Abstract
A cassiterite sample from Land’s End granite, SW England, contains cassiterite crystals rich in orthorhombic phases. A precise classification is not possible by mixing remnants of different polytypes. Obviously, the main phase is the polymorph type Pbcn (PbO2-type), which forms around 30 GPa. The possibility of the formation of polytypes of cassiterite at such high pressures is indicated by the proof of the presence of diamond. Some diamonds and graphite are rich in 13C, 28% and 11.45% respectively. Such a high concentration of 13C in diamond is only possible by effective isotope fractionation in a supercritical fluid. Significant higher 13C values, up to 95%, were found by the author.
Keywords
Land’s End Granite, Raman spectroscopy, Orthorhombic cassiterite, 13C-rich diamond and graphite, Supercritical fluid
Introduction
The naturally occurring form of cassiterite is usually tetragonal and crystallizes in the rutile-type structure. However, Thomas (2023a) has shown that cassiterite can also crystallize as an orthorhombic polytype and is widespread in the Erzgebirge and Schlaggenwald (Horni Slavkov, Czech Republic). Last time, a couple of experimental works on the different polytypes of cassiterites were performed [1-5]. From these experiments, it follows that the orthorhombic cassiterite types formed under high pressure and high temperature. Because the orthorhombic cassiterite of the Variscan tin deposits in the Erzgebirge region is part of the tetragonal cassiterite formed in the upper crustal region, it is, therefore, formally a low- pressure formation. The appearance of orthorhombic cassiterite here needs, thus, a plausible explanation [6,7]. Essential indicators for the occurrence of the high-pressure cassiterite polytypes are the paragenetic high-pressure minerals diamond, lonsdaleite, and others, which came from the mantle region via supercritical fluid, as well as the Lorenzian distribution of some trace and main elements [8]. Proofs for such new transport mechanisms are in Thomas et al. (2023a) [1]. In this contribution, we will show that orthorhombic cassiterite and diamond are not only related to the vast Variscan Erzgebirge region but also to the Cornubian Batholite and other places. Furthermore, we will give a further example of the diamond of the Land’s End granite batholite, England, which is exceptionally rich in 13C. Such strong isotope fractionation of carbon needs an explanation.
Sample Material and Raman Spectroscopy: Methodology
Sample Material
A concise description of the Land’s End Granite, their geology, mineralogy, and geochemistry was given by Drivenes (2022) [9]. We use an uncovered microprobe sample von Kristian Drivenes for the Raman spectroscopic studies. Before our studies, the owner removed the carbon coating from the sample with diamond paste. The main minerals are cassiterite, tourmaline (dravite, uvite, and olenite), quartz, rutile, and apatite (all determined with Raman spectroscopy). According to Drivenes (2022) [9], tourmalines contain Sn in relatively high concentrations, up to 2.53%. The owner of the sample has significant problems with the new findings.
Microscopy and Raman Spectroscopy
We performed all microscopic and Raman spectroscopic studies with a petrographic polarization microscope (BX 43) with a rotating stage coupled with the EnSpectr Raman spectrometer R532 (Enhanced Spectrometry, Inc., Mountain View, CA, USA) in reflection and transmission. The Raman spectra were recorded in the spectral range of 0–4000 cm-1 using an up-to-50 mW single-mode 532 nm laser, an entrance aperture of 20 µm, a holographic grating of 1800 g/mm, and spectral resolution ranging of 4 cm-1. Generally, we used an objective lens with a magnification of 100x: the Olympus long-distance LMPLFLN100x objective (Olympus, Tokyo, Japan). The laser power on the sample is adjustable down to 0.02 mW. The Raman band positions were calibrated before and after each series of measurements using the Si band of a semiconductor-grade silicon single-crystal (for the studies on cassiterite) and a water-clear diamond crystal (Mining Academy Freiberg: 2453/37 from Brazil) for the Raman studies on diamond. The run-to-run repeatability of the line position (based on 20 measurements each) is ±0.3 cm−1 for Si (520.4 ± 0.3 cm-1) and 0.4 cm-1 for diamond (1332.7 cm-1 ± 0.4 cm-1 over the range of 0–2000 cm-1). The FWHM = 4.26 ± 0.42 cm-1. FWHM is the Full-Width at Half Maximum. We also routinely determined the zero point of the Raman spectrometer for the first-order band position of the 13C-rich diamond.
