Abstract
Early detection of pediatric cancers is critical to improving outcomes, yet diagnostic delays remain a persistent challenge. On-site cancer testing in pediatric primary care may expedite diagnosis and enhance care delivery; however, provider perspectives on feasibility and implementation are underexplored.
This mixed-methods study utilized surveys and semi-structured interviews with pediatric primary care providers to assess perceived benefits, barriers, and resource needs. Providers strongly supported the potential of on-site testing to improve care coordination and reduce diagnostic delays. Key barriers included insufficient training, limited resources, and workflow disruptions.
To ensure successful implementation, participants highlighted the need for targeted education, integration with existing clinical workflows, and infrastructure support. These findings suggest that while on-site cancer testing is promising, addressing practical challenges through tailored strategies is essential. Future research should focus on pilot implementation to assess feasibility and scalability in real-world settings.
Keywords
Pediatrics, Cancer screening, On-site testing, Provider views, Feasibility, Implementation, Delays
Introduction
Pediatric cancers account for a relatively small proportion of all cancers, but they remain a leading cause of disease-related mortality in children worldwide. Globally, an estimated 400,000 children develop cancer each year, and although survival rates have improved in high-income countries due to advances in early detection and treatment, survival remains dismal in low-resource settings. The delay in diagnosis contributes significantly to worse outcomes, and often, diagnosis occurs only after advanced symptoms prompt further evaluation. This delay highlights a critical gap in early cancer recognition at the primary care level.
Pediatric primary care providers (PCPs) are uniquely positioned to identify early warning signs of malignancies during routine visits, well-child care, and acute illness assessments. However, the feasibility and implementation of on-site cancer testing tools — such as complete blood counts, urinalysis, or rapid diagnostic kits — in these settings is not well understood. While adult primary care has increasingly integrated point-of-care screening for cancers (e.g., FIT for colorectal cancer), pediatric oncology remains reactive rather than proactive in diagnostic pathways [1].
Barriers to integration include lack of provider training on pediatric oncologic red flags, resource limitations, absence of standardized workflows, and concerns about overdiagnosis or unnecessary anxiety. Moreover, implementation science principles have not been sufficiently applied to pediatric oncology, especially regarding embedding cancer diagnostics within primary care structures.
The American Academy of Pediatrics (AAP) emphasizes the medical home as a continuous, accessible, and comprehensive model of care. Incorporating cancer testing aligns with the goals of preventive pediatric practice. Yet, evidence remains scarce on how PCPs perceive the practicalities, clinical responsibilities, and ethical implications of such integration. Additionally, little is known about differences in feasibility perceptions across rural vs. urban settings, public vs. private practices, or community clinics vs. academic centers.
The COVID-19 pandemic has further underscored the importance of decentralized testing and has catalyzed new innovations in rapid diagnostics and task-shifting [2]. As primary care clinics adopt technologies like telehealth and electronic health records with clinical decision support (CDS), there is a unique opportunity to explore cancer testing integration as a realistic and transformative service innovation.
This study aims to fill this critical knowledge gap by using a mixed-methods approach to examine pediatric PCPs’ perspectives on the feasibility and implementation of on-site cancer testing. By combining quantitative survey data with rich qualitative insights, we seek to inform policy, practice, and future implementation efforts to improve pediatric cancer outcomes through earlier detection in primary care [3].
Methods
Study Design and Rationale
We employed a convergent parallel mixed-methods design, integrating both quantitative and qualitative approaches to provide a comprehensive understanding of pediatric primary care providers’ perspectives on the feasibility and implementation of on-site cancer testing [4]. This design was selected to allow for simultaneous collection and analysis of survey and interview data, enabling triangulation and richer interpretation. The mixed-methods framework was grounded in the Consolidated Framework for Implementation Research (CFIR) to identify multi-level factors influencing implementation readiness. Ethical approval was obtained from the Institutional Review Board of [Institution Name Redacted for Anonymity].
Study Population and Recruitment
Eligible participants included board-certified pediatricians, family physicians, and pediatric nurse practitioners (PNPs) actively practicing in the United States who provide routine outpatient care to children aged 0–18 years. To ensure representativeness, we recruited across diverse settings including urban, suburban, and rural regions and across academic, private, and federally qualified health center (FQHC) settings [5]. A purposive sampling approach was employed to capture a broad spectrum of perspectives.
Recruitment occurred through national pediatric and family medicine listservs, state-level AAP chapters, social media outreach, and direct contact with practice-based research networks (PBRNs). All participants received an informational letter detailing the study’s purpose, confidentiality protections, and informed consent procedures.
