Abstract
Epilepsy is one of the most common chronic neurological disease that characterized by recurrent, spontaneous brain seizures. Anti-epileptic drug is used clinically to control the epilepsy or reduce the frequency of the attacks. Liver is the primary and main organ for drug metabolism and elimination of several drugs such as anti-epileptic drugs (AEDs). Thus, drug-induced toxicity may occur. Since liver enzymes can serve as biological markers of hepatocellular injury, this study was aimed to evaluate the effect of anti-epileptic drugs used in patients treated at the Department of Neurology in Benghazi Children Hospital, on activities of liver enzymes; aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP). Out of 58 patients selected randomly in this study 38% of them were female with age ranged from four months to five years old. Male patients were more susceptible to the adverse effects than the female patients. Mode of therapy and age of the patient did not show any effect on the levels of enzyme changes. Sodium valproate was the frequent drug used and level of ALP of the majority of patient was elevated above the normal level. Routine screening of hepatic enzymes level during the chronic use of anti-epileptic drugs is recommended and the need for obtaining baseline liver function tests is also essential before starting anti-epileptic therapy in Libyan children.
Keywords
Anti-epileptic drug, Liver enzyme, Adverse effect, Child, Libya
Introduction
Epilepsy is one of the oldest neurological conditions known to mankind. It is characterized by recurrent, spontaneous brain seizures. Epilepsy affecting about 50 million individuals worldwide and 90% of them are from developing countries [1]. About, 70 – 80% of the patients who develop epilepsy may expect to have their seizures controlled with optimal anti-epileptic therapy [2]. All anti-convulsant medications are associated with adverse effects which may have significantly impact on the quality of life and contributing to non-compliance and, in rare circumstances be, potentially life-threatening [3]. Some anti-convulsants, particularly phenytoin and carbamazepine induce and increase the production of hepatic enzymes. This can result in clinically significant drug interactions by increasing the metabolism of some co-administered drugs. Other enzyme inducing anti-epileptics include phenobarbitone sodium and primidone. Topiramate and oxcarbazepine are inducers at high dose but at lower doses have some inhibiting properties. Sodium valproate is an inhibitor of specific isoenzymes and typically increases the concentrations of other anticonvulsants, particularly lamotrigine and the active metabolite of carbamazepine. Doses of lamotrigine should be halved while taking sodium valproate [4]. Since liver is the primary organ for drug metabolism and elimination for many antiepileptic drugs (AEDs) thus, drug-induced toxicity may occur. Hepatotoxic reactions, ranged from mild and transient elevations of hepatic enzymes to fatal hepatic failure. Chronic therapy with AED causes abnormalities in calcium metabolism, including hypocalcemia, hypophosphatemia, elevated levels of serum alkaline phosphatase and serum parathyroid hormone, reduced serum levels of biologically active vitamin D metabolites, radiologic evidence of rickets, and histologic evidence of osteomalacia [5].
It has been found that, carbamazepine, phenytoin and sodium valproate are associated with mild elevations of liver enzymes, which may occur in up to 50% of patients. Although this elevation is usually transitory or dose-related and do not appear to be associated with hepatocellular injury [6], the liver enzymes can serve as biological markers of hepatocellular injury [7]. Since the adverse metabolic effects of AED treatments have only recently received attention, the objectives of the present study are to investigate the pattern and the clinical effects of these drugs on the liver enzyme activities of young children admitted to Department of Neurology, Benghazi Children Hospital, second major city in Libya.
Materials and Methods
A descriptive serious case study was conducted on 58 medical record selected from Pediatric Neurology Unit at the Benghazi Children Hospital, Benghazi, Libya during 2016. The study included epileptic patients receiving anti-epileptic drugs only. Exclusion subjects: Epileptic patients who had concomitant liver diseases, using other drugs causing elevation of liver enzymes (e.g. antibiotics, anti-rheumatic drugs, statins and on steroidal anti-inflammatory drugs) were excluded from this study.
