middle-aged and older adults following traumatic brain injury predicts rapid and substantial declines in cognitive function (memory and processing speed), independent of age.
Introduction
The human serotonin 2A receptor (5HT2AR) plays a diverse role in cognition, learning and memory, appetite, and mood regulation [1]. In human post-mortem [2] and imaging studies [3] from Alzheimer’s dementia patients, 5HT2AR receptor binding was decreased generally in cerebral cortex [3] and in specific brain regions, i.e. temporal lobe [2] subserving memory. On the other hand, 5-HT2AR binding was increased in the temporal lobe from patients with vascular dementia compared to age-matched individuals [4].
A role for dysregulation of central 5-HT2AR signaling in Alzheimer’s dementia and the underlying mechanism is still poorly understood. We reported spontaneously occurring neurotoxic plasma IgG autoantibodies in older adults suffering with certain neurodegenerative disorders, e.g. Parkinson’s disease and dementia [5], including lifelong traumatic brain injury, sufferers affected with these specific neurodegenerative disorders [6]. The IgG autoantibodies displayed increased binding to a synthetic peptide corresponding to the second extracellular loop of the 5HT2AR and they caused accelerated neuroblastoma cell death in cell culture [5,7] by a mechanism involving sustained activation of G-protein coupled PLC/IP3R/Ca2+ signaling [5,7]. Here we tested a hypothesis that baseline presence of the agonist serotonin 2A receptor IgG autoantibodies in plasma in older adult TBI-sufferers predicts accelerated decline in neurocognitive functioning. There is currently no plasma biomarker(s) that can predict accelerated cognitive decline following TBI. We used a battery of neuropsychological tests performed at baseline and repeated two years later to test whether the baseline presence (vs.absence) of plasma serotonin 2A receptor autoantibodies predicts the rate of decline in working memory, processing speed and/or executive function in thirty-five middle-aged and older adult men veterans who had suffered a TBI.
Patients
Thirty-five middle-aged and older adult men (> 50 years old) were enrolled in the study between Sept 2019 and March 2020. A local Institutional Board Review-approved consent was obtained in all study participants prior to initiating study procedures. The patients underwent baseline blood drawing in the morning followed by administration of three neuropsychological tests (St. Louis University Mental Status, Digit Symbol Substitution Test, and the Trails-Making Test Part B). Each of the cognitive tests was repeated at mean interval of 2 years (range 22-26 months) following baseline testing. The baseline clinical characteristics in the study cohort were previously reported [6]. Most patients had suffered a mild direct force TBI. A few patients had a history of moderate TBI with loss of consciousness > 30 minutes or longer. Five patients reported multiple TBI exposures.
Blood Drawing
Blood was drawn in the morning after an overnight fast. Plasma or serum was stored at -20°C or used immediately in protein-A affinity chromatography to obtain IgG fraction.
Protein-A Affinity Chromatography
Protein A chromatography was carried out as previously reported [5].
Human Serotonin 2A Receptor Peptide
An 18-meric linear synthetic peptide corresponding to the second extracellular loop of the human 5-HT2AR was synthesized at Lifetein, Inc (Hillsborough, NJ), catalog number 701781. It had purity of 95% or greater.
Enzyme Linked Immunosorbent Assay for 5-HT2AR Autoantibodies
Linear synthetic human 5-HT2AR receptor peptide was used as the solid phase antigen. A 1/40th dilution of human protein-A eluate fraction was incubated in the presence of antigen as previously described [7]. Binding more than two-fold above assay background level (blank= 0.05 absorbance units, AU) was defined as indicative of the baseline ‘presence’ of 5-HT2AR plasma autoantibodies. In a subset of patients, serial dilution of protein A eluate fraction (1/40th, 1/160th, 1/320th) was performed to assess the autoantibody titer.
Protein Concentration
Protein concentration was determined using a bicinchoninic assay (Thermo-Fischer, Inc) as previously reported [5].
Neuropsychological Tests
The St. Louis Mental Status examination [8], digit symbol substitution test [9] and the Trails-making Test, Part B (TMT-B) [10,11] were administered at baseline and repeated at the two-year study interval. Change in neurocognitive test scores was calculated as the difference between year 2 and baseline raw scores. A negative value for the difference is indicative of a decline in cognitive test performance (SLUMs, and DSST).