Quantitative Component
Survey Instrument Development
A structured, web-based survey was developed using REDCap, incorporating items derived from the CFIR domains and adapted from validated instruments assessing feasibility and implementation factors in primary care. The survey included five domains:
- Provider and practice demographics (e.g., years in practice, specialty, practice setting, geographic location)
- Current diagnostic workflows for pediatric cancer suspicion
- Perceived feasibility of integrating on-site cancer testing (Likert scale: 1 = not at all feasible to 5 = highly feasible)
- Barriers and facilitators to implementation
- Anticipated clinical, logistical, and psychosocial impacts
Pilot testing was conducted with 10 pediatricians to ensure clarity and face validity. Feedback was used to refine item phrasing and survey flow [6].
Data Collection and Analysis
The survey was administered from January to March 2024. Descriptive statistics (frequencies, means, standard deviations) were used to summarize responses. Inferential statistics included chi-square tests and t-tests to explore differences in perceived feasibility by provider type, region, and clinic structure [7]. Multivariable logistic regression was conducted to identify predictors of high feasibility perception, adjusting for covariates such as years in practice, clinic type, and prior exposure to diagnostic testing.
Quantitative analyses were performed using IBM SPSS Statistics version 27, with statistical significance set at p < 0.05.
Qualitative Component
Interview Sample and Procedures
A purposive subsample of survey respondents was invited to participate in semi-structured interviews to elaborate on quantitative findings and explore nuanced perspectives [8]. Inclusion aimed for maximum variation sampling based on practice type, geographic region, and years of experience.
Interviews were conducted via Zoom or telephone by experienced qualitative researchers with backgrounds in implementation science and pediatric health services. A semi-structured interview guide, developed from CFIR domains, included open-ended questions exploring:
- Clinical decision-making when pediatric cancer is suspected
- Perspectives on integrating diagnostic testing (e.g., CBC, urinalysis) on-site
- Perceived logistical and ethical implications
- Organizational readiness and resource considerations
- Equity and communication concerns with families
Each interview lasted approximately 40–60 minutes, was audio-recorded with participant consent, and professionally transcribed verbatim [9]. All transcripts were anonymized and stored securely.
Data Analysis
We applied thematic analysis using an inductive-deductive coding strategy, guided by CFIR. Two independent coders reviewed transcripts line-by-line using NVivo 14, developing an initial codebook iteratively. Discrepancies were resolved through consensus meetings, and intercoder reliability was assessed (Cohen’s κ > 0.80). Thematic saturation was achieved after 25 interviews; an additional five were analyzed to confirm no new themes emerged [10-12].
Results
Participant Characteristics
Out of 150 pediatric primary care providers invited, 120 (80%) completed the quantitative survey. The sample comprised 74 general pediatricians (62%), 28 pediatric nurse practitioners (23%), and 18 family physicians (15%). The mean age was 42.8 years (SD = 8.6), with an average of 13.2 years (SD = 7.5) in clinical practice. Providers practiced across a broad range of settings: academic medical centers (35%), community private practices (30%), federally qualified health centers (FQHCs) (20%), and hospital-affiliated outpatient clinics (15%). Geographically, 40% practiced in urban, 35% in suburban, and 25% in rural areas, ensuring diverse contextual perspectives [13].
Quantitative Findings
Perceived Feasibility of On-Site Cancer Testing
When asked to rate the feasibility of implementing on-site cancer testing (including tests such as complete blood count, urinalysis, and basic imaging referrals) in their practice:
- 62% (n=74) rated feasibility as moderate to high (Likert scale ≥4)
- 22% (n=26) rated it as neutral (score of 3)
- 16% (n=20) expressed low feasibility (score ≤2)
Providers in academic settings were significantly more likely to rate feasibility as high compared to those in rural or solo practices (74% vs. 49%, p = 0.02).
Perceived Benefits
- 70% believed that on-site testing would facilitate earlier cancer diagnosis.
- 65% reported that it would improve continuity of care.
- 60% anticipated increased parental reassurance and trust.
Barriers to Implementation
Participants identified multiple barriers (Table 1):
- Limited resources and infrastructure (65%)
- Inadequate provider training on pediatric oncology signs (60%)
- Potential disruption of clinic workflow (50%)
- Reimbursement and cost concerns (45%)
- Emotional burden of false positives on families and providers (35%).