Results
Orthorhombic Cassiterite
Usually, cassiterite in the classic Variscan tin deposits grows in the tetragonal rutile form (Figure 1). So, the occurrence and the proof of orthorhombic cassiterite in the upper crust raises a lot of questions regarding the formation processes. Explanations are in Thomas 2023a [1,6-8]. A Raman spectrum of typical tetragonal cassiterite from the Tannenberg mine near Mühlleiten/ Vogtland, Germany is shown in Figure 1.
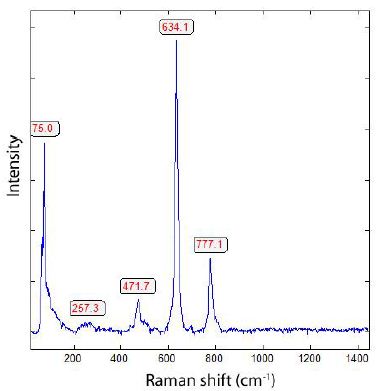
Figure 1: Typical Raman spectrum of a tetragonal cassiterite crystal (Tannenberg mine near Mühlleiten/Vogtland, Germany). A high tourmaline content characterizes this deposit.
Five Raman lines are characteristic of the Raman spectrum of the tetragonal cassiterite (Figure 1): 777.1 cm-1 (medium), 634.1 cm-1 (very strong), 471.7 cm-1(weak), 257.3 cm-1 (very weak), and 75 cm-1 (strong). The main line at 634.1 cm-1 has a FWHM of 12.6 cm-1. According to Chukanov and Vigasina (2020) [10], using a 633 nm He- Ne laser, four lines are typical for tetragonal cassiterite: 842, 776, 635s, and 475 cm-1. Bands at lower Raman shift values (between 100 and 200 cm-1) are mostly not given (for example, in the RRUFF database [11]).
Figure 2 shows a typical Raman spectrum of the orthorhombic cassiterite from Land’s End Granite, characterized by very strong bands in the low-frequency range.
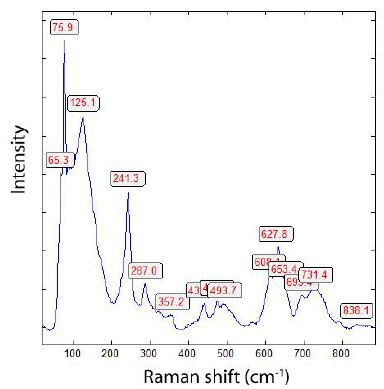
Figure 2: Raman spectrum of orthorhombic cassiterite from Land’s End’s Granite. The strong Raman bands at 75.9 and 125.1 are typical for the polymorph cassiterite Pbcn type (PbO2-type).
Typical for this Raman spectrum is the strong band at about 125.1 cm-1 and the medium bands at 627.8 cm-1 and 241.3 cm-1, characteristical for the orthorhombic Pbcn polymorph of cassiterite [5]. Table 1 shows the mean and the standard deviation for measured Raman bands of the Pbcn cassiterite type.
Table 1: Results of the Raman measurements on different cassiterite crystals (orthorhombic Pbcn type) from Land’s End, Cornwall, SW England.
|
Band (cm-1) |
Intensity (rel.) | Raman mode |
n |
|
76.6 ± 2.5 |
vs | Ag | 22 |
| 119.5 ± 2.3 | vs | B2g |
13 |
|
236.9 ± 5.3 |
s | Ag | 25 |
| 439.0 ± 2.3 | s – m | B2g |
15 |
|
608.7 ± 3.5* |
m – w | B2g | 26 |
| 631.1 ± 1.8 | vs – m | B1g, B2g |
22 |
|
835.9 ± 2.9** |
w -vw | B2g |
19 |
| vs – very strong, s – strong, m – medium, w – weak, vw – very weak; n – measured crystals | |||
|
Some Raman lines are not stable under the 532 nm laser (50 mW on the sample). The best data are obtainable with a low laser power of 4 mW on the sample at a long measuring time of 2000 or 4000 s. *This Raman band is a shoulder of the 631.1 band and can be a remnant of the Pnma-I (cotunnite) cassiterite type. ** This Raman band is not present in the computed Raman modes (Balakrishnan et al., 2022) [5]. According to Hellwig et al. (2003) [2], that band is a high-pressure remnant of the Pnnm CaCl2 type of cassiterite (14.8 GPa). |
|||
The data in Table 1 are representative of orthorhombic cassiterite of the Pbcn polytype (PbO2-type). After Shieh et al. (2006) [3], the transition of polymorphs is sluggish. Therefore, we find that besides the Pbcn-type, remnants of the cotunnite and CaCl2-type. Generally, the tetragonal cassiterites (P42/mnm) containing orthorhombic cassiterite phases also include the orthorhombic cassiterite polytypes: Pnnm (CaCl2), Pbcn (PbO2), Pbca (ZrO2), and Pnma-I. The Pbcn and Pbca polytypes are metastable phases converted into more stable polymorphs under certain experimental conditions [5]. Nevertheless, we find these phases efficiently with the help of Raman spectroscopy. According to Girao (2018) [4], the essential phase transformations of tetragonal cassiterite happen at 14.8 GPa to the CaCl2-type (Pnnm) and at 30 GPa to the PbO2-type (Pbcn).