Higher raw score in the TMT-B is indicative of worse cognitive performance. Lay staff was trained in the administration of neuropsychological test battery prior to testing study participants. Normative data for the TMT-B, adjusted for age and education level [12], were used to convert raw scores to a scaled score in order to make comparison between the present results and results reported in non- TBI population having similar mean age and co-morbidities [13].
Medication Use
Medications to treat highly prevalent conditions, e.g. diabetes (78%) included a wide range of different classes including insulin, incretins, GLP- 1 agonists, and SGLT2 inhibitors. Baseline anti-depressant medication use (in 33% of patients) included: selective serotonin reuptake inhibitors, tetracyclic antidepressants, and atypical antipsychotics.
Statistics
Comparisons were made using paired t-test (Tables 1, 2 and Figure 3); multivariable linear regression analysis was conducted using SAS 9.4 (SAS Institute Inc, Cary, NC) (Tables 3-5).
Table 1: Baseline characteristics in eighteen male TBI patients who completed baseline and 2-year follow up cognitive testing.
|
Risk factor |
Antibody (13) | No Antibody (5) |
P-value |
| Serotonin 2AR peptide binding (AU)+ |
0.129 ± 0.029 |
0.07 ± 0.02 |
0.001* |
| Age (years) |
66.4 ± 7.5 |
63.8 ± 9.3 |
0.56* |
| Beck depression inventory |
19.2 ± 10.4 |
14.4 ± 13.5 |
0.47 |
| Education (12+ yrs) (yes/no) |
12/1 |
5/0 |
1.00^ |
| Diabetes (yes/no) |
11/2 |
3/2 |
0.53 |
| Hypertension (yes/no) |
8/5 |
3/2 |
1.00 |
| Moderate-severe obesity (BMI >/=34 kg/ m2)(yes/no) |
5/8 |
1/4 |
0.62 |
| Antidepressant med (yes/no) |
4/9 |
2/3 |
1.00 |
Results are mean ± SD or number; AU-absorbance units +Binding to an 18-meric linear synthetic peptide comprised of the 2nd extracellular loop of the human serotonin 2A receptor in a 1/40th dilution of the protein-A eluate of plasma was determined as described in Materials & Methods.
*Student’s t-test.
^Fischer’s exact test.
Table 2: Mean two-year change in cognitive test performance on the St. Louis University Mental Status, Digit Symbol Substitution test, and Trails Making Test Part B according to baseline presence or absence of plasma autoantibody binding to serotonin 2A receptor peptide.
|
Test |
Antibody | No Antibody |
P-value* |
| SLUMS |
-4.5 + 3.26 (13) |
-0.6 + 1.14 (5) |
0.018 |
| DSST |
-5.45 + 5.0 (11) |
2.25 + 1.71 (4) |
0.011 |
| TMT-B |
51.45 +58.3 (11) |
1.25 + 29.6 (4) |
0.129 |
*Student’s t-test. Results are mean ± SD.
( ) number of participants.
SLUMS- St Louis University Mental Status test. DSST- Digit Symbol Substitution test.
TMT-B Trails-making Test Part B.
Table 3: Age-adjusted model of risk factors associated with 2-year change in St. Louis University mental status exam in male TBI sufferers.
|
Estimate |
Standard Error |
P-value |
|
| Age (years) |
-0.048 |
0.089 |
0.598 |
| Serotonin2AR Antibody (present/absent) |
-3.712 |
1.527 |
0.028 |
N=18 TBI patients.
Table 4: Age-adjusted model of risk factors associated with 2-year change in Digit Symbol Substitution test score in male TBI sufferers.
|
Estimate |
Standard Error |
P-value |
|
| Age (years) |
-0.143 |
0.1449 |
0.343 |
| Serotonin2AR Antibody (present/absent) |
-7.711 |
2.591 |
0.012 |
N=15 TBI patients.
Table 5: Age-adjusted model of risk factors associated with 2-year change in Trails making Test Part B score in male TBI sufferers.
|
Estimate |
Standard Error |
P-value |
|
| Age (years) |
-0.6407 |
1.679 |
0.7095 |
| Serotonin2AR Antibody (present/absent) |
51.67 |
32.28 |
0.135 |
N=15 TBI patients.