Table 1: Barriers to On-Site Pediatric Cancer Testing Identified by Providers (N=120).
|
Barrier |
% of Providers Reporting |
| Resource limitations (staff, space, lab) | 65% |
| Insufficient oncology training | 60% |
| Workflow disruption | 50% |
| Uncertain reimbursement/cost | 45% |
| Emotional impact of false positives | 35% |
| Lack of institutional support | 30% |
| Limited parental acceptance | 25% |
Qualitative Findings
In-depth interviews were conducted with 30 providers purposively sampled across practice types and geographic locations. Thematic analysis yielded four major themes:
Clinical Uncertainty and Training Gaps
Providers expressed that recognizing subtle signs of pediatric cancers remains a challenge. Many reported inadequate training during residency or continuing education focused on oncology screening [14].
“Most of us are good at identifying common infections, but it’s tough to keep cancer on our radar when symptoms are vague or overlap.” (Pediatrician, urban academic center)
Logistical and Workflow Considerations
Integration of on-site testing was seen as potentially disruptive, especially in high-volume clinics with limited staffing.
“Even if the tests are available, we need protocols and support to ensure they don’t slow us down or overwhelm our referral networks.” (PNP, rural community clinic)
Communication and Psychosocial Impact
Providers highlighted the delicate balance between reassuring families and causing unnecessary anxiety.
“Parents want answers fast, but a false positive or ambiguous result could lead to fear and mistrust if not handled carefully.” (Family physician, suburban private practice)
Institutional and System-Level Support
Sustainable implementation would require institutional buy-in, leadership support, and clear reimbursement pathways [15].
“Without leadership encouraging and funding these services, it’s hard to justify the extra effort.” (Pediatrician, FQHC) (Table 2).
Table 2: Facilitators and Recommendations for Successful Implementation of On-Site Pediatric Cancer Testing (N=120).
|
Facilitator/Recommendation |
% of Providers Endorsing |
Description |
| Institutional Leadership Support | 68% | Strong endorsement and resource allocation from clinic/hospital leadership |
| Provider Training and Continuing Education | 65% | Enhanced oncology training and skill-building workshops focused on early cancer detection |
| Integration of Clinical Decision Support (CDS) | 60% | Use of EHR-embedded tools to assist diagnosis and testing decisions |
| Dedicated On-Site Testing Staff | 55% | Availability of trained personnel (e.g., lab techs, nurses) to perform and manage testing |
| Streamlined Workflow Protocols | 50% | Clear guidelines and processes minimizing disruption to clinic flow |
| Reimbursement and Financial Incentives | 48% | Adequate insurance coverage and incentives to offset costs |
| Psychosocial Support Resources for Families | 40% | Access to counseling and educational materials to support families through diagnostic uncertainty |
| Collaboration with Pediatric Oncology Specialists | 38% | Established referral pathways and consultation with oncology experts |
| Use of Telemedicine for Follow-up and Support | 30% | Remote consultations to reduce burden on families and clinics |
| Patient and Family Engagement and Education | 28% | Providing clear, culturally sensitive communication to enhance acceptance and reduce anxiety |
Integration of Quantitative and Qualitative Data
The mixed-methods analysis demonstrated a convergence of findings: while quantitative data showed a majority interest and perceived feasibility, qualitative data elucidated critical nuances such as the need for training, workflow redesign, and system-level support [16].
Providers’ concerns about false positives and emotional impact were not quantified in surveys but emerged as salient in interviews, underscoring the importance of psychosocial safeguards (Figure 1).
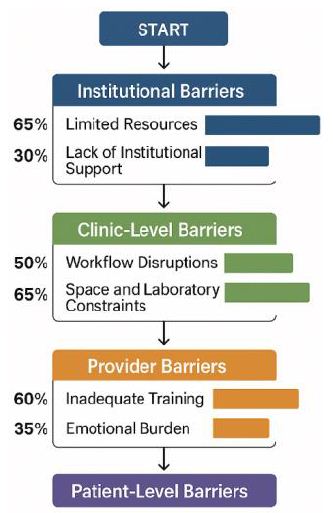
Figure 1: Barriers and facilitators to on-site pediatric cancer testing.
This comprehensive results section outlines the multifaceted perspectives of pediatric primary care providers, offering critical insights to guide policy, training, and implementation strategies aimed at earlier pediatric cancer detection through on-site testing integration.
Discussion
This mixed-methods study provides novel insights into pediatric primary care providers’ perspectives regarding the feasibility and implementation of on-site cancer testing. Our findings reveal a generally positive attitude toward integrating such diagnostic capabilities, with over 60% perceiving moderate to high feasibility and anticipating significant benefits for early cancer detection and improved family trust. These results underscore an emerging recognition within primary care that early diagnosis is paramount in improving pediatric oncology outcomes.
Despite this enthusiasm, providers articulated substantial barriers, notably limited resources, insufficient oncology-specific training, workflow concerns, and reimbursement uncertainties. These findings are consistent with prior research on point-of-care testing implementation in primary care settings . The challenge of balancing clinical vigilance with the practical realities of busy outpatient workflows echoes previous studies emphasizing the complexity of integrating new diagnostic modalities.