Liver enzyme assessed
Laboratory investigations were investigated to assess the liver enzyme activities which include: alanine aminotransferase (ALT, reference range of 5.0 – 41.0 U/L), aspartate aminotransferase (AST, reference range of 5.0 – 41.0 U/L) and alkaline phosphatase (ALP, reference range of 40.0 – 129.0 U/L). Data was presented as a descriptive analysis (mean and standard deviation).
Results and Discussion
From a total number of pediatric patients admitted to the Department of neurology in Benghazi Children Hospital, only 58 patients from randomly selected files were included. Out of total, 36 patients were males and the rest of them (n = 22) were female patients; accordingly, as demonstrated in (Figure 1), the majority of children admitted were male and formed 62% of total. The age of the included patients were ranged from four months up to 17 years old. They were divided into three sub-groups according to their ages as following: group 1: up to five years old (n = 36, 62.0%), group 2: included children from 6 to 10 years old (n = 9, 15.5%) and group 3: from 11 to 17 years old (n = 13, 23.5%) as shown in (Figure 2).
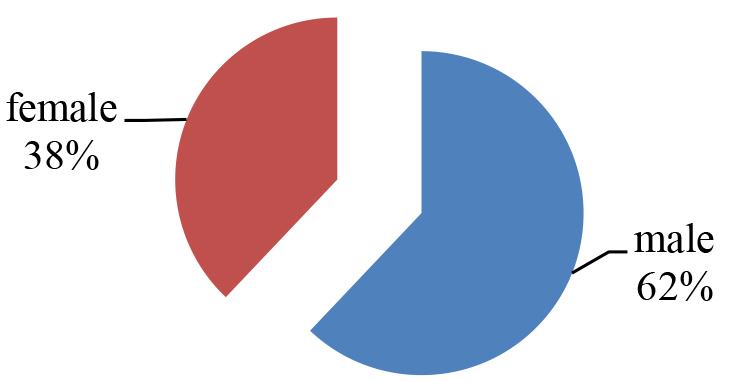
Figure 1. The percentage of male and female.
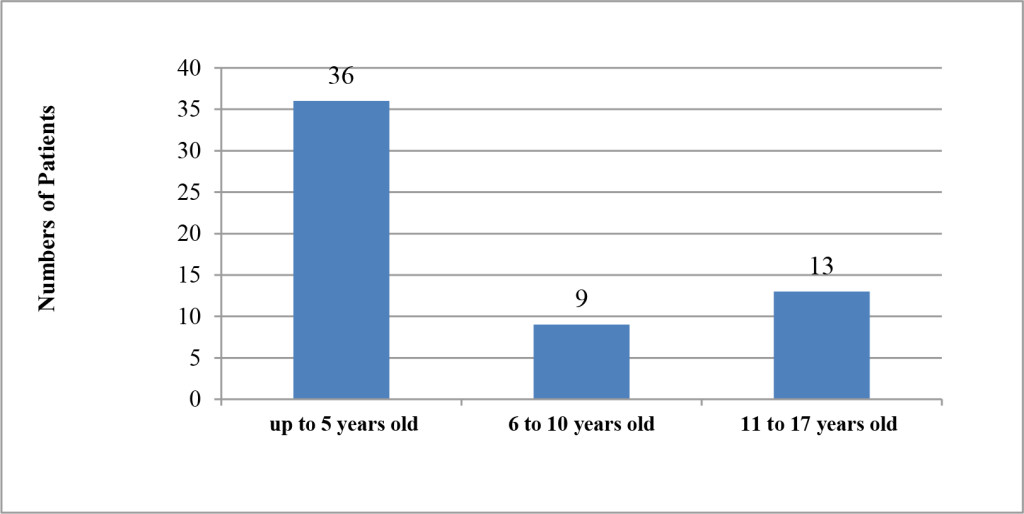
Figure 2. Distribution of patients with regard to Age.
According to the records, several AEDs with different mechanisms of action were used for treatment of the admitted patients (8). The most frequently drug used, as shown in table 1, was sodium valproate which administered by 42 of the patients, while only one patient was receiving clonazepam. The number of patients which received phenytoin, carbamazepine, diazepam and levetiracetam was almost equal for each drug. While, phenobarbital, lamotrigine and clonazepam were the lowest frequency in use (Table 1).