Table 2: 13C-rich diamonds and 13C-rich graphite calculated according to Enkovich et al., 2016 [12] and Gutierrez et al., 2014 [14], from Raman measurements (means).
Diamond:
|
Origin |
Raman band (cm-1) | 13C in diamond (%) | n |
References |
| Land’s End |
1318.8 |
28.0 |
3 |
This work |
| Land’s End |
1328.0 ± 2.8 |
12.5 |
10 |
This work |
| Ehrenfriedersdorf |
1286.7 ± 6.5 |
95.1 |
10 |
Thomas (2025) [8] |
| Bornholm |
1311.1 ± 4.2 |
52.1 |
62 |
Thomas (2024c) [15] |
| Ehrenfriedersdorf, Beryl |
1318.4 ± 6.1 1328.6 ± 5.6 |
36.0
10.9 |
19 14 |
Thomas et al. (2023b) [16] |
| Zinnwald |
1313.9 ± 6.1 |
46.1 |
18 |
Thomas (2025) [8] |
| Reinbolt Hills |
1312.1 ± 2.5 |
50.0 |
16 |
Thomas (2023c) [17] |
Graphite:
|
Origin |
Raman band (cm-1) | 13C in graphite (%) | n |
References |
| Land’s End |
1573.9 |
11.45 |
3 |
This work |
| Land’s End |
– |
– |
– |
This work |
| Bornholm |
1562. 6 ± 10.82 |
29.70 |
10 |
Thomas (2024c) [14] |
| Ehrenfriedersdorf |
1518.1 ± 0.8 |
99.90 |
10 |
Thomas (2025) [8] |
| EhrenfriedersdorfBeryl |
1569.8 ± 5.2 |
18.1 |
13 |
Thomas et al. (2023b) [18] |
| Zinnwald |
1521.5 ± 8.5 |
96.0 |
10 |
Thomas (2025) [19] |
| Reinbolt Hills |
1575.3 |
9.2 |
1 |
Thomas (2023c) [17] |
13C-Rich Diamond
During the study of the sample, blue tourmaline crystals are striking. In the black cores of some blue tourmaline crystals, we found 13C-rich diamonds. The Raman spectrum is shown in Figure 3. That 13C-rich diamond was used for deleting the microprobe carbon film is improbable. The effort to produce 13C-rich diamond for sample preparation is enormous and is therefore highly unlikely.
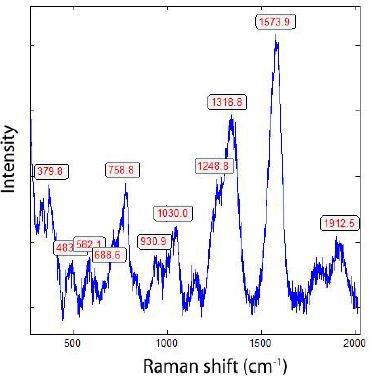
Figure 3: Raman spectrum of a 13C-rich diamond crystal in blue tourmaline with the first- order diamond band at 1318.8 cm-1. Also, the graphite G-band at 1573.9 cm-1 is relatively low. The FWHM are 48.8 and 60 cm-1 respectively.
As a first approximation, according to Enkovich et al. (2016) [12], results in a 13C concentration for the diamond of 28% and the graphite of 11.45%. That is an unusually high 13C value. Because the sample is repeatedly cleaned with diamond paste, remnants of diamond are possible. Therefore, a comparison with a similar diamond paste was necessary. Figure 4 shows a Raman spectrum of such a diamond from the paste used. The graphite band is nearby, wholly missing.