Results
Baseline Clinical Characteristics in Older Adult Male TBI Cohort
The thirty-five TBI patients who underwent baseline blood drawing for autoantibody determination had mean age of 64.8 ± 8.4 years (not shown in Table 1). Total 18 patients completed both baseline and at least one of the three neuropsychological tests at the two-year follow-up visit. In thirteen patients a 2-year change in neurocognitive function could not be determined for several reasons: three patients failed to undergo baseline cognitive testing, two older patients (both having PD) died prior to their 2-year follow-up testing date, and eight patients were ‘lost to follow-up testing’ because of transportation or another social issue. In four additional patients, data was excluded either because of a co-morbid CNS pathology (e.g. focal cortical deficit, lacunar infarcts) independently associated with progressive cognitive decline (n=3) or because baseline plasma autoantibody neurotoxicity had specificity for a different G-protein coupled receptor than 5HT2AR (n=1).
Mean plasma autoantibody binding to a serotonin 2A receptor peptide corresponding to the second extracellular loop was significantly increased in patients having baseline autoantibody vs. those lacking baseline autoantibody (0.129 ± 0.029 vs. 0.07 ± 0.02; P= 0.001; Table 1). Patients having or not having baseline 5-HT2AR peptide plasma autoantibodies did not differ significantly in their mean age, education level, medical co-morbidities, baseline depressive symptoms (Beck Depression Inventory score) or anti-depressant medication use (Table 1). Association between baseline presence of 5HT2AR autoantibodies and 2-year cognitive change we next compared mean change in the individual two-year neurocognitive test score in patient who had presence or absence of baseline plasma 5-HT2AR autoantibodies. Baseline presence of autoantibodies was associated with significantly larger mean decrease in test performance in the St. Louis University Mental Status Exam (P=0.018), and in the Digit Symbol Substitution Test (P= 0.011; Table 2) after two years follow-up. There was no significant difference in mean (two-year) change in Trails-Making Test, Part B test score in patient subgroups having vs. lacking baseline autoantibodies (Table 2).
These findings are further illustrated in Figures 1 and 2. Baseline and two-year SLUMs test scores were relatively stable and unchanged in all five TBI patients who lacked plasma 5-HT2AR peptide binding autoantibodies (Figure 1A). SLUMs scores fell sharply over the same two- year interval in eleven of thirteen patients who had baseline significant autoantibody (Figure 1B). Five of eleven antibody-positive patients experienced a substantially large two-year decline in test score to result in having met or exceeded the test’s diagnostic threshold for ‘dementia.’
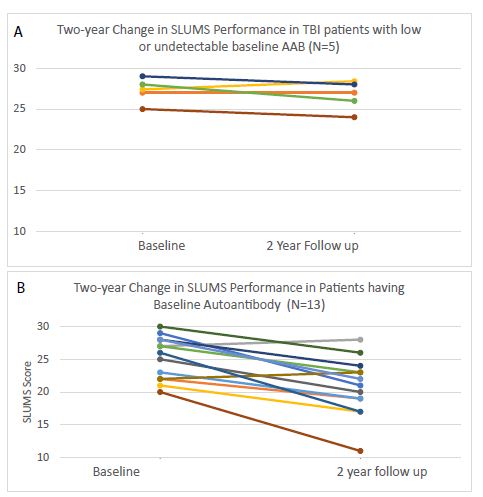
Figure 1: Change in SLUMS test performance in TBI patients in the absence A) or presence B) of baseline plasma serotonin 2A receptor autoantibodies.
Antibody-positive, TBI patients also experienced a significant decline in Digit Symbol Substitution test performance after two years (Figure 2A). The (two-year) DSST test performance was stable or improved somewhat in patients lacking baseline plasma 5-HT2AR peptide binding autoantibodies (Figure 2B).
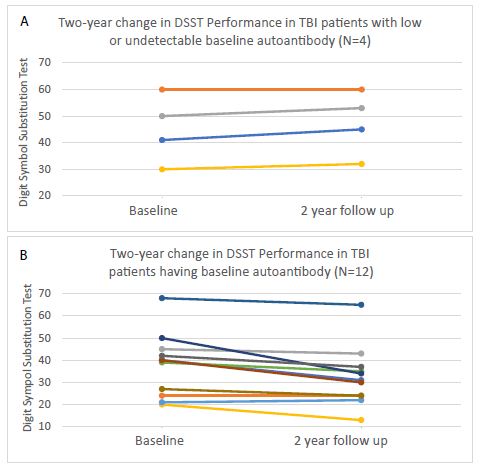
Figure 2: Change in Digit Symbol Substitution Test Performance in TBI patients having A) low, undetectable or B) present baseline plasma serotonin 2A receptor autoantibodies.