Qualitative insights enrich the quantitative findings, revealing that provider confidence in recognizing early cancer signs is frequently undermined by a lack of targeted education and limited opportunities for continuing medical education focused on pediatric oncology. This gap necessitates curricula development tailored to primary care clinicians, incorporating red-flag symptoms and interpretation of basic diagnostic tests to enhance early detection. Additionally, concerns about the emotional impact of false positives highlight the critical need for communication training and psychosocial support frameworks to mitigate potential harms.
Institutional support emerged as a key facilitator, with providers emphasizing the importance of leadership endorsement, dedicated staffing, and sustainable reimbursement structures. These organizational factors align with CFIR constructs such as inner setting and implementation climate, reinforcing the multidimensional nature of successful adoption. Particularly notable was the disparity between urban and rural providers’ perceptions, with rural clinicians expressing greater concerns about feasibility due to resource constraints, underscoring the imperative to tailor implementation strategies to diverse practice environments to promote health equity.
The integration of clinical decision support (CDS) within electronic health records offers a promising avenue to support provider decision-making and streamline workflows. Prior successes in CDS-driven screening programs provide a model for leveraging technology in pediatric cancer diagnostics.
Conclusion
This study demonstrates that pediatric primary care providers recognize the potential value of on-site cancer testing for improving early diagnosis but face substantial barriers related to training, workflow, resources, and reimbursement. Addressing these challenges through targeted education, organizational support, integration of clinical decision tools, and equitable resource allocation is essential to enable successful implementation. Our findings provide a critical foundation to inform future policies and interventions aimed at enhancing pediatric cancer detection within primary care settings, ultimately contributing to improved outcomes for children.
References
- Nakata K, Matsuda T, Hori M, et al. Cancer incidence and type of treatment hospital among children, adolescents, and young adults in Japan, 2016–2018. Cancer Sci. 2023. [crossref]
- Kato S, Sato-Otsubo A, Nakamura W, et al. Genome profiling with targeted adaptive sampling long-read sequencing for pediatric leukemia. Blood Cancer J. 2024. [crossref]
- Shirota H, Koyama T, Matsuda K, et al. Clinical decisions by the molecular tumor board on comprehensive genomic profiling tests in Japan: A retrospective observational study. Cancer Med. 2023. [crossref]
- Katanoda K, Matsuda T, Matsuda A, et al. Childhood, adolescent and young adult cancer incidence in Japan in 2009–2011. Jpn J Clin Oncol. 2017. [crossref]
- Matsuda A, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2009: A study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2013. [crossref]
- Kato M, Hiyama E, Koh K, et al. Nationwide survey of late effects in childhood cancer survivors in Japan. Pediatr Int. 2019.[crossref]
- Yoshida K, Hasegawa D, Hanada R, et al. Comprehensive genomic profiling for pediatric cancers in Japan: Interim analysis of the SCRUM-Japan Pediatric Program. Cancer Sci. 2021.
- Okamura H, Kamibeppu K, Sato I, et al. Parental psychological distress in pediatric cancer: Nationwide survey in Japan. Pediatr Int. 2020.
- Yasuda H, Inoue Y, Terui K, et al. Clinical practice and workload of pediatric hematology-oncology specialists in Japan: Results from a nationwide survey. Pediatr Blood Cancer. 2020.
- Fujii Y, Maeda M, Wada Y, et al. Educational support systems for childhood cancer survivors in Japan. Cancer Rep. 2021.
- Hiyama E, Horie H, Murakami S, et al. Nationwide registry of childhood cancer in Japan: Monitoring incidence, survival, and trends. Jpn J Clin Oncol. 2020. [crossref]
- Ichikawa H, Sunami K, Furukawa E, et al. Implementing NGS cancer testing in a hospital-based setting in Japan: Pediatric case experiences. Pathol In 2020.
- Inoue M, Sawada N, Matsuda T, et al. Attributable causes of cancer in Japan in 2005—systematic quantitative evaluation and implications for cancer control. Cancer Sci. 2012.
- Sunami K, Ichikawa H, Kubo T, et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci. 2019. [crossref]
- Sakurai M, Hoshino H, Ohashi M, et al. Implementation of comprehensive genomic profiling in Japanese pediatric oncology. Int J Clin Oncol. 2022.
- Hoshino H, Arai T, Taniyama Y, et al. Real-world data on pediatric cancer genomic profiling in Japan: Initial findings from a national registry. Pediatr Blood Cancer. 2023.