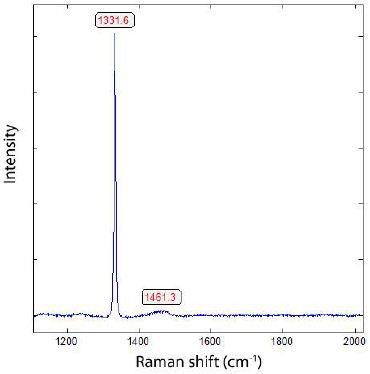
Figure 4: First order Raman spectrum of diamond of a DP diamond paste B from H. Struers, Chemiske Laboratorium, Copenhagen, Denmark. [2.2% 13C].
The first-order diamond band of the DP diamond paste B is at 1331.9 ± 0.22 cm-1, and the FWHM is 5.4 ± 0.2 cm-1. Thomas et al. (2023a) [13] listed the Raman spectroscopic result for technical diamonds used for geological sample preparation. Such a substantial shift from 1332.7 cm-1 to 1318.8 cm-1 could not observed. To be sure, we scrutinized the zero point of the Raman spectrometer steadily. Besides this, for the sample’s extreme 13C values, there are also 12C-rich nano-diamonds (mostly in cassiterite) present. From 10 different crystals, a mean of 1328.0 ± 2.8 cm-1 and a FWHM of 33.3 ± 12.9 cm-1 result. According to the mean comparison (0.999) between the 12C-rich diamond in cassiterite and the diamond used for polishing, a significant difference results. The same holds for the FWHM values.
Interpretation
Usually, in upper-crust tin deposits, neither orthorhombic cassiterites nor diamonds are present. Explanations for those unusual observations are in Thomas (2023a [1,8,9] and Thomas et al. (2023) regarding the different contributions to these problems. The strong 13C isotope enrichment in diamond and graphite is a further challenge. The simplest explanation is the interaction between the Earth’s mantle and the crust via supercritical fluids. Such fluids are brought from mantle depth not only water, CO2, and CH4 but also solid mineral phases such as foreign substances like diamonds, lonsdaleite, high- pressure SiO2, cassiterite polymorphs, etc. Because supercritical fluids are characterized by high diffusion rates and extremely low viscosity, isotope fractionation should also be extensive.
Table 2 summarizes the current results of the measured 13C-rich diamonds and graphite. The 13C-values of the measured diamonds and related graphite are uncommonly high and are nowhere near the “classic” diamonds and graphite data [20].
Discussion
Thomas and coauthors show that in upper crust mineralizations, especially in the Variscan tin deposits of the Erzgebirge, however, also at other places (Bornholm pegmatite, Land’s End Sn deposit, and Reinbolt Hills pegmatite), there are indications of the interaction between deep mantle and upper crust [19] via supercritical fluids. A huge problem is that most natural diamonds have low 13C concentrations. According to Cartigny (2005) [20], the abundance for 12C is about 98.9% and for 13C 1.1%. Therefore, the ratios are expressed in parts per thousand relative to an internationally accepted standard. However, the finding of very high 13C values in natural diamond and graphite is a novum and needs an acceptable interpretation. Here, we interpret the formation of 13C-rich diamond and graphite as a result of a strong isotope fractionation of the species CO2 and/or CH4 in the supercritical fluid. Maybe the formation of this type of diamond and graphite happens directly at the upper crust during the transition from supercritical to the undercritical state. That is supported by the finding of SiC-whisker, diamond, and graphite in beryl [16,18]. The highest 13C concentration was found by the author in the cassiterite sample (Sn- 70) from the Sauberg mine near Ehrenfriedersdorf [8], and high 13C values results also for diamond in cassiterite from Zinnwald (sample Sn-23). Using the data from Enkovich et al. (2016) [12], the results for 13C of the Sauberg-diamond is 99.9% and for the Zinnwald (sample Sn-23) 46.1%. More work is necessary to clear some open questions.
Conclusion
There are clear hints that some diamonds, together with SiC, grow at low pressure and moderate temperatures [18] via supercritical fluids. The transition from the supercritical to the under-critical state is a scientific challenge. The proof of the diamond formation at one- atmosphere pressure and relatively low temperatures (1025°C) by Gong et al. (2024) is a clear paradigm shift. Our results aim in the same direction.