Best-fitting Model of Predictors of Cognitive Decline in Adult TBI
In multivariable linear regression analysis that adjusted for age, baseline presence (vs.absence) of 5-HT2A receptor peptide binding autoantibody was a significant predictor ((P=0.028) of the rate of decline in SLUMS test performance (Table 3). Serotonin 2A receptor peptide autoantibody positivity was also a significant predictor (P=0.0116) of the rate of decline in DSST test performance (Table 4), but it did not significantly predict (P=0.139) the rate of decline in Trail-making Test Part B performance (Table 5). Baseline diabetes, hypertension, moderate-severe obesity, depressive symptomatology (Beck depression inventory score) or anti-depressant medication use was not significantly associated with the rate of decline in neurocognitive test performance. There was no significant interaction effect of (age x baseline antibody presence) on the rate of neurocognitive decline.
Titer in Representative Autoantibody-positive or -negative TBI Patients
Protein-A eluate was assayed at two different concentrations in a subset of antibody-positive (n=3) and antibody-negative (n=2) patients. Mean binding to the 5-HT2AR linear synthetic peptide enzyme linked immunosorbent assay (ELISA) was significantly higher (P<0.05) in ~1 µg/mL concentration (1/160th dilution) of IgG obtained from three ‘antibody-positive’ TBI patients compared to an identical concentration of IgG from two ‘antibody-negative’ TBI patients (Figure 3).
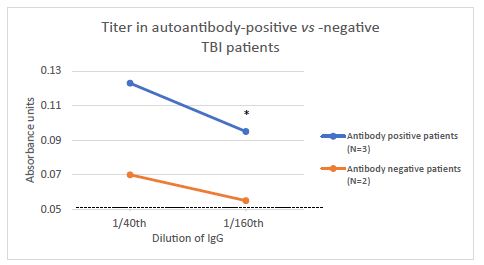
Figure 3: Titer of plasma serotonin 2A receptor autoantibodies in representative patients having baseline low, undetectable or elevated autoantibodies. Protein-A eluates from three representative antibody-positive or two representative antibody-negative patient plasmas were assayed at the indicated dilutions in the serotonin 2A receptor peptide ELISA as described in Materials and Methods. Mean binding in the ELISA (at 1/160th dilution=~1 µg/mL IgG) of the protein-A eluate from patients having baseline autoantibodies was significantly increased (*P < 0.05) compared to patients not having baseline autoantibodies.
Discussion
Chronic inflammation is a hallmark feature in sporadic forms of human neurodegenerative diseases, including Parkinson’s disease and Alzheimer’s dementia. Systemic inflammation was associated with increased plasma serotonin 2A receptor autoantibodies in a number of different disease conditions [5,7,14] including TBI [6].
The present finding that (binding in an enzyme linked immunosorbent assay (ELISA) specific for the 5HT2AR second extracellular loop) identified plasma serotonin 2A receptor autoantibodies as a predictive biomarker of accelerated cognitive decline in a subset of middle-aged and older adult male TBI patients may have future utility in health monitoring post-TBI exposure.
Baseline plasma serotonin-2A receptor autoantibody positivity likely reflects long-lasting effects from IgG autoantibodies, which exerted both potent endothelial toxicity and neurotoxicity in cell culture experiments [5]. Much larger studies in more diverse populations are needed to confirm whether the steep trajectory of decline in neurocognitive performance (observed in older male veterans harboring serotonin 2A receptor agonist antibody) and having multiple co-morbidities may be experienced by other populations. Diabetes, hypertension and obesity may promote peripheral inflammation, which could drive (in part) autoantibody formation. We used published age- and education-adjusted TMT-B test scores from the Veterans Affairs Diabetes Trial, a study in a large non-TBI population of older adult advanced type 2 diabetes (mean age 60 year) [13] as comparison to the current TBI population having similar age and metabolic co-morbidities. Plasma serotonin 2A receptor autoantibody-positive TBI patients experienced approximately 3.4-fold greater decline in age- and education-scale TMT-B performance after only two years compared to the five-year decline in TMT-B performance reported in a non-TBI, obese, diabetic hypertensive population [13]. This suggests that prevalent medical co-morbidities, diabetes, obesity, hypertension, may not contribute much to the substantial decline experienced by TBI patients harboring serotonin 2A receptor autoantibodies.