Table 1. Frequency of antiepileptic drugs prescribed for the patients.
|
Anti-epileptic drug |
Frequency of use No. (%) |
|
Sodium Valproate |
42 (72.4%) |
|
Phenytoin |
18 (31.0%) |
|
Carbamazepine |
17 (29.3%) |
|
Diazepam |
16 (27.5%) |
|
Levetiracetam |
15 (25.8 %) |
|
Phenobarbital |
6 (10.3%) |
|
Lamotrigine |
3 (5.1)% |
|
Clonazepam |
1 (1.7%) |
Most of the patients included in this study were belongs the group 1 and represent 62% of all the patients, while the percentage of patients belongs to group 2 and group 3 were 15.5% and 22.4%, respectively. The mean age for each group was 2.50 ± 1.65, 7.89 ± 1.54 and 12.54 ± 1.90 in that order. In order to assess whether AEDs have changed the level of liver enzyme activities of the admitted patients; the results of investigation recorded in patient’s files were tabulated to evaluate and abnormal levels of AST, ALT and ALP. According to (Figure 3), out of the total number of patients respected; only 9 (16%) children of them had no changes in their enzymes level while, the level of same enzymes of 49 (84%) patients were increased to be more than the normal range (Table 2).
Table 2. frequency with percentage of Libyan patients for abnormal liver enzyme activities.
|
Enzyme |
AST |
ALT |
ALP |
|
No. of patients with changed enzyme levels |
17 |
3 |
47 |
|
Percentages |
29.30% |
5.20% |
81.00% |
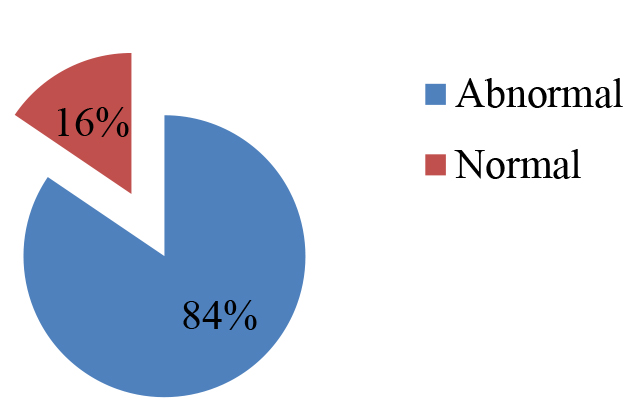
Figure 3. Percentage of patients with normal and abnormal enzyme level
Our finding was in line with the study performed on 2010 which reported that liver enzymes were elevated as a result of AEDs adverse reaction [2]. It was reported that hepatotoxic reactions ranged from mild and transient elevations of hepatic enzymes to fatal hepatic failure [6] and the level of liver enzymes can serve as biological markers of hepatocellular injury as confirmed by Ahmed and Siddiqi [7]. Accordingly, present results indicate that AEDs may leads to hepatocellular injury which appeared as elevation in level of liver enzymes. It has been found that, levels of both AST and ALP enzyme in all the groups have been increased above the normal range, whereas, ALT level has elevated in only group 1 (Table 3). The present results also indicated that ALP level of the majority of patients (81%) was increased to be more than normal range meanwhile, AST was above the range in 29.3 % of patients. Only three patients (5.20%) had high level of AST.
Table 3. Abnormal level of liver enzyme in each group (Mean ±STD).
|
Enzyme Levels in U/L |
AST |
ALT |
ALP |
|
group 1 |
52.77 ± 15.26 |
52.73 ± 12.13 |
218.00 ± 59.14 |
|
group 2 |
48 |
– |
192.25 ± 64.51 |
|
group 3 |
54.33 ± 9.16 |
– |
190.91 ± 43.50 |
It has been reported that, sodium valproate is more hepatotoxic than other AEDs, phenytoin and Carbamazepine [8], and according to our findings shown in table 2, subsequently, it could be proposed that the elevation in lever enzyme was a results of sodium valproate, phenytoin and Carbamazepine administration. The number of patients (female /male) had abnormal level enzyme in each group were illustrated in (Table 4). It has been found that percentage of boys with abnormal level of liver enzyme formed a higher percentage of the total patients included see table 4. The number of boys was approximately double of girl’s in both groups 1 and 3 while it was 3 fold in group 2 as seen in table. Since there was a marked difference between male and female patients in our result, it may indicate that male is more susceptible to liver injury due to AEDs.