Acknowledgment
The author thanks Kristian Drivenes from the Geological Survey of Norway, Trondheim, for the loan of the Land’s End sample. A discussion with Adolf Rericha helped with some clarifications.
References
- Thomas R (2023a) Unusual cassiterite mineralization, related to the Variscan tin mineralization of the Ehrenfriedersdorf deposit, Aspects in Mining & Mineral Science. 11: 1233-1236.
- Hellwig H, Goncharov AF, Gregoryanz E, Mao H, Hemley RJ (2003) Brillouin and Raman spectroscopy of the ferroelastic rutile-to CaCl2 transition in SnO2 at high Physical Review B 67: 174110-1174110-7.
- Shieh SR, Kubo A, Duffy TS, Prakapenka VB, Shen G (2006) High-pressure phases in SnO2 to 117 Phys. Rev. B. 73: 014105-1–014105-7.
- Girano HT (2018) Pressure-induced disorder in bulk and nanometric SnO2. Material Université de Lyon. Pg: 139.
- Balakrishnan K, Veerapandy V, Fjellvag H, Vajeeston P (2022) First-principles exploration into the physical and chemical properties of certain newly identified SnO2 ACS Publ. 7: 10382-10393.
- Thomas R (2024a) The CaCl2 -to-rutile phase transition in SnO from high to low pressure in nature. Geol Earth Mar Sci. 6: 1-4.
- Thomas R (2024b) Rhomboedric cassiterite as inclusions in tetragonal cassiterite from Slavkovský les – North Bohemia (Czech Republic). Geol Earth Mar Sci 6: 1-6.
- Thomas R (2025) Extremely 13C-rich diamond in orthorhombic cassiterites in the Variscan Erzgebirge, Saxony/Germany. Geology, Earth and Marine Sciences. 7: 1-5.
- Drivenes K (2022) Sn-rich tourmaline from the Land’s End granite, SW Journal of Geosciences. 67: 173-189.
- Chukanov NV, Vigsina MF (2020) Vibrational (Infrared and Raman) Spectra of Minerals and Related Compounds. Springer. Pg: 1370.
- Lafuente B, Downs, RT, Yang H, Stone N (2015) The power of database: the RRUFF In: Armbruster T, Danisi RM (eds.). Highlights in mineralogical crystallography. Berlin. Pg: 1-30.
- Enkovich PV, Brazhkin VV, Lyapin SG, Novikov AP, Kanada H, et (2016) Raman spectroscopy of isotopically pure (12C, 13C) and isotopically mixed (12.5C) diamond single crystals at ultrahigh pressures. Journal of Experimental and Theoretical Physics. 123: 443-451.
- Thomas R, Davidson R, Rericha A, Recknagel U (2023a) Ultahigh-pressure mineral inclusions in a crustal granite: Evidence for a novel transcrustal transport mechanism. Geosciences. 94: 1-13.
- Gutierrez G, Le Normand F, Aweke F, Muller D, Speisser C, et (2014) Mechanism of thin layers graphite formation by 13C implantation and annealing. Appl Sci. 4: 180- 194.
- Thomas R (2024c) 13C-rich diamond in a pegmatite from Ronne, Bornholm Island: Proof for the interaction between mantle and Geol Earth Mar Sci. 6: 1-3.
- Thomas R, Recknagel U, Rericha A (2023b) A moissanite-diamond-graphite paragenesis in a small beryl-quartz vein related to the Variscan tin-mineralization of the Ehrenfriedersdorf deposit, Germany. Aspects in Mining & Mineral Science. 11: 1310-1319.
- Thomas R (2023c) Diamond in pegmatitic sillimanite from Reinbolt Hills/East Geol Earth Mar Sci 5: 1-3.
- Thomas R (2023b) Growth of SiC whiskers in beryl by a natural supercritical VLS Aspects in Mining & Mineral Science. 11: 1292-1297.
- Thomas R, Rericha A (2025) Extreme element enrichment by the interaction of supercritical fluids from the mantle with crustal rocks. Minerals. 33: 1-10.
- Cartigny P (2005) Stable isotopes and origin of Elements. 1: 77-84.
- Gong Y, Luo D, Choe M, Seong WK, Bakharev P, et (2024) Growth of diamond in liquid metal at 1 atmosphere pressure. Nature. 629: 348-354.