Although all three neurocognitive tests measure different cognitive domains: TMT-B-executive function, DSST-processing speed, and SLUMS-short-term episodic and working memory, each test is sensitive to decreased cognitive functioning resulting from impaired brain functioning. Perhaps owing to larger variance in TMT-B test performance (compared to other two tests) a larger sample size may be required to have adequate power to detect a statistically significant difference in two-year rate of test performance between serotonin 2A receptor plasma autoantibody-positive vs.antibody-negative patients.
Vascular dementia (normally less common than Alzheimer’s dementia in the general population) may have been increased because of the high prevalence of vascular risk factors in our population [15]. Eight of thirty-five patients had incidental brain imaging studies which revealed evidence of small or large vessel disease. Among them, three patients had brain imaging findings (lacunar infarcts, large volume loss) and/or cardiovascular risk factors suggesting probable vascular- type dementia. All three patients had baseline SLUMs test score in the range of dementia, and baseline plasma was negative for the presence of 5-HT2AR autoantibodies. Aging and Alzheimer’s dementia (AD) are associated with increased blood brain barrier permeability [16,17] and ‘small vessel disease’ may coexist together with AD. Three of four patients in our study who demonstrated MRI ‘white matter hyper intensities’ which is an early marker of small vessel disease predictive of progressive cognitive decline [15] harbored elevated plasma serotonin 2A receptor autoantibodies in the circulation. Serotonin 2A receptor agonist autoantibodies display potent endothelial cell toxicity in vitro [5] suggesting they could have a role in increased microvascular permeability underlying small vessel disease in a subset of stroke/dementia patients [18].
The second extracellular loop of the serotonin 2A receptor is a conserved region [19] located near the receptor orthostatic binding pocket, which is thought to play a role in preventing constitutive GPCR receptor activation [19]. Plasma autoantibodies, which display increased binding in this GPCR regulatory region of the 5HT2AR caused neuroblastoma and endothelial cell death via long-lasting activation of Gq11/IP3R/Ca2+ signaling. Sequence alignment in the trace amine family of conserved GPCRs [19] indicates that the alpha1 adrenergic receptor family of GPCRs share considerable homology to the 5-HT2AR in a second extracellular loop sub region adjacent to a highly conserved cysteine residue. Because alpha 1 adrenergic receptor activation typically is positively coupled to Gq11/IP3R/Ca2+ signaling we explored whether any of the TBI plasma IgG autoantibodies may be comprised of additional (alpha1adreneric) receptor-targeting specificity We used an acute neurite retraction assay [5] in which the phenotypic response was completely inhibitable by antagonists of Gq11/IP3R/Ca2+ pathway signaling molecules or Rho A/Rho kinase to screen the protein-A eluates from thirty of thirty-five TBI patients’ plasma. Specificity for either the 5-HT2AR or alpha 1 adrenergic receptor was determined by the ability of several hundreds’ Nano molar concentration of a highly selective antagonist, prazosin (alpha 1 AR) or M100907 (5-HT2AR) to substantially (70% or more) prevent IgG-evoked neurite retraction. The protein-A eluate from one TBI patient who suffered multiple TBI exposures and experienced progressive cognitive decline appeared to harbor only alpha1 AR-targeting IgG, lacking either 5-HT2AR-like bioactivity (in vitro) or binding to the 5-HT2AR peptide in the ELISA. Protein-A eluate(s) from four other patients (two of whom had also experienced repetitive TBI exposures, i.e. athletic neurotrauma harbored both alpha 1 AR- and 5-HT2AR-targeting IgG, and the titer of 5-HT2AR autoantibodies was significantly increased compared to that in plasma from single TBI patients [6]. Taken together, these data suggest that repetitive TBI and chronic neuroinflammation may promote ‘epitope spreading’ to include one or more closely related antigens belonging to the conserved, trace amine superfamily of GPCRs, which can positively couple to Gq11/IP3R/Ca2+ signaling. An enzyme-linked immunosorbent assay which specifically detects binding to the second extracellular loop sub region in the alpha1 adrenergic receptor homologous to the region in the 5-HT2AR (targeted by nearly all TBI autoantibodies) could provide a direct test for this possibility.
The present study has several limitations including its small size and relatively homogeneous patient population. The findings may only reflect the experience of middle-aged and older men patients who suffered a single or repetitive direct force, mild TBI exposures.