Table 4. Male and female who had abnormal level of liver enzyme in each group.
|
Groups/gender |
Female |
Male |
|
Group 1 |
27.70% |
55.50% |
|
Group 2 |
22.20% |
66.60% |
|
Group 3 |
30.80% |
61.50% |
Regarding mode of therapy; the epileptic patients were divided into 2 subgroups; patients who were treated by receiving a single AED (monotherapy) and patients who received multiple AEDs (polytherapy). The effect of mode of therapy, in term of single and multiple drug use, in the level of liver enzyme been evaluated. Thus, as demonstrated in (Figure 4); 17 patients (29.3%) were on single therapy (7 of them were on sodium valproate and 5 were on carbamazepine while only 3 and 2 of were on diazepam and phenytoin respectively). Level of respected enzyme of 14 of them has been elevated to become above the normal range. The rest of the patients were on multiple therapy (receive two or more AEDs) and were represented 70.7 % of the patients. Enzymes level of 36 of these patients was affected.
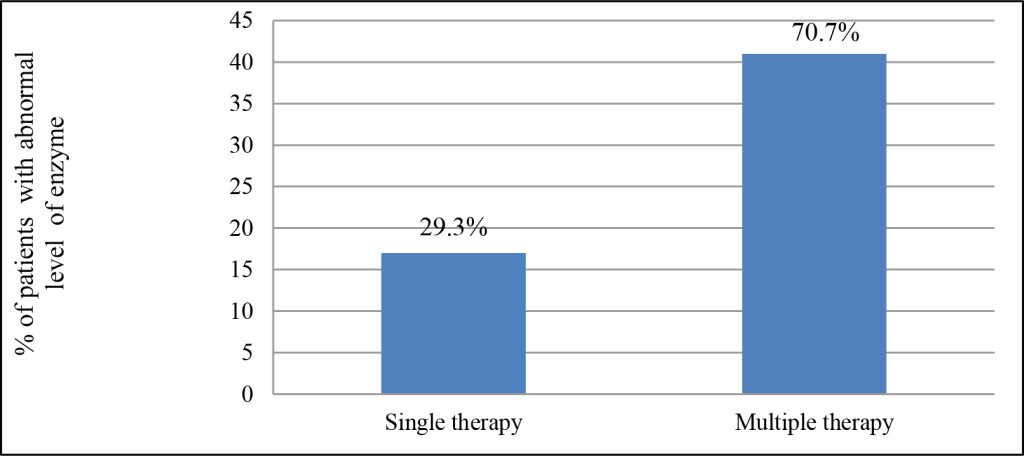
Figure 4. Frequency (percentage) of the patients received different mode of therapy.
Regarding single therapy, the level of liver enzyme of 14 patients has increased and mean level of AST, ALT and ALP were 47.15, 42 and 231.88, respectively. Similarly, the levels of respected enzymes of patients (n = 36) on multiple therapies were also increased to become 55.96, 58.1 and 198.33 in the same order as shown in (Figure 5).