To our knowledge, this is the first report that plasma 5-HT2AR neurotoxic autoantibodies (determined in an epitope-specific ELISA) [7] appear to predict rapid substantial decline in neurocognitive performance in middle-aged and older adult men TBI sufferers. Traditional risk factors which present in midlife, e.g. hypertension [20] are thought to promote accelerated cognitive decline for several decades prior to observable clinical effects. A plasma biomarker that predicts rapid decline in neurocognitive function may enable earlier identification of a high-risk subset of TBI patients for closer health monitoring and avoidance of ongoing TBI exposures. Since G-protein, coupled receptors are highly druggable targets the availability of a predictive plasma biomarker could assist in the future evaluation of candidate drug therapies aimed at slowing neurodegeneration in TBI- sufferers.
Acknowledgements
This work is Supported by a grant from the New Jersey Commission on Brain Injury Research NJCBIRPIL007 to MZ.
References
- Zhang G, Stackman RW Jr (2015) The role of serotonin 5-HT2A receptors in memory and cognition. Front Pharmacol. 6: 225. [crossref]
- Lai MK, Tsang SW, Alder JT, et (2005) Loss of serotonin 5-HT2A receptors in the postmortem temporal cortex correlates with rate of cognitive decline in Alzheimer’s disease. Psychopharmacology. 179(3): 673-677. [crossref]
- Blin J, Baron JC, Dubois B, et (1993) Loss of brain 5-HT2 receptors in Alzheimer’s disease. In vivo assessment with positron emission tomography and [18F] setoperone. Brain. 116 (3): 497-510. [crossref]
- Elliott MS, Ballard CG, Kalaria RN, et al. (2009) Increased binding to 5-HT1A and 5-HT2A receptors is associated with large vessel infarction and relative preservation of cognition. Brain. 132(7): 1858-1865. [crossref]
- Zimering MB (2018) Circulating Neurotoxic 5-HT2A Receptor Agonist Autoantibodies in Adult Type 2 Diabetes with Parkinson’s J Endocrinol Diabetes. 5(2). [crossref]
- Zimering MB, Pulikeyil AT, Myers CE, et al. (2020) Serotonin 2A Receptor Autoantibodies Increase in Adult Traumatic Brain Injury In Association with Neurodegeneration. J Endocrinol Diabetes. 7(1): 1-8. [crossref]
- Zimering MB (2019) Autoantibodies in Type-2 Diabetes having Neurovascular Complications Bind to the Second Extracellular Loop of the 5-Hydroxytryptamine 2A Endocrinol Diabetes Metab J. 3(4): 118. [crossref]
- Tariq SH, Tumosa N, Chibnall JT, et al. (2006) Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder-a pilot Am J Geriatr Psychiatry. 14(11): 900-910. [crossref]
- Wechsler D (2008) WAIS-III Administration and Scoring Manual. San Antonio, TX: Psychological Corporation. [crossref]
- Lezak MD, Howieson DB, Bigler ED, et (2012) Neuropsychological Assessment. 5th ed New York: Oxford University Press.
- Strauss E, Sherman EMS, Spreen O (2006) A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. [crossref]
- Tombaugh TN (2004) Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 19: 203-214. [crossref]
- Zimering MB, Knight J, Ge L, et al. (2016) VADT Predictors of Cognitive Decline in Older Adult Type 2 Diabetes from the Veterans Affairs Diabetes Trial. Front Endocrinol(Lausanne). 7: 123. [crossref]
- Zimering MB (2021) Severe COVID-19 Pneumonia is Associated with Increased Plasma Immunoglobulin G Agonist Autoantibodies Targeting the 5-Hydroxytryptamine 2A Endocrinol Diabetes Metab J. 5(1): 1-9. [crossref]
- Debette S, Seshadri S, Beiser A, et al. (2011) Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 77(5): 461-468. [crossref]
- Farrall AJ, Wardlaw JM (2009) Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging. 30(3): 337-352. [crossref]
- Black S, Gao F, Bilbao J (2009) Understanding white matter disease: imaging- pathological correlations in vascular cognitive impairment. Stroke. 40(3): S48-S52. [crossref]
- Zimering MB (2010) Recurrent macular edema and stroke syndrome in type 1 diabetes mellitus with potent endothelial cell inhibitory autoantibodies. Endocr Pract. 16(5): 842-850. [crossref]
- Michino M, Beuming T, Donthamsetti P, et al. (2015) What can crystal structures of aminergic receptors tell us about designing subtype-selective ligands?. Pharmacol Rev. 67(1): 198-213. [crossref]
- Swan GE, DeCarli C, Miller BL, et (1998) Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 51: 986-993. [crossref]