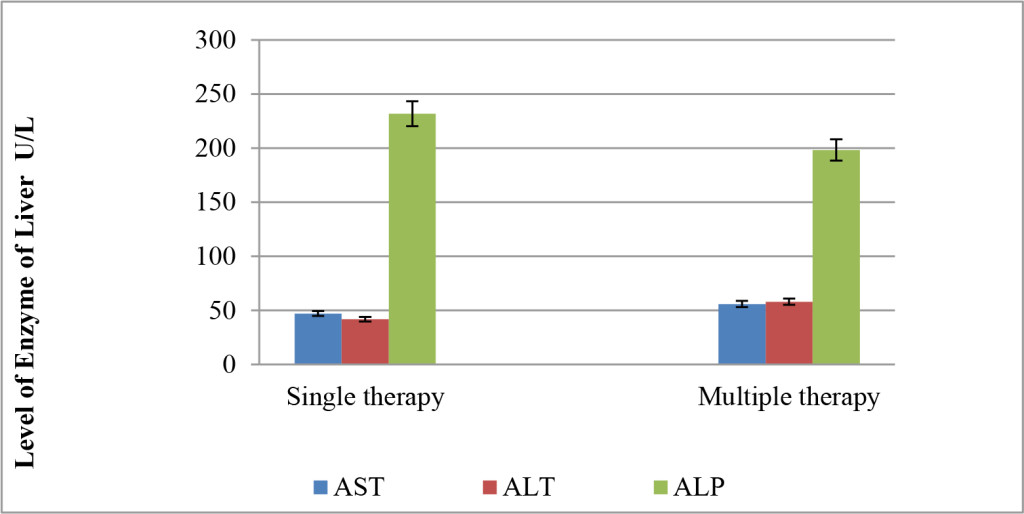
Figure 5. Effect of mode of therapy in the level of liver enzymes.
According to the present finding, it seems more likely that there is no marked change between AST and ALT levels in both mode of therapy. Although the level of ALP of patients on single therapy was higher than that who received more than one AED, however, with no marked difference. It has found that receiving more than one AEDs may decrease inducing enzyme liver but remain higher than normal range. Therefore it could said that mode of therapy did not affect the level of respected enzymes. However, there is no clear answer on whether to combine AEDs but different strategies are needed in different patients to control epilepsy and adverse effects [9, 10].
Conclusion and Recommendation
In Libya, male patients were more admitted to neurology unit and age of the patients was ranged from four months to five years old. Multiple therapies have been used to control epilepsy more than single treatment. Sodium valproate was the most frequent drug used followed by phenytoin and carbamazepine. Mode of therapy and age did not show any effect in level of enzyme during the treatment with AEDs. On contrast, the difference in gender has a marked effect in the level of respected enzyme as male was more susceptible to liver injury during AEDs treatment. Elevation in the levels of liver enzyme (AST and ALP) to 2-3 fold during AEDs was confirmed and precautions should be taken when using anti-epileptic drugs in Libyan epileptic patients. Routine screening of hepatic enzymes level during chronic use of anti-epileptic drugs is recommended and the need for obtaining baseline liver function tests is essential before starting antiepileptic therapy.
References
- Kumar H, Chandi M, Kandar C, Das S K, Lakshmkanta Ghosh L, et al. (2012) Epilepsy and its management: A review. J Pharma Sci Tech 1: 20–26.
- Naithani M, Chopra S, Lsomani B, Ksingh R (2010) Studies on adverse metabolic effects of antiepileptics and their correlation with blood components. Curr Neurobiol 1: 117–120.
- Tatum WO (2010) Antiepileptic drugs: adverse effects and drug interactions. Continuum (Minneap Minn) 16: 136–158. [crossref]
- Stein MA, Kanner AM (2009) Management of newly diagnosed epilepsy: a practical guide to monotherapy. Drugs 69: 199–222. [crossref]
- Farhat G, Yamout B, Mikati MA, Demirjian S, Sawaya R, et al. (2002) Effect of antiepileptic drugs on bone density in ambulatory patients. Neurology 58: 1348–1353. [crossref]
- Arroyo S, de la Morena A (2001) Life-threatening adverse events of antiepileptic drugs. Epilepsy Res 47: 155–174. [crossref]
- Ahmed SN, Siddiqi ZA (2006) Antiepileptic drugs and liver disease. Seizure 15: 156–164. [crossref]
- Hussein RS, Soliman RH, Ali AM, Tawfeik MH, Abdelhaleem ME (2013) Effect of anti-epileptic drugs on liver enzymes. Beni-suef Univ J Basic App Sci 2: 14–19.
- Sherif FM (2015) Pharmacological profile of the GABA-transaminase inhibitor vigabatrin. World Journal Pharmacy Pharmaceutical Sciences 4: 139–148.
- Stephen LJ, Brodie MJ (2012) Antiepileptic drug monotherapy versus polytherapy: pursuing seizure freedom and tolerability in adults. Curr Opin Neurol 25: 164–172. [crossref]